Anguilliform Locomotion across a Natural Range of Swimming Speeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Experimental Setup
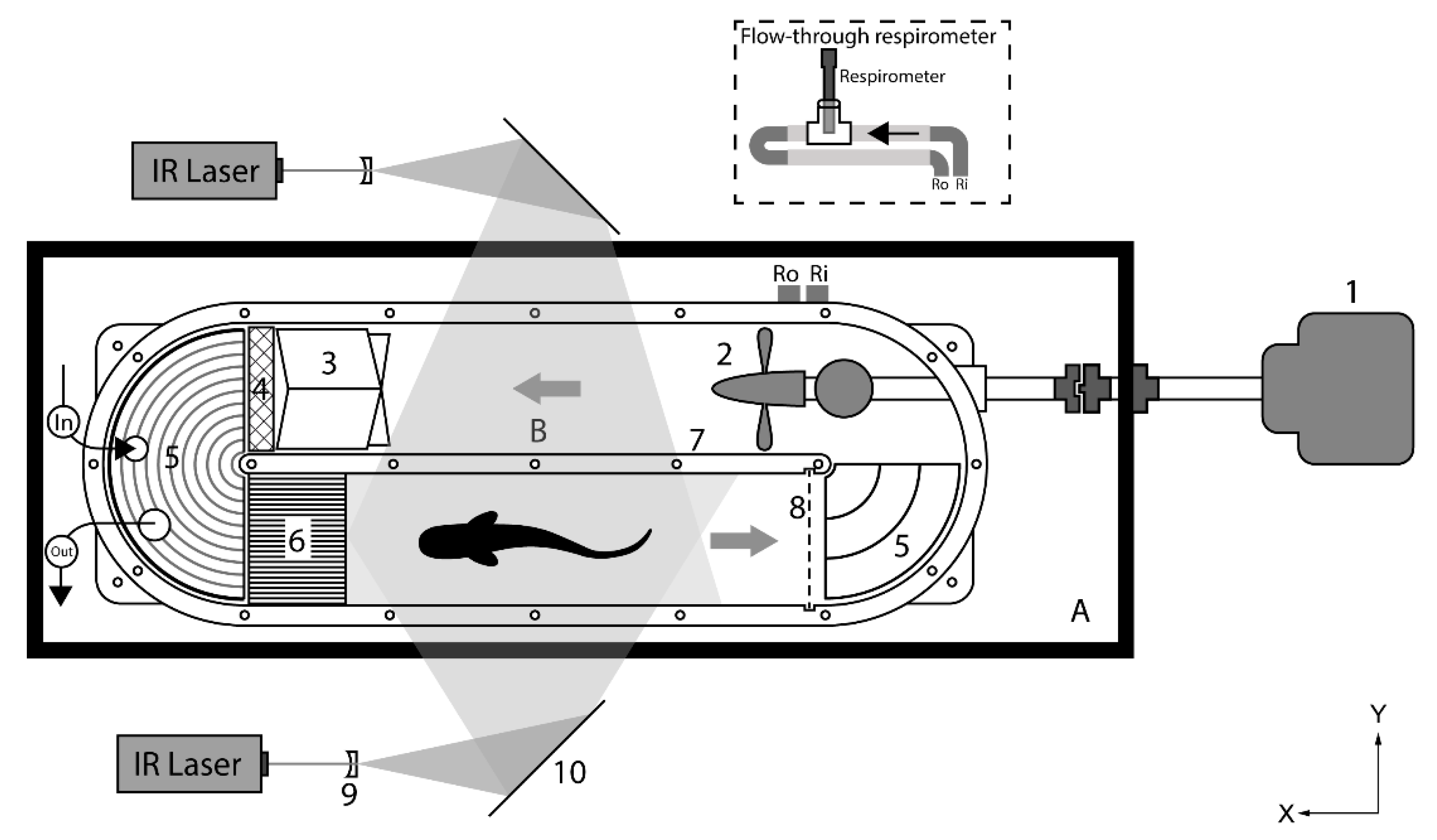
2.3.1. Swim Tunnel Respirometer
2.3.2. Respirometry
2.3.3. PIV Experiments
2.4. Kinematics, Pressure, and Force Calculations
2.5. Statistics
3. Results
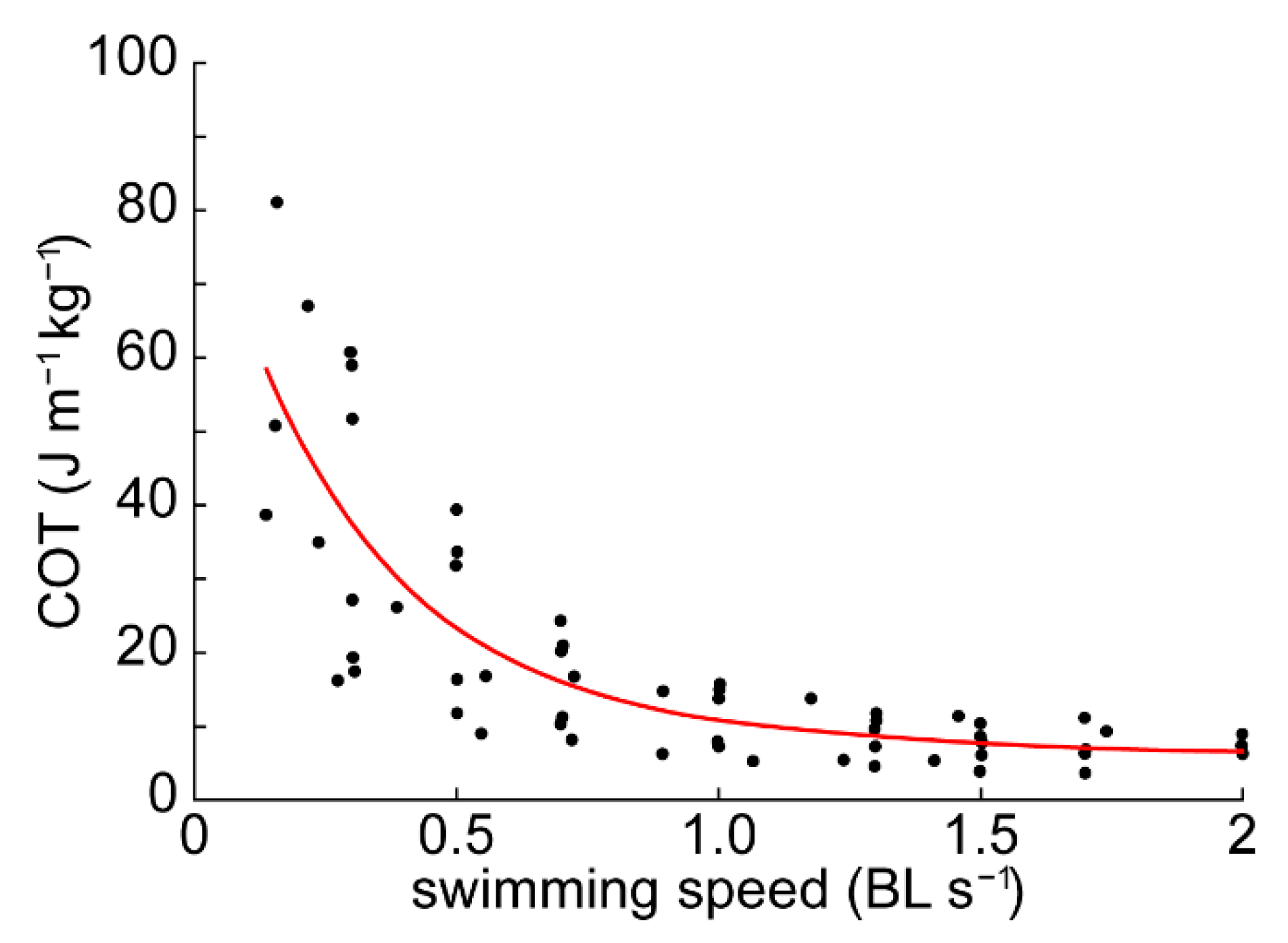
3.1. Cost of Transport
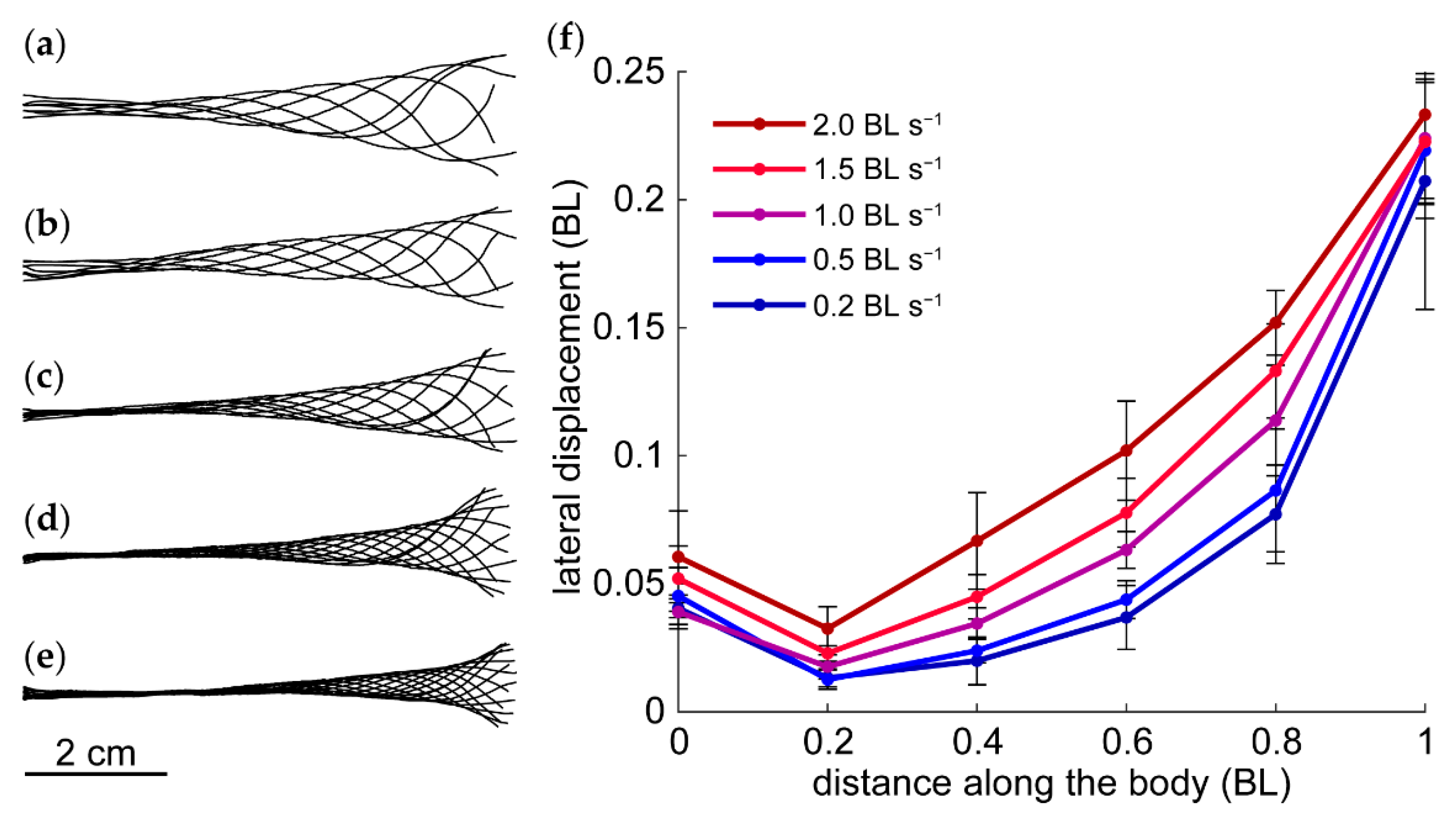
3.2. Kinematics
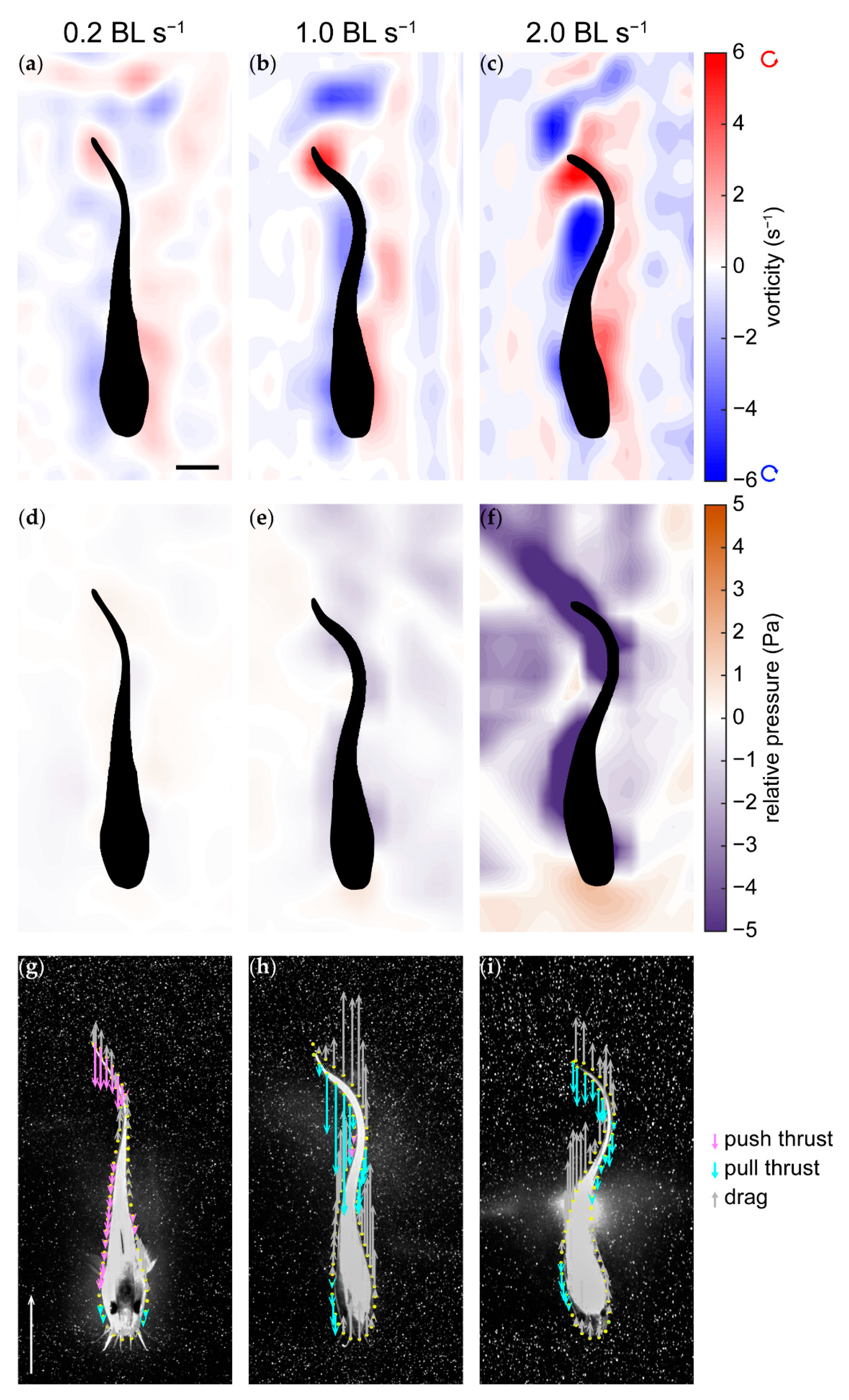
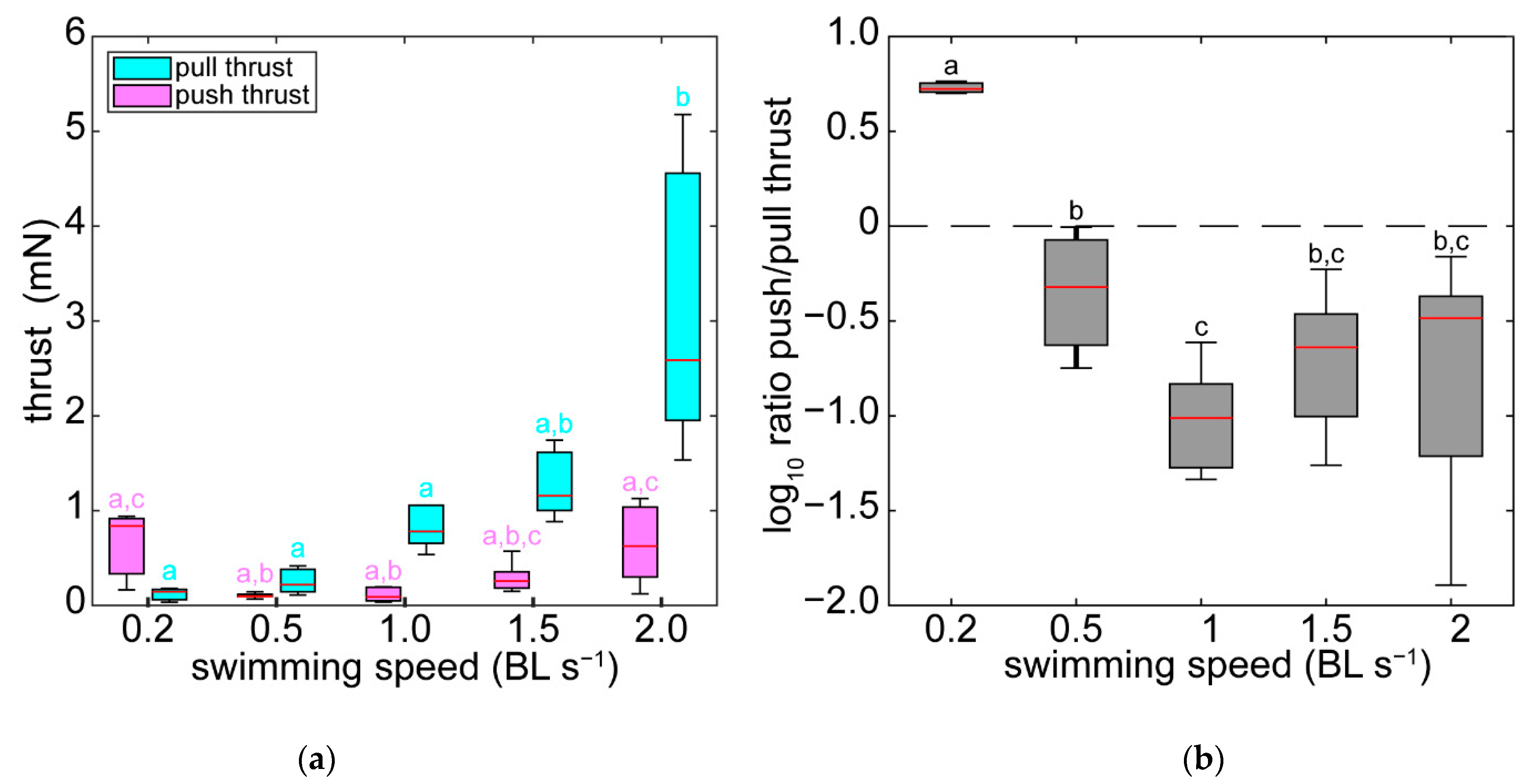
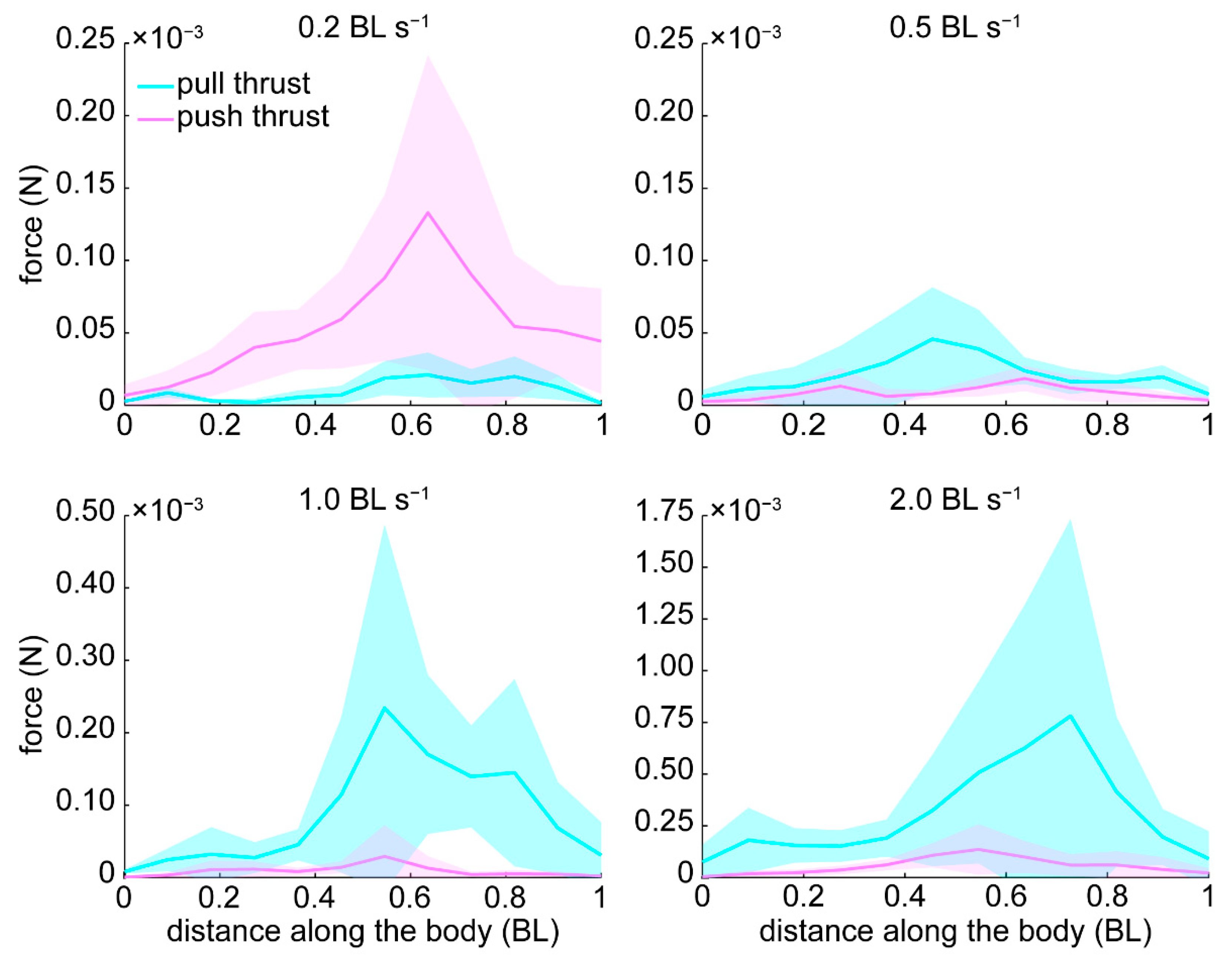
3.3. Pressure and Forces
4. Discussion
4.1. Energetics and Efficiency
4.2. Speed-Dependent Kinematics and Near-Body Vorticity
4.3. Pressure and Thrust Resulting from Varying Hydrodynamic Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Ginneken, V.; Antonissen, E.; Müller, U.K.; Booms, R.; Eding, E.; Verreth, J.; van den Thillart, G. Eel migration to the Sargasso: Remarkably high swimming efficiency and low energy costs. J. Exp. Biol. 2005, 208, 1329–1335. [Google Scholar] [CrossRef]
- van den Thillart, G.; Palstra, A.; van Ginneken, V. Simulated migration of European silver eel; swim capacity and cost of transport. J. Mar. Sci. Technol. 2007, 15, 1–16. [Google Scholar]
- Palstra, A.; van Ginneken, V.; van den Thillart, G. Cost of transport and optimal swimming speed in farmed and wild European silver eels (Anguilla anguilla). Comp. Biochem. Phys. A 2008, 151, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gray, J. Studies in animal locomotion: II. The relationship between waves of muscular contraction and the propulsive mechanism of the eel. J. Exp. Biol. 1933, 10, 88–104. [Google Scholar]
- Sigvardt, K.A. Spinal mechanisms in the control of lamprey swimming. Am. Zool. 1989, 29, 19–35. [Google Scholar] [CrossRef]
- Williams, T.L.; Grillner, S.; Smoljaninov, V.V.; Wallén, P.; Kashin, S.; Rossignol, S. Locomotion in lamprey and trout: The relative timing of activation and movement. J. Exp. Biol. 1989, 143, 559–566. [Google Scholar]
- Wardle, C.; Videler, J.; Altringham, J. Tuning in to fish swimming waves: Body form, swimming mode and muscle function. J. Exp. Biol. 1995, 198, 1629–1636. [Google Scholar]
- Warren, C.E.; Davis, G.E. Laboratory studies on the feeding, bioenergetics, and growth of fish. Or. State Univ. Agric. Exp. Station Spec. Rep. 1967, 230, 175–214. [Google Scholar]
- Steffensen, J.F.; Johansen, K.; Bushnell, P.G. An automated swimming respirometer. Comp. Biochem. Phys. A. 1984, 79, 437–440. [Google Scholar] [CrossRef]
- Brett, J.R.; Groves, T.D.D. Physiological energetics. Fish Physiol. 1979, 8, 280–352. [Google Scholar]
- Noca, F.; Shiels, D.; Jeon, D. Measuring instantaneous fluid dynamic forces on bodies, using only velocity fields and their derivatives. J. Fluid Struct. 1997, 11, 345–350. [Google Scholar] [CrossRef]
- Gurka, R.; Liberzon, A.; Hefetz, D.; Rubinstein, D.; Shavit, U. Computation of pressure distribution using piv velocity data. In Proceedings of the 3rd International Workshop on Particle Image Velocimetry, Santa Barbara, CA, USA, 16 September 1999; Adrian, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Lauder, G.V.; Tytell, E.D. Hydrodynamics of undulatory propulsion. Fish Physiol. 2005, 23, 425–468. [Google Scholar]
- van Oudheusden, B.W.; Scarano, F.; Roosenboom, E.W.M.; Casimiri, E.W.F.; Souverein, L.J. Evaluation of integral forces and pressure fields from planar velocimetry data for incompressible and compressible flows. Exp. Fluids 2007, 43, 153–162. [Google Scholar] [CrossRef]
- Dabiri, J.O.; Bose, S.; Gemmell, B.J.; Colin, S.P.; Costello, J.H. An algorithm to estimate unsteady and quasi-steady pressure fields from velocity field measurements. J. Exp. Biol. 2014, 217, 331–336. [Google Scholar] [CrossRef]
- Lucas, K.N.; Dabiri, J.O.; Lauder, G.V. A pressure-based force and torque prediction technique for the study of fish-like swimming. PLoS ONE 2017, 12, e0189225. [Google Scholar] [CrossRef]
- Gemmell, B.J.; Colin, S.P.; Costello, J.H.; Dabiri, J.O. Suction-based propulsion as a basis for efficient animal swimming. Nat. Commun. 2015, 6, 8790. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, B.J.; Fogerson, S.M.; Costello, J.H.; Morgan, J.R.; Dabiri, J.O.; Colin, S.P. How the bending kinematics of swimming lampreys build negative pressure fields for suction thrust. J. Exp. Biol. 2016, 219, 3884–3895. [Google Scholar] [CrossRef]
- Müller, U.K.; Smit, J.; Stamhuis, E.; Videler, J. How the body contributes to the wake in undulatory fish swimming: Flow fields of a swimming eel (Anguilla anguilla). J. Exp. Biol. 2001, 204, 2751–2762. [Google Scholar]
- Tytell, E.D.; Lauder, G.V. The hydrodynamics of eel swimming: I. Wake structure. J. Exp. Biol. 2004, 207, 1825–1841. [Google Scholar] [CrossRef] [PubMed]
- Clos, K.T.D.; Dabiri, J.O.; Costello, J.H.; Colin, S.P.; Morgan, J.R.; Fogerson, S.M.; Gemmell, B.J. Thrust generation during steady swimming and acceleration from rest in anguilliform swimmers. J. Exp. Biol. 2019, 222, jeb212464. [Google Scholar] [CrossRef]
- Lim, J.L.; Winegard, T.M. Diverse anguilliform swimming kinematics in Pacific hagfish (Eptatretus stoutii) and Atlantic hagfish (Myxine glutinosa). Can. J. Zool. 2015, 93, 213–223. [Google Scholar] [CrossRef]
- Svendsen, M.B.S.; Bushnell, P.G.; Steffensen, J.F. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish Biol. 2016, 88, 26–50. [Google Scholar] [CrossRef]
- Elliott, J.M.; Davison, W. Energy equivalents of oxygen consumption in animal energetics. Oecologia 1975, 19, 195–201. [Google Scholar] [CrossRef]
- Flammang, B.E.; Lauder, G.V.; Troolin, D.R.; Strand, T. Volumetric imaging of shark tail hydrodynamics reveals a three-dimensional dual-ring vortex wake structure. Proc. R. Soc. B Biol. Sci. 2011, 278, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, B.J.; Adhikari, D.; Longmire, E.K. Volumetric quantification of fluid flow reveals fish’s use of hydrodynamic stealth to capture evasive prey. J. R. Soc. Interface 2014, 11, 20130880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batchelor, G.K. An Introduction to Fluid Mechanics; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Wang, J.; Zhang, C.; Katz, J. GPU-based, parallel-line, omni-directional integration of measured pressure gradient field to obtain the 3D pressure distribution. Exp. Fluids 2019, 60, 58. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Pearson: Bergen, NJ, USA, 1999; pp. 35–424. [Google Scholar]
- William, F.; Beamish, H. Migration and spawning energetics of the anadromous sea lamprey, Petromyzon marinus. Environ. Biol. Fishes 1979, 4, 3–7. [Google Scholar] [CrossRef]
- Daniel, T.L. Unsteady aspects of aquatic locomotion. Am. Zool. 1984, 24, 121–134. [Google Scholar] [CrossRef]
- Di Santo, V.; Kenaley, C.P.; Lauder, G.V. High postural costs and anaerobic metabolism during swimming support the hypothesis of a U-shaped metabolism-speed curve in fishes. Proc. Natl. Acad. Sci. USA 2017, 114, 13048–13053. [Google Scholar] [CrossRef]
- Webb, P.W. Control of posture, depth, and swimming trajectories of fishes. Integr. Comp. Biol. 2002, 42, 94–101. [Google Scholar] [CrossRef]
- Di Santo, V.; Blevins, E.L.; Lauder, G.V. Batoid locomotion: Effects of speed on pectoral fin deformation in the little skate, Leucoraja erinacea. J. Exp. Biol. 2017, 220, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Kendall, J.L.; Lucey, K.S.; Jones, E.A.; Wang, J.; Ellerby, D.J. Mechanical and energetic factors underlying gait transitions in bluegill sunfish (Lepomis macrochirus). J. Exp. Biol. 2007, 210, 4265–4271. [Google Scholar] [CrossRef]
- Webb, P.W. Stability and maneuverability. Fish Physiol. 2005, 23, 281–332. [Google Scholar]
- Schmidt-Nielsen, K. Locomotion: Energy cost of swimming, flying, and running. Science 1972, 177, 222–228. [Google Scholar] [CrossRef]
- Palstra, A. Energetic Requirements and Environmental Constraints of Reproductive Migration and Maturation of European Silver Eel (Anguilla anguilla L.). Ph.D. Thesis, University of Leiden, Leiden, The Netherlands, 2006. [Google Scholar]
- Goolish, E.M. The metabolic consequences of body size. In Biochemistry and Molecular Biology of Fishes; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 4, pp. 335–366. [Google Scholar]
- Triantafyllou, G.S.; Triantafyllou, M.S.; Grosenbauch, M.A. Optimal thrust development in oscillating foils with application to fish propulsion. J. Fluids Struct. 1993, 7, 205–224. [Google Scholar] [CrossRef]
- Triantafyllou, M.S.; Triantafyllou, G.S. An efficient swimming machine. Sci. Am. 1995, 272, 40–46. [Google Scholar] [CrossRef]
- Taylor, G.K.; Nudds, R.L.; Thomas, A.L.R. Flying and swimming animals cruise at a Strouhal number tuned for high power efficiency. Nature 2003, 425, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Eloy, C. Optimal Strouhal number for swimming animals. J. Fluids Struct. 2012, 30, 205–218. [Google Scholar] [CrossRef]
- Tytell, E.D. The hydrodynamics of eel swimming II. Effect of swimming speed. J. Exp. Biol. 2004, 207, 3265–3279. [Google Scholar] [CrossRef]
- Anderson, J.M.; Streitlien, K.; Barrett, D.S.; Triantafyllou, M.S. Oscillating foils of high propulsive efficiency. J. Fluid Mech. 1998, 360, 41–72. [Google Scholar] [CrossRef]
- Wang, Z.J. Vortex shedding and frequency selection in flapping flight. J. Fluid Mech. 2000, 410, 323–341. [Google Scholar] [CrossRef]
- Müller, U.K.; Stamhuis, E.J.; Videler, J.J. Riding the waves: The role of the body wave in undulatory fish swimming. Integr. Comp. Biol. 2002, 42, 981–987. [Google Scholar] [CrossRef]
- Yu, C.L.; Hsu, Y.H.; Yang, J.T. The dependence of propulsive performance on the slip number in an undulatory swimming fish. Ocean Eng. 2013, 70, 51–60. [Google Scholar] [CrossRef]
- Gillis, G. Undulatory locomotion in elongate aquatic vertebrates: Anguilliform swimming since Sir James Gray. Am. Zool. 1996, 36, 656–665. [Google Scholar] [CrossRef]
- Gillis, G. Anguilliform locomotion in an elongate salamander (Siren intermedia): Effects of speed on axial undulatory movements. J. Exp. Biol. 1997, 200, 767–784. [Google Scholar]
- D’Aout, K.; Aerts, P. A kinematic comparison of forward and backward swimming in the eel Anguilla anguilla. J. Exp. Biol. 1999, 202, 1511–1521. [Google Scholar]
- Lighthill, M.J. Hydromechanics of aquatic animal propulsion. Annu. Rev. Fluid Mech. 1969, 1, 413–466. [Google Scholar] [CrossRef]
- Lighthill, M.J. Large-amplitude elongated-body theory of fish locomotion. Proc. R. Soc. B Biol. Sci. 1971, 179, 125–138. [Google Scholar]
- Weihs, D. The mechanisms of rapid starting of slender fish. Biorheology 1973, 10, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Weihs, D. Design features and mechanics of axial locomotion in fish. Am. Zool. 1989, 29, 151–160. [Google Scholar] [CrossRef]
- Gillis, G.B. Environmental effects on undulatory locomotion in the American eel Anguilla rostrata: Kinematics in water and on land. J. Exp. Biol. 1998, 201, 949–961. [Google Scholar]
- Williams, T.L.; Bowtell, G.; Carling, J.C.; Sigvardt, K.A.; Curtin, N.A. Interactions between muscle activation, body curvature and the water in the swimming lamprey. In Biological Fluid Dynamics; Ellington, C.P., Pedley, T.J., Eds.; The Company of Biologists Ltd.: Cambridge, UK, 1995; pp. 49–59. [Google Scholar]
- Liao, J.C. Swimming in needlefish (Belonidae): Anguilliform locomotion with fins. J. Exp. Biol. 2002, 205, 2875–2884. [Google Scholar] [PubMed]
- Webb, P.W. Form and function in fish swimming. Sci. Am. 1984, 251, 72–83. [Google Scholar] [CrossRef]
- Müller, U.K.; van Leeuwen, J.L. Undulatory fish swimming: From muscles to flow. Fish Fish. 2006, 7, 84–103. [Google Scholar] [CrossRef]
- Li, G.; Müller, U.K.; van Leeuwen, J.L.; Liu, H. Body dynamics and hydrodynamics of swimming fish larvae: A computational study. J. Exp. Biol. 2012, 215, 4015–4033. [Google Scholar] [CrossRef]
- Sfakiotakis, M.; Lane, D.; Davies, J. Review of fish swimming modes for aquatic locomotion. IEEE J. Oceanic Eng. 1999, 24, 237–252. [Google Scholar] [CrossRef]
- Lauder, G.V.; Drucker, E.G. Forces, fishes, and fluids: Hydrodynamic mechanisms of aquatic locomotion. News Physiol. Sci. 2002, 17, 235–240. [Google Scholar] [CrossRef]
- Borazjani, I.; Sotiropoulos, F. On the role of form and kinematics on the hydrodynamics of self-propelled body/caudal fin swimming. J. Exp. Biol. 2010, 213, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Lauder, G.V.; Flammang, B.; Alben, S. Passive robotic models of propulsion by the bodies and caudal fins of fish. Integr. Comp. Biol. 2012, 52, 576–587. [Google Scholar] [CrossRef]
- Wen, L.; Lauder, G. Understanding undulatory locomotion in fishes using an inertia-compensated flapping foil robotic device. Bioinspir. Biomim. 2013, 8, 046013. [Google Scholar] [CrossRef]

| Parameters | Symbols | Units | 0.2 BL s−1 | 0.5 BL s−1 | 1.0 BL s−1 | 1.5 BL s−1 | 2.0 BL s−1 | p-Value |
|---|---|---|---|---|---|---|---|---|
| Reynolds number | Re | 1319 ± 406 | 2767 ± 316 | 5430 ± 556 | 8152 ± 782 | 10,835 ± 1033 | <0.05 | |
| amplitude envelope area | A | BL2 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.013 | 0.10 ± 0.01 | <0.05 |
| wavelength | λ | BL | 0.62 ± 0.01 | 0.68 ± 0.02 | 0.71 ± 0.02 | 0.73 ± 0.01 | 0.75 ± 0.01 | <0.05 |
| period | T | s | 0.55 ± 0.04 | 0.47 ± 0.02 | 0.35 ± 0.01 | 0.28 ± 0.01 | 0.24 ± 0.01 | <0.05 |
| frequency | f | s−1 | 1.83 ± 0.14 | 2.14 ± 0.08 | 2.84 ± 0.08 | 3.64 ± 0.13 | 4.21 ± 0.18 | <0.05 |
| body-wave speed | V | BL s−1 | 1.14 ± 0.09 | 1.45 ± 0.04 | 2.01 ± 0.08 | 2.67 ± 0.09 | 3.16 ± 0.13 | <0.05 |
| slip | 0.18 ± 0.01 | 0.35 ± 0.01 | 0.50 ± 0.02 | 0.57 ± 0.02 | 0.64 ± 0.03 | <0.05 | ||
| Strouhal number | St | 1.88 ± 0.23 | 0.93 ± 0.03 | 0.63 ± 0.02 | 0.54 ± 0.01 | 0.49 ± 0.02 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tack, N.B.; Du Clos, K.T.; Gemmell, B.J. Anguilliform Locomotion across a Natural Range of Swimming Speeds. Fluids 2021, 6, 127. https://doi.org/10.3390/fluids6030127
Tack NB, Du Clos KT, Gemmell BJ. Anguilliform Locomotion across a Natural Range of Swimming Speeds. Fluids. 2021; 6(3):127. https://doi.org/10.3390/fluids6030127
Chicago/Turabian StyleTack, Nils B., Kevin T. Du Clos, and Brad J. Gemmell. 2021. "Anguilliform Locomotion across a Natural Range of Swimming Speeds" Fluids 6, no. 3: 127. https://doi.org/10.3390/fluids6030127
APA StyleTack, N. B., Du Clos, K. T., & Gemmell, B. J. (2021). Anguilliform Locomotion across a Natural Range of Swimming Speeds. Fluids, 6(3), 127. https://doi.org/10.3390/fluids6030127






