Abstract
Nanofluids are obtained by dispersing nanoparticles and dispersant, when present, in a base fluid. Their properties, in particular their stability, however, are strictly related to several other parameters, knowledge of which is important to reproduce the nanofluids and correctly interpret their behavior. Due to this complexity, the results appear to be frequently unreliable, contradictory, not comparable and/or not repeatable, in particular for the scarcity of information on their preparation. Thus, it is essential to define what is the minimum amount of information necessary to fully describe the nanofluid, so as to ensure the possibility of reproduction of both their formulation and the measurements of their properties. In this paper, a literature analysis is performed to highlight what are the most important parameters necessary to describe the configuration of each nanofluid and their influence on the nanofluid’s properties. A case study is discussed, analyzing the information reported and the results obtained for the thermophysical properties of nanofluids formed by water and TiO2 nanoparticles. The aim is to highlight the differences in the amount of information given by the different authors and exemplify how results can be contradictory. A final discussion gives some suggestions on the minimum amount of information that should be given on a nanofluid to have the possibility to compare results obtained for similar nanofluids and to reproduce the same nanofluid in other laboratories.
1. Introduction
Huge research efforts have been devoted in the last 20 years to the study of nanofluids, from the point of view of preparation methods, stabilization of the colloidal suspension, thermophysical properties, modelling, etc. Their use is now envisaged for a large variety of applications in many sectors, ranging from agriculture, for the enhancement of crop nutrition and protection [1], medicine, for cancer therapy [2], to environmental chemistry, for wastewater decontamination [3] and mechanical engineering, for enhancing tribological and thermophysical features of lubricants [4]. However, since the first formulation of the concept of nanofluid [5] two decades ago, heat transfer applications are the most studied due to the possibility offered by dispersion of solid nanoparticles in common thermal fluids, such as water, oil and water−ethylene/propylene glycol mixtures, to significantly increase their thermal conductivity and eventually their heat transfer coefficient. This is a very important issue in all engineering applications, where efficient cooling is a strong need to reduce costs, size, energy consumption and environmental impact of the systems. From this point of view, the application of nanofluids look attractive in all sectors of engineering as new generation coolants, e.g., for engine cooling [6], solar water heating [7], heating and cooling of buildings [8], refrigeration and air conditioning [9], cooling of electronics [10], power generation [11] and many others [12]. For heat transfer applications, the most important properties to be considered are thermal conductivity and viscosity, because heat transfer coefficients, pressure drops and eventually the energetic efficiency of the systems strongly depend on them. However, nanofluids are very complex objects, whose properties and performance depend on a significant number of parameters: material, concentration, shape and size of the nanoparticles, type and concentration of dispersants, preparation methods [13,14,15,16,17,18,19]. The situation is also complicated by the fact that many of these parameters interact with each other. In the last two decades, numerous articles have been published that analyze, from both a theoretical and an experimental point of view, the influence of these parameters. Numerous reviews are also available, which attempt to summarize the results available in the literature by critically evaluating them [20,21,22,23,24]. However, despite the importance of each of the parameters listed above on the performance of individual nanofluids, some or many of them are often overlooked in the literature, at least among the publicly reported information. This makes the results obtained difficult to interpret, as well as preventing the reproduction of the nanofluids by other laboratories. The purpose of this article is not to perform a comprehensive review of the literature on nanofluids for heat transfer applications. With the support of selected articles, however, we want to try to answer some important questions regarding the knowledge we have on nanofluids and how the literature describes them: what are the parameters necessary to describe a nanofluid? What is their influence on the thermophysical properties? Do the articles available in the literature describe in an exhaustive way the parameters that characterize a nanofluid, allowing its reproduction? Are the results obtained from the measurements of the thermophysical properties consistent?
First of all, the relevance of the individual parameters characterizing the nanofluids, will be analyzed on the basis of selected papers found in the literature, showing their potential effect on the main thermophysical properties (thermal conductivity and viscosity) for the purposes of heat exchange. Although it is almost impossible to quantify this effect univocally, it is still possible to establish orders of magnitude and/or identify trends. As a second step, through the analysis of a case study (i.e., water-based nanofluids with dispersion of titanium oxide nanoparticles), the spectrum of results achieved by different authors for the same kind of nanofluid is investigated. Twenty papers have been found in the literature reporting measurements on thermal conductivity and/or viscosity of nanofluids obtained dispersing TiO2 nanoparticles in water. Each paper describes the materials used and the methodology of preparation of the nanofluid. The scope of the second part of the present paper is to evaluate the completeness of the information available on the studied nanofluids, together with the comparison of the results obtained for the thermophysical properties, in particular thermal conductivity. In this way, it will be possible to highlight the reproducibility of a single nanofluid by other laboratories, one of the basic requirements to control and confirm the scientific results. Moreover, the analysis aims at highlighting whether the measurements of the thermophysical properties are coherent with each other or if they look contradictory on the basis of the available information.
2. Nanofluid Parameters Influencing Thermophysical Properties
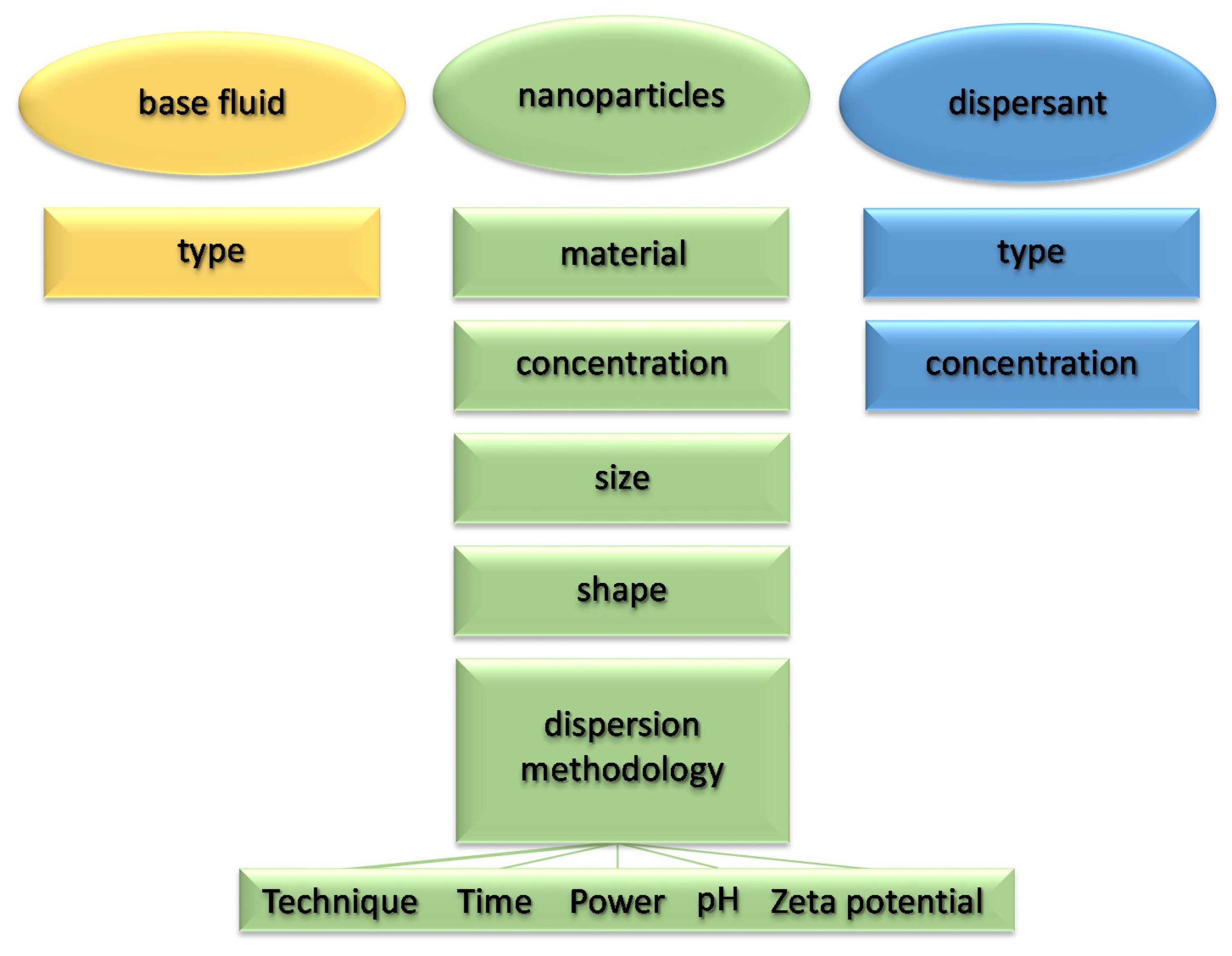
In the first part of the article, without proposing a further systematic review of the works available in the literature, we want to highlight the effects of the various parameters characterizing the nanofluid on the main thermophysical properties (thermal conductivity and viscosity) for the purposes of heat exchange. Figure 1 summarizes the main parameters affecting the thermophysical properties of nanofluids.
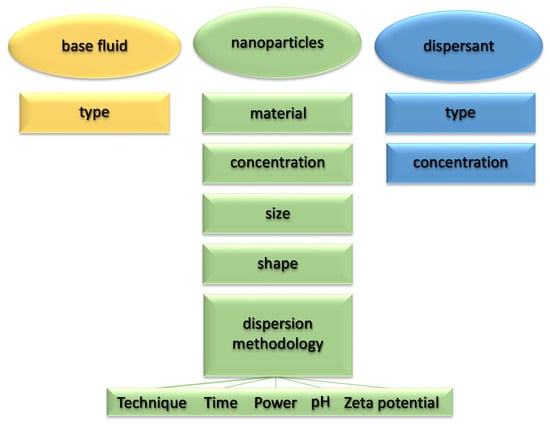
Figure 1.
Main parameters affecting the thermophysical properties of nanofluids.
2.1. Methods of Preparation
Before starting the analysis of the main parameters characterizing nanofluids, it is worth describing shortly the methods of preparation of a nanofluid, because they significantly affect the stability of the colloidal suspension and its thermophysical properties. Consequently, the preparation method is an essential step in order to obtain nanofluids with good performance. More detailed information on these methodologies can be obtained by the reader referring to the reviews available in the literature (e.g., [13,24,25]). The methods available are various, but they can be divided into two main categories that are distinct by the number of steps required to complete the procedure: one-step methods and two-step methods.
2.1.1. Two-Step Method
It is the most commonly used method for the production of nanofluids, generated by dispersing nanoparticles in the base liquid until a homogeneous suspension is obtained. The main problem is caused by the considerable Van der Waals attractive forces, which can create agglomerations of considerable size and are difficult to reduce. The first phase of the process consists of the production of the nanoparticles, which can be done by applying different techniques: sol−gel method, microemulsion, hydrothermal synthesis and others [24]. The second phase consists of the dispersion of the nanoparticle powder in the liquid. After carefully weighing the amount of powder necessary to obtain the desired concentration in the pre-established amount of base fluid, the procedure generally involves gradually pouring the nanoparticles into the liquid, stirring the solution with a magnetic stirrer until the nanoparticles are completely dispersed. We then proceed with the application of ultrasound to the solution, in order to counteract the attractive intermolecular forces by breaking the clusters and thus reducing their size. In this way, the stability of the suspension is increased but it is also possible to improve the thermophysical properties. It should be noted that the sonication time (which can vary from a few tens of minutes to a few hours) and the sonication power (typically around a few hundred Watts) are important for an optimal dispersion of the nanoparticles [26]. In some studies, it has been shown that increasing the sonication time decreases the size of the particles, but generally after a certain time no reduction is observed. In addition to sonication, other mechanical methods can be employed for cluster reduction, e.g., high shear homogenization, stirred bead milling, high speed mixing [27,28]. However, mechanical methods alone are not always sufficient to guarantee adequate stability to the nanofluid. Chemical methods are then used, which can improve stability, but in turn affect the thermophysical properties of the nanofluid, as will be seen later. The first approach consists of using a dispersant to increase the repulsive forces between the nanoparticles, reducing the likelihood that they form agglomerates [13]. With the same purpose, another method consists of modifying the pH of the solution, adding suitable substances to make the solution more acidic or more basic: in this way the Zeta potential of the solution can be increased in order to guarantee a good stabilization [29]. These techniques (stirring, mechanical disintegration, use of dispersants, modification of the pH) are often used together to maximize the stability of the nanoparticles. At the same time, however, they alter the thermophysical properties of the nanofluid, even worsening its performance. A more detailed discussion on these techniques and their effect on the properties of the nanofluid is made in paragraph 3.2 of the article. It is interesting to note, looking at Table 1, that, at least in the case of water-based nanofluids and TiO2 nanoparticles, the two-step method is the one used in all cases considered and that sonication is the most used mechanical technique. In addition to its relative simplicity, the main advantage of the two-step technique over the one-step technique is its cost-effectiveness, as the powdered nanoparticles nowadays are produced on a large scale at relatively low cost. On the other hand, the tendency to agglomerate particles both in the powder state and during the storage of the nanofluid, forces the application of mechanical or chemical dispersion techniques which, among other things, do not allow adequate control over the shape and size of the nanoparticles [25].
2.1.2. One-Step Method
One-phase methods aim to limit or overcome the problem of the agglomeration of nanoparticles that can occur with the two-phase method in the various phases that characterize it (storage, pouring, dispersion and drying of the nanoparticles). The basic idea is to obtain the nanofluid by generating the nanoparticles directly inside the base fluid, simultaneously carrying out synthesis and dispersion of the nanoparticles in the fluid. Over the past two decades, various one-step techniques have been developed in an attempt to reduce costs and expand the types of nanofluids that can be produced with this methodology. Physical vapor deposition (PVD) was the first one-step method applied to nanofluids, it was developed by Choi and Eastman in 2001 [30] and is still the most widely used. With this technique, the nanoparticle material (e.g., Cu) is evaporated inside a vacuum chamber and condenses directly into the base liquid. The main limitation is given by the possibility of using only high boiling fluids. A similar but more advanced technique, developed later, is pulsed wire evaporation (PWE) [31]. Further developments have been obtained with submerged arc techniques, the SANSS (submerged arc nanoparticle synthesis system) [32] and the ASNSS (arc spray nanoparticle synthesis system) [33]. These techniques are based on the evaporation of the nanoparticle material (e.g., titanium), immersed in the base liquid inside a vacuum chamber, by means of an electric arc at a very high temperature (of the order of 10,000 K). The nanofluids thus obtained show high stability and better dispersion of the nanoparticles, as well as a more regular shape. Another type of one-step technique is constituted by the Laser Ablation method, [34] with the PLA (pulsed laser ablation) variant [35]. In this technique, a laser beam of suitable intensity and wavelength strikes the material of the nanoparticles immersed in the base fluid, causing the production of nanometric particles that are directly dispersed in the base fluid. Finally, [35] proposed a technique that allows a very stable dispersion of nanoparticles to be obtained directly within the base fluid by microwave irradiation of a solution of the base fluid containing the reagents and a dispersant. In general, the main advantage of one-step methods is to obtain the nanofluid by producing and dispersing the nanoparticles directly in the base fluid, without the need for intermediate steps which, as seen for the two-step methods, can lead to the formation of agglomerates. In addition, very stable nanofluids can be obtained without the need to add dispersants. Finally, these techniques allow for much better control of the size and shape of the dispersed nanoparticles. The main disadvantage of one-step methods is their high cost, although cheaper technologies are being developed. Furthermore, they can only be produced in batches and in small quantities and therefore are not yet suitable for industrial production. Finally, from the point of view of quality, the nanofluid produced with these techniques may contain residues of reagents that have not been transformed, negatively affecting the performance of the nanofluids in their applications.
2.2. Effect of Nanoparticle Concentration and Morphology on Thermophysical Properties
Nanoparticles added to the base fluid are characterized by the material of which they are made, their size, shape and concentration. Each of these parameters influence the stability and the thermophysical properties of the nanofluid and thus its performance.
2.2.1. Nanoparticle Concentration
The concentration of nanoparticles dispersed in the base fluid, expressed as volumetric or a mass fraction, is one of the main parameters influencing the thermophysical properties of the nanofluid. Practically all the results available in the literature refer the measurements of the thermophysical properties to the nanoparticle concentration. In general, even if with some exceptions, increasing concentration determines an increase of both thermal conductivity and viscosity (e.g., [15,26,36,37,38]). This is a problem in terms of the nanofluid’s applications, since heat transfer is influenced by both properties, but with contrary effects: higher thermal conductivity improves heat exchange, while higher viscosity worsens heat exchange and increase the amount of energy required to circulate the nanofluid, due to the higher friction factor. Thus, when designing a nanofluid, these two properties should be considered together, searching for the conditions to optimize the overall performance of the nanofluid.
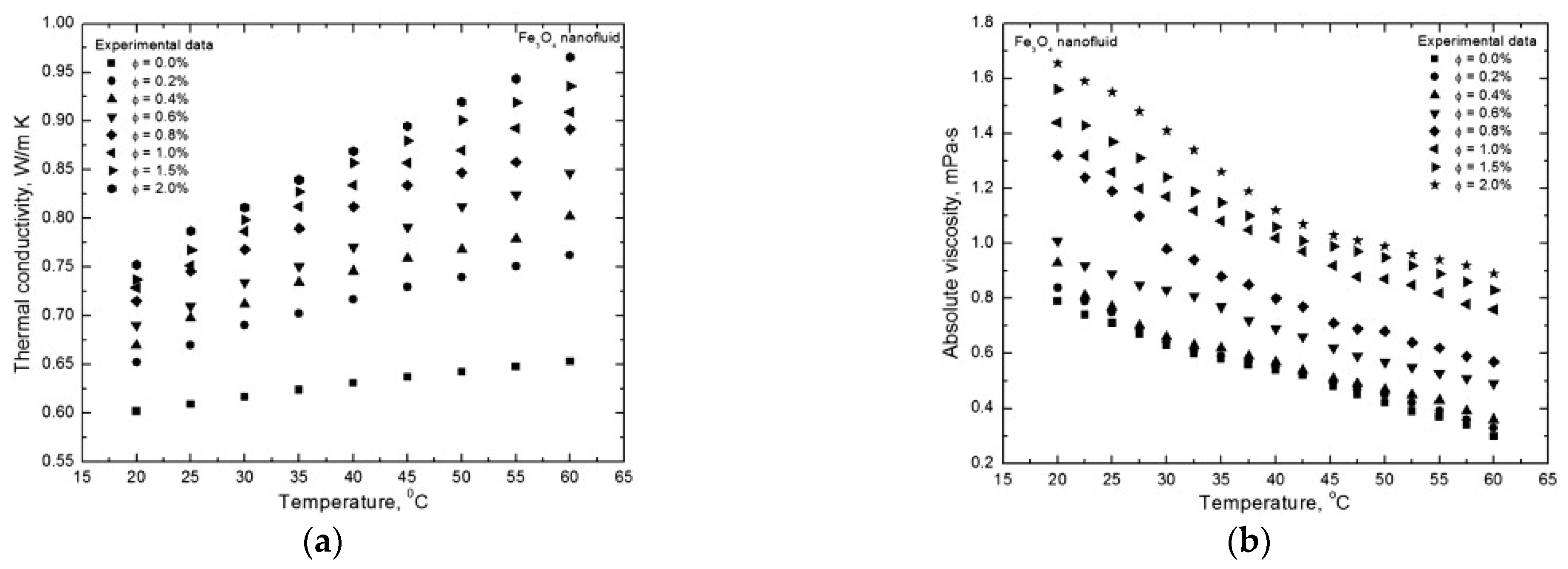
Just to exemplify the effect of particle concentration, Figure 2 shows a typical behavior for the thermal conductivity and the viscosity of a nanofluid in relation to the nanoparticle volume fraction [39]. For both thermal conductivity and viscosity, it is evident the enhancement produced by increasing the volume fraction of Fe3O4 nanoparticles at given temperatures. It is worth noting here that temperature is a very important parameter, since it strongly influences all the thermophysical properties of nanofluids. However, it is not an intrinsic parameter of the nanofluid in itself.
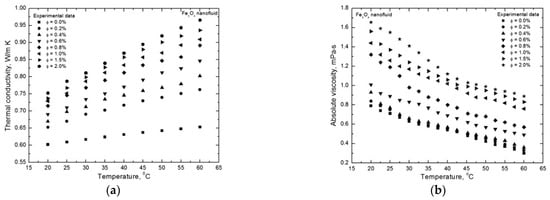
Figure 2.
Typical behavior of (a) thermal conductivity and (b) viscosity as a function of nanoparticle volumetric fraction at various temperatures [39].
2.2.2. Nanoparticle Size
The influence of nanoparticle size on thermal conductivity and viscosity of nanofluids has been explored in several papers (e.g., [37,40,41,42]). The size of nanoparticles depends on the way they are produced and dispersed in the base fluid. The size of nanoparticles can vary within the fluid due to the formation of clusters of more or less compact particles, the size of which can possibly be reduced using sonication: by increasing the sonication time, it is possible to reduce the size of the particles, as shown also experimentally (e.g., [26]). In order to control size and shape of the nanoparticles dispersed in the base fluid it is very useful to apply detection techniques such as DLS, XRD, SEM or TEM.
With reference to nanofluid thermal conductivity, the enhancement compared to the base fluid, determined by the interaction between nanoparticles and base liquid, is generated by the cumulative effect of various mechanisms [41,43]: Brownian motion, ordered base fluid molecules layering at the interface solid−liquid, nature of heat transport (fonons), high nanoparticle specific surface area.
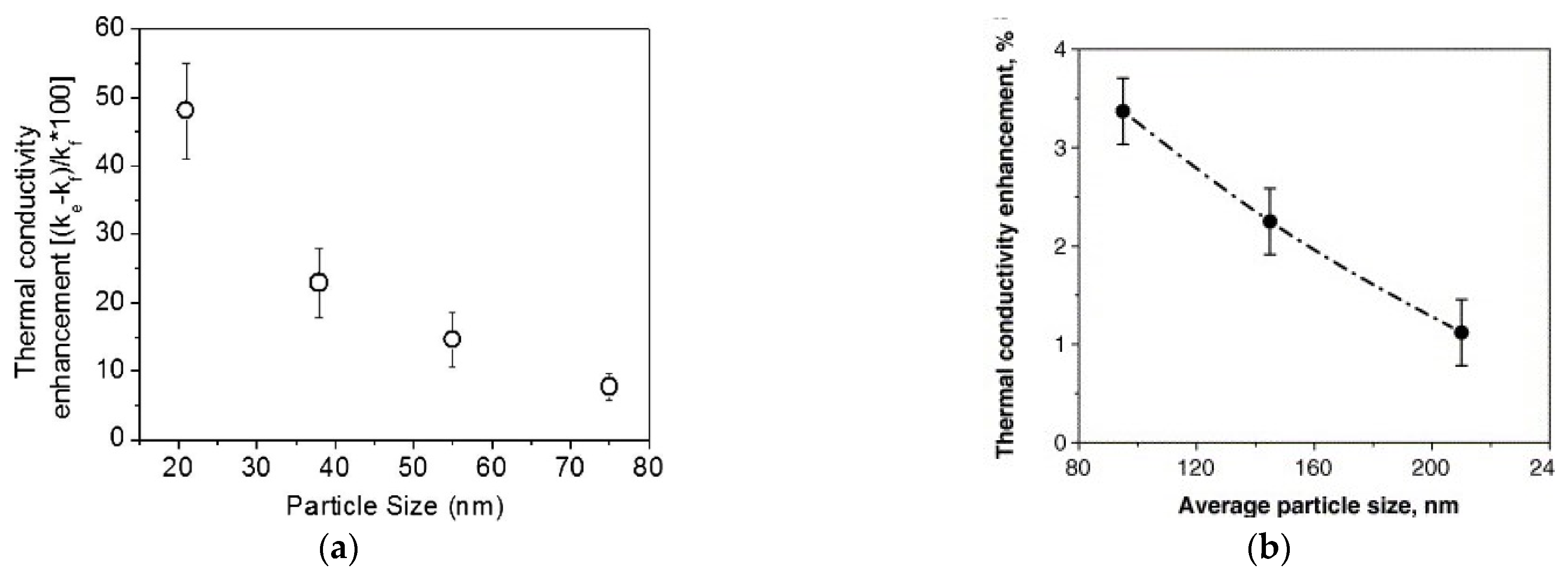
Nanoparticle size, besides strongly affecting the stability of nanofluids, intervenes in some of these mechanisms, influencing the transmission of heat between liquid and nanoparticles. For example, size is important in Brownian motion: the smaller the size of the particle, the greater its agitation speed, favoring heat exchange. In addition, smaller particles, at equal concentration in the liquid, also increase the exchange surface ratio at the interface between solid and liquid, helping the heat transfer [37]. From the theoretical point of view, supported by several experimental achievements, smaller particles increase the enhancement of thermal conductivity. Figure 3a shows the thermal conductivity enhancement of a gold dispersed (0.00026 vol%) water-based nanofluid as a function of nanoparticle size. In this case, the influence of nanoparticles is striking, since thermal conductivity enhancement increases from around 10% for particle size of 75 nm to 50% for particle size of 20 nm [41].
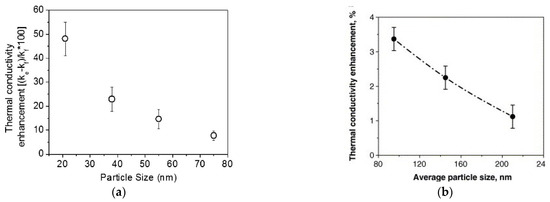
Figure 3.
Thermal conductivity enhancement percentage of two different nanofluids as a function of particle size (a) [41] (b) [44].
However, a much lower increase is shown by a water-based nanofluid where gold is substituted by TiO2 nanoparticles [44]. In this case (Figure 3b), enhancement increased only by 1.2% to 3.5%, when nanoparticle size decreased from 210 nm to 95 nm. However, this behavior cannot be generalized, because other experimental results show an inverse relation between size and thermal conductivity enhancement (e.g., [45]). This suggests that the influence of size on thermal conductivity enhancement is related to other parameters, e.g., nanoparticle material, dispersant, sonication.
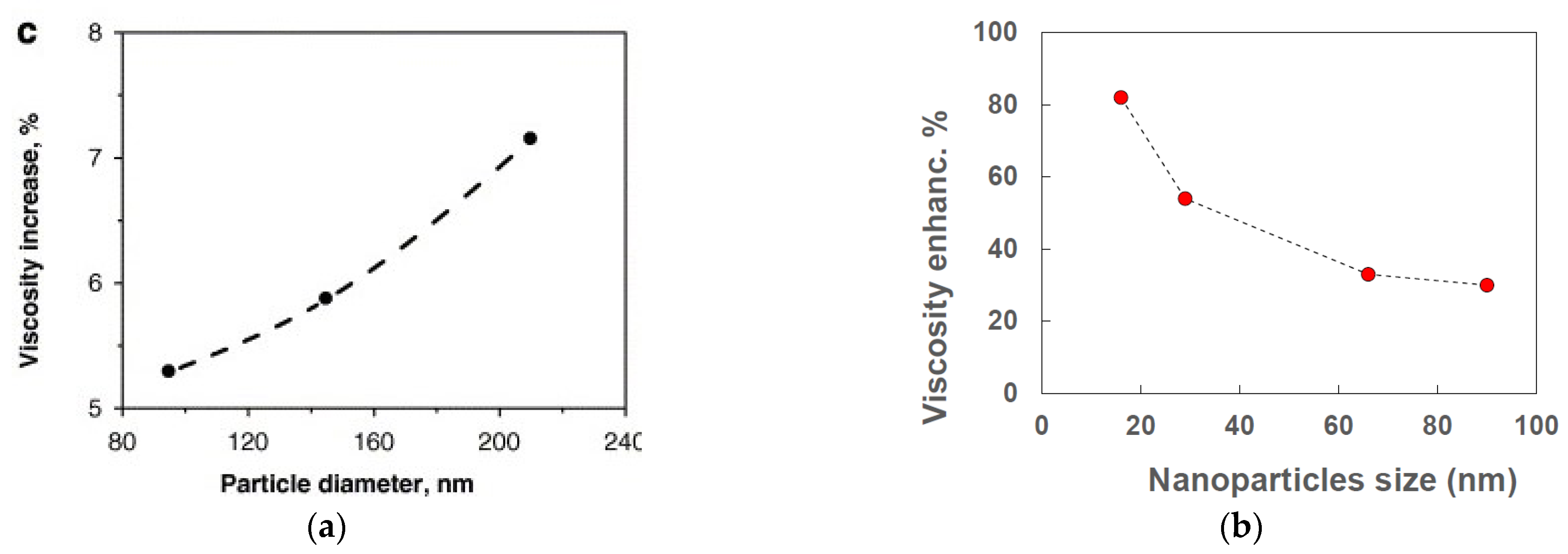
Particle size is a key parameter also for the viscosity of nanofluids. However, as highlighted by some reviews about the viscosity of nanofluids [14,39], the results reported in the literature show contradictions with reference to the dependence of viscosity on particle size.
In some cases, viscosity increases by increasing particle size (e.g., [44,46]), while in others viscosity increases by decreasing particle size (e.g., [40,42]). Figure 4a reports the results obtained by [44] for a water-based nanofluid with TiO2 nanoparticles at 0.6% concentration by volume. Viscosity enhancement increased from 5.3%, for 95 nm particle size, up to 7.2% for 210 nm particle size. Figure 4b shows the viscosity enhancement as a function of nanoparticle size in a water-based nanofluid with αSi nanoparticles at 4.1% by volume [40]. In contrast with the previous case, a decrease of viscosity enhancement was obtained from around 85 to 30% by increasing size from 16 to 90 nm. It is worth noting that the same paper reports the thermal conductivity enhancement as a function of particle size, with increasing enhancement at increasing size of the particles. Thus, the behavior is the opposite of that shown in Figure 3. This suggests that each nanofluid has its own behavior with reference to nanoparticle size.
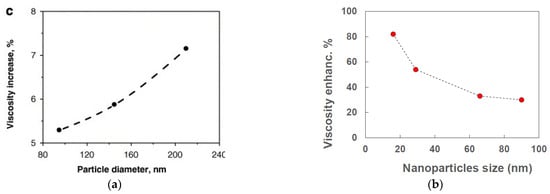
Figure 4.
Influence of nanoparticle size on (a) viscosity [44] and (b) thermal conductivity and viscosity [40] of two different nanofluids.
2.2.3. Nanoparticle Shape
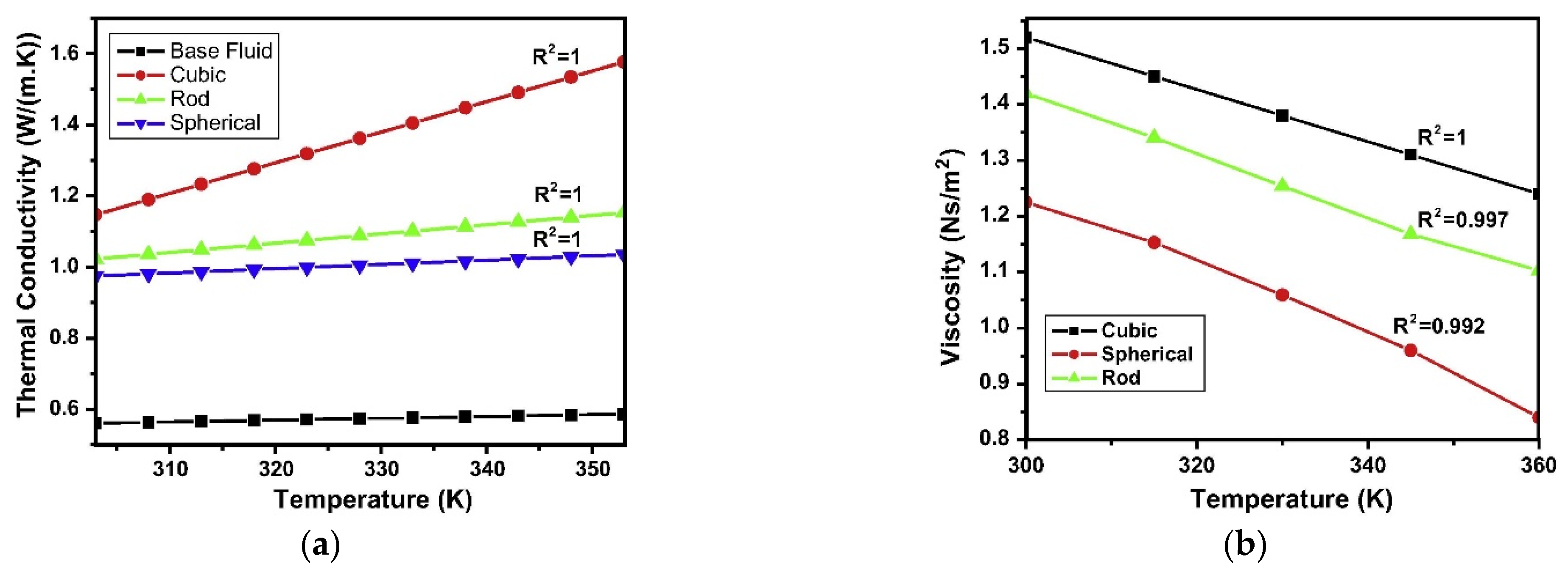
Besides size, the shape of nanoparticles is another morphological aspect that can influence the properties of nanofluids. Some studies are available in the literature showing this effect on thermal conductivity and viscosity [15,17,47]. To exemplify the influence of nanoparticle shape, Figure 5a shows the results obtained with three different nanoparticle shapes in a TiO2 water-based nanofluid [15]: cubic, rod and spherical. Passing from cubic to spherical nanoparticles, the thermal conductivity significantly enhances by around 20% at 303 K up to around 60% at 353 K. The authors suggest this result is related to the different surface to volume ratios for the different shapes, as highlighted also by [47], which enhance the heat transfer. At the same time, viscosity is also influenced by nanoparticle shape. Figure 5b reports the results obtained again by [15] for the viscosity of a TiO2 water-based nanofluid. Cubic shape gives the highest viscosity amongst the three different shapes considered, with a viscosity increase around 25% at 303 K in comparison to spherical shape. This increase becomes over 50% at 360 K. A possible explanation is related to the different mass moment of inertia and frontal cross-sectional area of particles with different shapes [15].
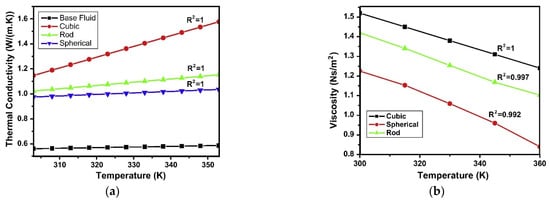
Figure 5.
Effect of nanoparticle shape on (a) thermal conductivity and (b) viscosity [15].
2.3. Effect of Dispersion Methodology on Thermophysical Properties
One of the most important features of a nanofluid is the stability of the nanoparticles’ suspension over time. Stability is essential to guarantee the functionality and the performance of nanofluids. Agglomerated particles and their sedimentation degrade the thermophysical properties and reduce the technological applicability of nanofluids (e.g., sedimentation can determine the obstruction of minichannels). Nanoparticles in suspension in the base liquid are subject to several forces. Van der Waal attractive force and gravitational force, which induce agglomeration and sedimentation, are countered by buoyancy force and electrostatic repulsive force, which tend to keep nanoparticles in suspension and prevent agglomeration [13]. Nanofluids must then be prepared in such a way that forces acting against agglomeration and sedimentation are prevalent. This is possible by adopting a proper methodology to produce the nanofluid. When the two-step method is applied, agglomeration is commonly countered by extensive ultrasonication and stirring. Moreover, to favor the repulsion forces, dispersants can be added to the suspension with different mechanisms of stabilization: electrostatic, steric and electrosteric, a combination of the first two. Various kind of surfactants can be added: anionic (e.g., SDS, SDBS), cationic (e.g., CTAB), nonionic (e.g., Span 80, Tween 20) and polymeric (e.g., PVP, PVA, GA). The details of the mechanisms of stabilization are out of the scope of this paper and can be found, for example, in [13]. What interests us here is that both ultrasonication and adding of dispersants can significantly affect the thermophysical properties of nanofluids, as reported by [16].
2.3.1. Effect of Dispersants
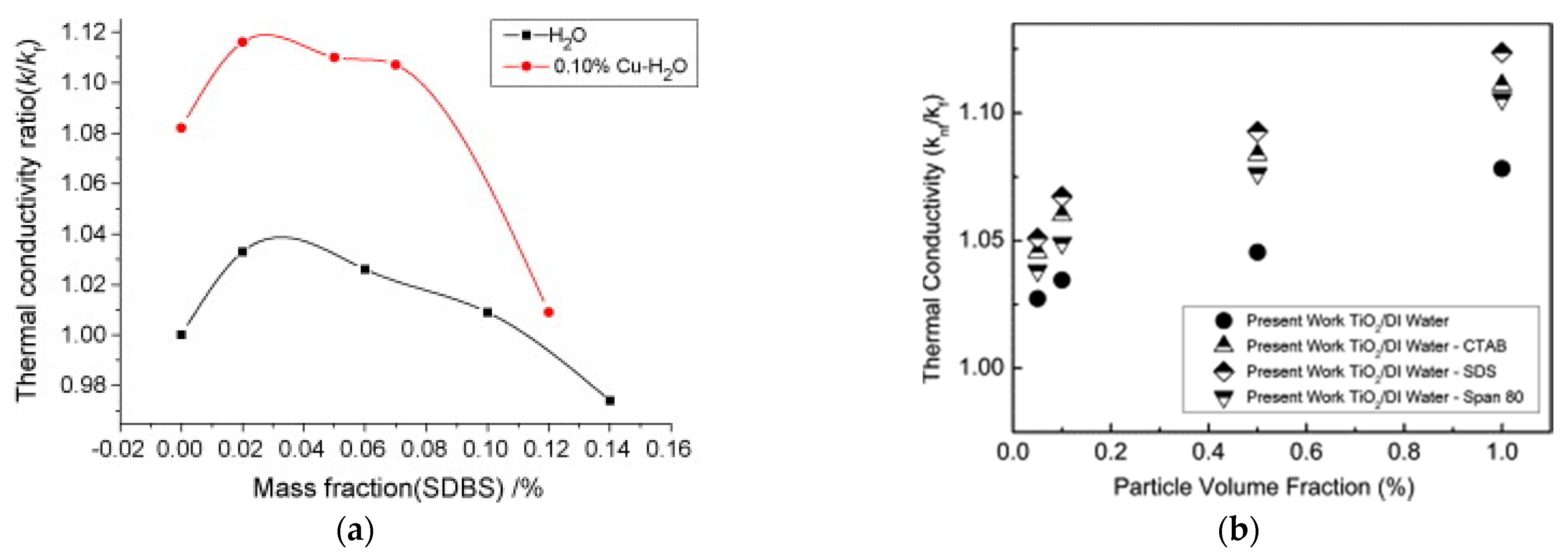
Figure 6a describes the influence of the SDBS concentration on thermal conductivity of Cu-H2O suspension at 0.1 wt% [29]. Adding surfactant to the base fluid increases the thermal conductivity ratio up to a maximum at around 0.03 wt%. Then, it progressively decreases. A similar behavior is shown by the Cu-H2O suspension up to SDBS concentration 0.07 wt%. For higher SDBS concentration, the thermal conductivity ratio of the suspension decreases quickly, becoming similar to that of the base fluid at 0.12 wt%. This means that an excess of surfactant negatively affects the thermal conductivity of the Cu-H2O suspension, due to the progressive accumulation of surfactant on the particle surface reduction, with corresponding reduction of the heat transfer area.
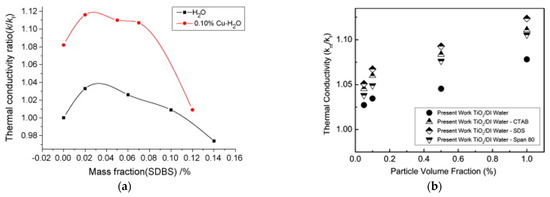
Figure 6.
Effect of (a) surfactant concentration [29] and (b) type of surfactant [48] on thermal conductivity.
At the same time, the type of surfactant can also influence the thermal conductivity enhancement of a nanofluid. For example, [48] added to the same TiO2-water nanofluid different types of surfactant, namely the cationic CTAB, the anionic SDS and the nonionic Span 80. Figure 6b highlights that surfactant significantly enhances thermal conductivity compared to the nanofluid without surfactant. In particular, the highest enhancement is achieved with the low molecular weight SDS, while lower enhancement is achieved with the CTAB and Span 80, with increasing molecular weight. Lower molecular weight of surfactant is one of the possible reasons for higher heat transfer enhancement, as suggested by [49].
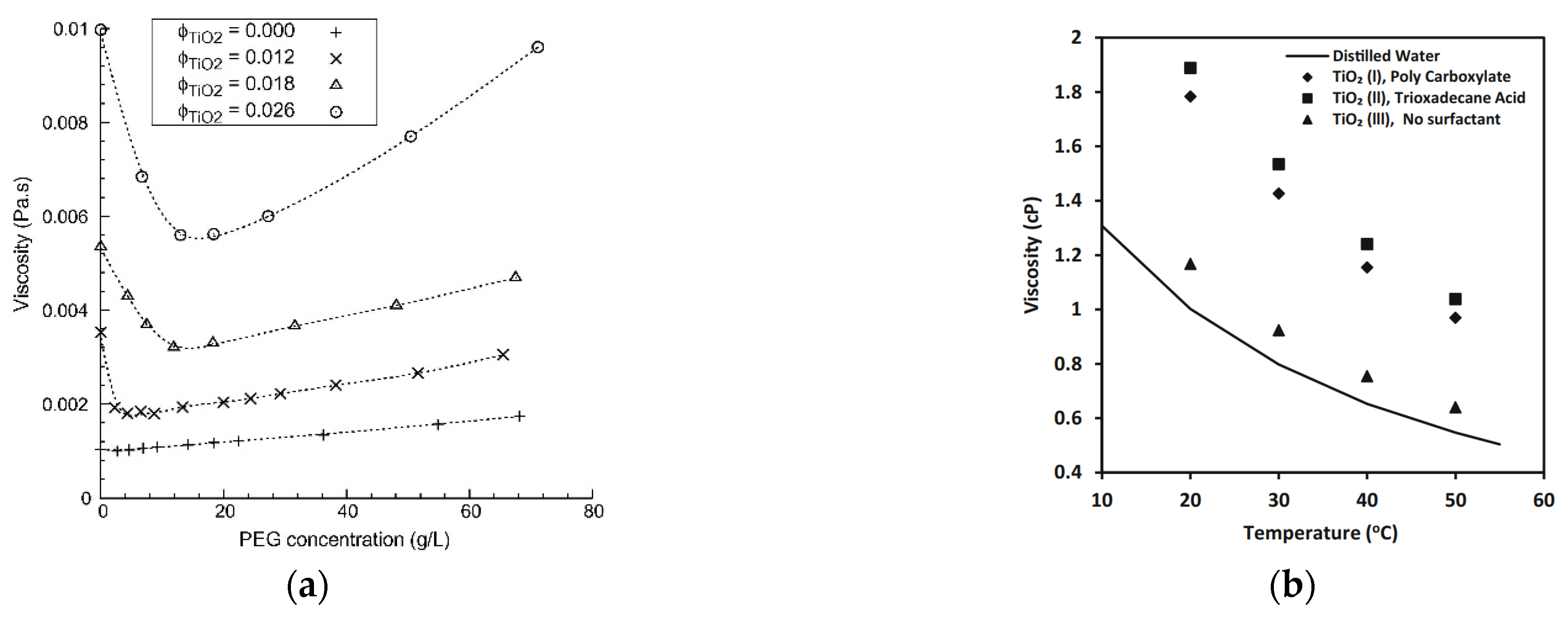
As thermal conductivity is influenced, so is the viscosity of nanofluids, by the amount and type of surfactant in the suspension. For example, [50] added different amounts of PEG 2000 to a suspension of titanium dioxide in water, at three different volumetric concentrations of titanium with the results shown in Figure 7a. It was evident that for every volumetric concentration of titanium dioxide, adding low amounts of PEG to the suspension rapidly reduced the viscosity to a minimum. This minimum was achieved at different PEG concentrations for each TiO2 volumetric fraction. Higher PEG 2000 negatively affected the viscosity that increased continuously. This means, again, that the amount of surfactant in the nanofluid must be optimized to get the best performance in terms of viscosity.
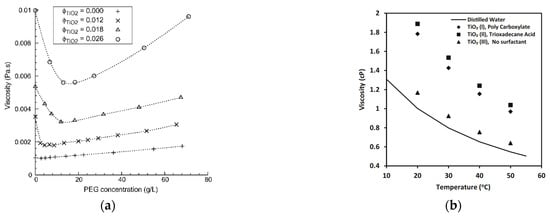
Figure 7.
Effect of dispersant (a) concentration [50] and (b) type [51] on the viscosity of different nanofluids.
Figure 7b shows the effect of different types of surfactant on the viscosity of a TiO2-water nanofluid with a nanoparticle mass fraction of 9 wt% [51]. The TiO2(III) nanofluid, without surfactant, had the lowest increase in viscosity, around 5%, in respect to the base fluid, but was less stable than the two nanofluids with surfactant. Adding surfactant, the viscosity of nanofluids increased significantly. TiO2(II) nanofluid with trioxadecane acid as surfactant, had a viscosity 61% higher than the nanofluid without surfactant. However, the TiO2(I) nanofluid, with polycarboxylate as surfactant, showed viscosity around 5% lower than TiO2(II), suggesting that also the type of surfactant must be properly selected to minimize the viscosity enhancement.
2.3.2. Effect of Cluster Reduction by Ultrasonication, Milling, High Pressure Homogenization
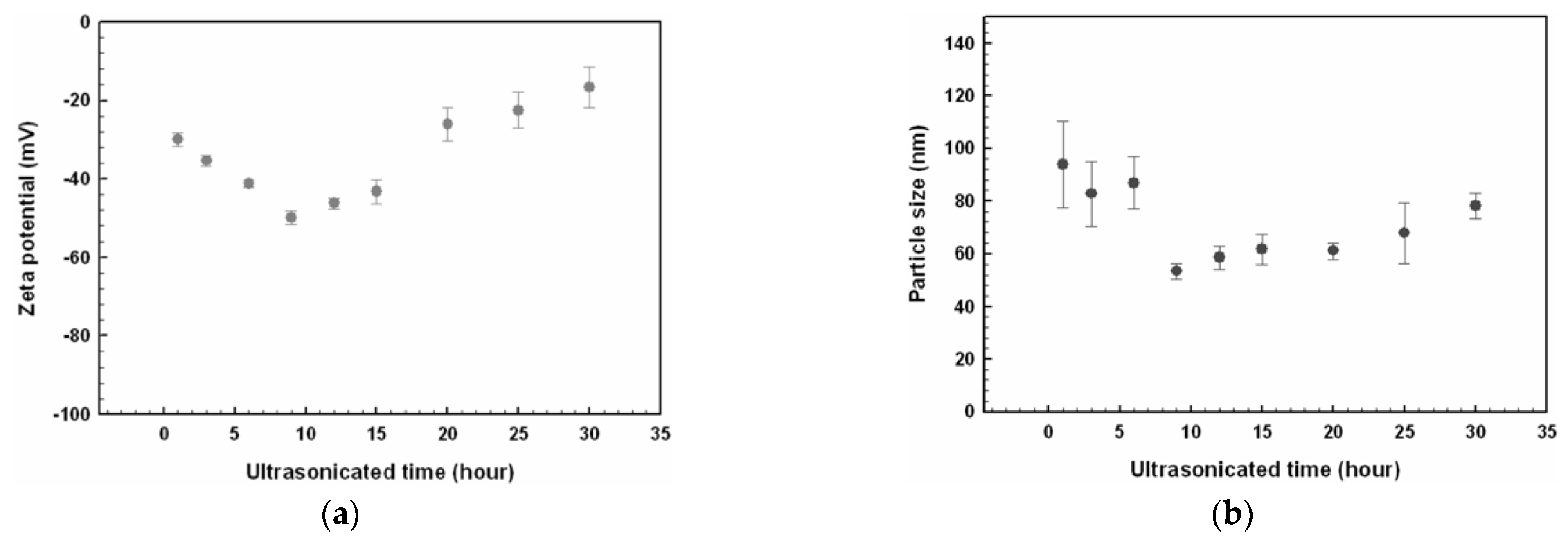
Even if the nominal size of nanoparticles to be dispersed in the base fluid is below 100 nm, by applying the two-step method of preparation they tend to agglomerate in clusters much bigger than the original nanoparticles, compromising the stability of the suspension. Different techniques can be applied to disaggregate nanoparticle clusters: ultrasonication, ball milling, homogenization [15,27,28,52]. Disaggregation of nanoparticles changes not only the size, but to some degree, all the properties of the nanofluid. This is exemplified by Figure 8, where the effect of ultrasonication time on Zeta potential and size of the nanoparticles is shown for an ethylene glycol-based nanofluid with dispersion of CuO nanoparticles [26].
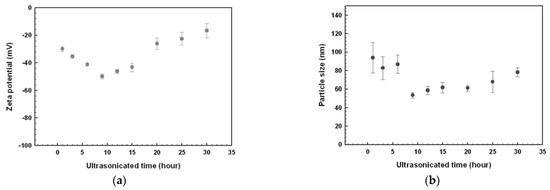
Figure 8.
Effect of ultrasonication time on (a) Zeta potential value and (b) nanoparticle size [26].
Figure 8a shows that sonication time influences the Zeta potential and then the stability of the suspension, with an optimal ultrasonication time of 9 h, where the Zeta potential is minimum (maximum in absolute value): then, the nanofluid has the highest stability, as described in next paragraph. At the same time, this minimum corresponds also to the minimum in nanoparticle size, as shown in Figure 8b, suggesting a relationship between these two quantities.
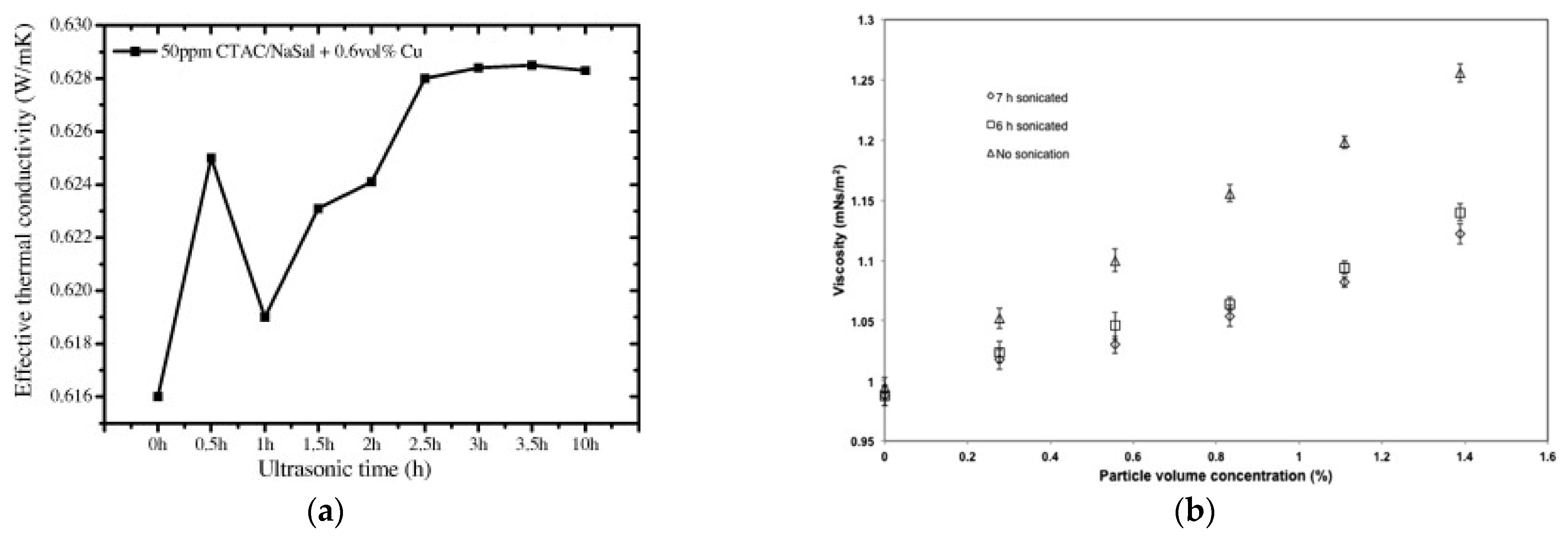
Thus, it is not surprising that ultrasonication time has similar effects also on the thermophysical properties of nanofluids, namely thermal conductivity and viscosity, as exemplified in Figure 9. As shown by [53], the thermal conductivity of a water-based nanofluid with 0.6% vol. of Cu nanoparticles and 50ppm of CTAC/NaSal dispersant, increased continuously for ultrasonication time up to 3 h (Figure 9a). For longer times, up to 10 h, the thermal conductivity was practically constant, suggesting that 3 h is the optimal time of ultrasonication for this property, corresponding to the most uniform dispersion of the nanoparticles inside the base fluid. Viscosity is also altered by ultrasonication time, being strictly connected to the size of nanoparticles. Figure 9b shows the change in viscosity due to ultrasonication in a TiO2-water dispersion [28]. After 6 h and 7 h of ultrasonication, the viscosity was clearly reduced compared to the non-ultrasonicated nanofluid. In this case, it is not possible to define an optimal time, but what is clear is the importance of a size reduction and better size distribution of nanoparticles inside the nanofluid obtained by ultrasonication.
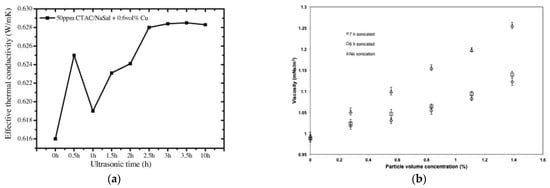
Figure 9.
Effect of ultrasonication time on (a) thermal conductivity [53] and (b) viscosity [28] for different nanofluids.
2.3.3. Effect of pH and Zeta Potential
An important indicator of stability is the Zeta potential, that is, the potential at the slipping plane between the liquid layer enveloping the nanoparticle and the bulk fluid. Its magnitude is related to the repulsive force acting on different particles: when the potential is low, attractive forces between particles can predominate, determining agglomeration and sedimentation. Zeta potential should be at least ± 30 mV to give a stable dispersion. For lower values, the dispersion tends to be unstable. Zeta potential is a parameter strictly connected to the solution pH [13,18]. By adjusting pH, which affects the electrical charge density on the nanoparticle surface, it is possible to increase the absolute value of Zeta potential and then the stability of the nanofluid.
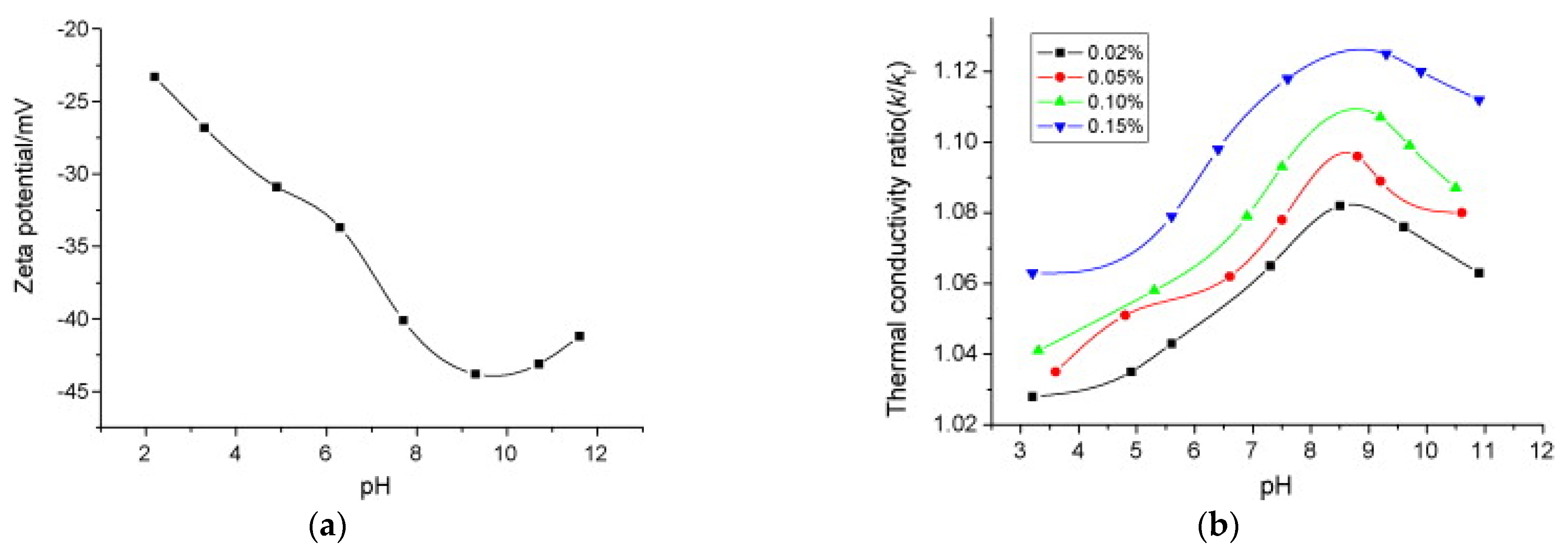
Figure 10a exemplifies the typical relation between pH and Zeta potential [29]. It is evident that pH clearly influences Zeta potential, with a rapid decrease (increase in absolute value) of the Zeta potential at increasing pH, with a minimum (maximum absolute value) at −45 mV in this case. It is worth underlining that a too low (acid solution) or too high (basic) pH can create technological problems due to possible corrosion of the materials. Considering the reference value of ± 30 mV, the nanofluid becomes stable at pH > 4, with maximum stability at pH around 9. Here, it is interesting to highlight the effect of pH on the thermophysical properties of the nanofluids. Again [29] measured the thermal conductivity enhancement of Cu-H2O suspension with different Cu mass fractions as a function of pH (Figure 10b). The maximum thermal conductivity enhancement is achieved at pH = 9, in correspondence to the maximum Zeta potential. [19] suggests that this enhancement is primarily related to the surface charge state of the particles, which is directly connected to pH and Zeta potential. However, it is relevant here to note that the thermal conductivity ratio increases significantly (from 1.06 to 1.12) in the range of pH between 3 and 9. This suggests that pH is another important parameter to be considered in nanofluid design: it should be optimized, not only to improve the stability of the solution, but also to obtain the best performance of the nanofluid in terms of heat transfer.
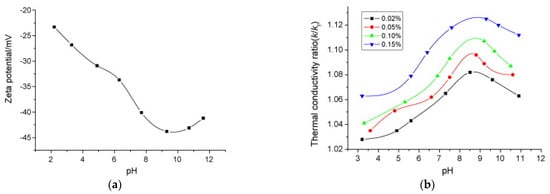
Figure 10.
Effect of pH on (a) Zeta potential and (b) thermal conductivity of Cu-H2O suspensions with SDBS surfactant at 0.05 wt% [29].
3. A Case Study: Thermophysical Properties of TiO2-Water Nanofluids as a Function of Preparation Parameters
As discussed in the previous part of the paper, every nanofluid is characterized by several parameters, each of them having an influence on its thermophysical properties and final performance. Thus, it is interesting to analyze if these parameters are always described and quantified in the literature or if there is a lack of information when a nanofluid is studied. Moreover, it is interesting to evaluate if the results obtained for the thermophysical properties are coherent with each other and to try to understand what the reason is for the possible contradictions. Thus, in this part of the paper, the information available in published papers regarding water-based nanofluids with dispersion of TiO2 nanoparticles will be analyzed as a case study. The amount of information available in each paper studying this kind of nanofluid will be reported and critically evaluated. Moreover, the results obtained by the measurements of thermal conductivity, will be described and compared to evaluate their mutual consistency.
3.1. Analysis of Parameters Characterizing the TiO2-Water Nanofluids
Nanofluids made by dispersion of TiO2 nanoparticles in water are amongst the most studied typology of nanofluid: 20 papers [28,38,44,48,51,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] were found in the open literature, reporting information on nanofluid preparation and results for the measurements of their thermophysical properties, in particular thermal conductivity and viscosity. It is not excluded that other papers are available in the literature, but the amount of papers found can be considered statistically relevant for our purposes. Table 1 synthetizes the information available in each paper for the main parameters described in the previous section of the paper: each column corresponds to one parameter and reports the corresponding data declared in each paper. It is worth mentioning that in all the cases here considered, the two-step method was used to prepare the nanofluids, thus the parameters referred to the second step of this methodology. With reference to nanoparticles, volumetric/mass concentration, shape and size were considered. The other parameters considered regard the dispersant (type and concentration) and the dispersion methodology (dispersion technique, time and power of stirring/sonication/homogenization, monitoring methodology applied to determine the size of nanoparticles or clusters in the nanofluid, pH and Zeta potential). In the following, the data reported in Table 1 will be analyzed and discussed.
3.1.1. Nanoparticles Concentration
In all papers, TiO2-water nanofluids were prepared within a certain range of volumetric/mass concentrations of nanoparticles, with the aim to describe their properties in dependence of concentration as the main structural parameter. Most of the concentrations ranged between 0.1% to 5%, and only in two cases they reached 10% or 20%. This is clearly related to the problem of stability and the strong enhancement of viscosity determined by high nanoparticle concentrations.
3.1.2. Nanoparticles Shape
Nine papers [27,28,44,56,57,58,63,64,66] out of 20 did not declare the shape of the nanoparticles. All the remaining used spherical nanoparticles. Only one paper [54] considered two different shapes, spherical and rod, to compare their effect on stability and thermophysical properties.
3.1.3. Nanoparticle Size
All the papers declared the nominal size of TiO2 nanoparticles dispersed in the nanofluid. It ranged from 5 nm to 76 nm. However, as discussed in the previous part of the paper, particles tended to create clusters of various dimensions depending on the dispersion methodology. Information on the actual dimension of clusters was even more important than the nominal size to interpret the results obtained for the thermophysical properties. Amongst the papers here discussed, almost all monitored the size of particles and clusters by some technique (TEM, DLS, XRD, EDX). However, it is worth noting here that techniques like TEM or SEM can determine the size of dry particles/clusters only, not of those actually in suspension inside the liquid. Seven papers reported the size range of clusters within the dispersion, mostly measured applying the dynamic light scattering (DLS) technique. Even if DLS measured the equivalent hydrodynamic diameter for non-spherical particles, this information was very useful for addressing the preparation of the nanofluid and the interpretation of the results obtained for the thermophysical properties. Since clusters of nanoparticles are the actual structures of the particle present in the nanofluid, more information should be obtained on their size and shape. At the same time, correlating properties and performance of the nanofluid to the nominal size of the primary nanoparticles can be misleading, since this dimension does not necessarily correspond to the real structure of the nanoparticles/cluster in the dispersion.
3.1.4. Dispersant Type and Concentration
In nine papers [38,48,51,54,59,62,63,66,67] out of 20, the studied nanofluids were obtained adding surfactants of various kinds. The most used was CTAB, that is a cationic surfactant. Other surfactants used are oleic acid, PEG600, acetic acid, SDS, Span 80, polycarboxylate, trioxadecane acid, PVP. The concentrations of dispersants are quite different and depend mostly on the type of surfactant and the nanoparticle concentration, as there is some proportionality between the optimal surfactant concentration and the nanoparticle concentration. It is interesting to note that 10 papers [27,48,51,55,56,58,60,61,64,67] studied nanofluids without surfactants, based on the idea that surfactant somehow alters the properties of the nanofluid and misleads in results interpretation. However, in one case the observed strong increase in viscosity was attributed to the absence of surfactant [56]. In four cases [28,44,57,65] the possible presence or the concentration of surfactants was not declared at all. Finally, in four papers [48,51,54,63] a comparison between two or more surfactants was performed, with the aim to identify the most suitable to stabilize the suspension and analyze the effect on the thermophysical properties. In particular, [48] concluded that the presence of a surfactant significantly improved the thermal conductivity of the studied nanofluids, however with a hierarchy of the different surfactants considered. However, [51], analyzing the same TiO2-water nanofluid without or in the presence of two different surfactants, observed a strong increase in viscosity due to the addition of surfactants.
3.1.5. Dispersion Methodology
As already said, in all the studies here considered the two-step methodology was applied to prepare the various TiO2-water nanofluids. In the large majority of cases, ultrasonication only was used to disperse nanoparticles, but in some cases [28,44,48,61,63,65] a combination of different techniques (stirring, ultrasonication, homogenization, milling) was applied. However, this was not enough to identify and understand the effect on the nanofluids’ properties. The aim of this step was also to disaggregate as much as possible the clusters of nanoparticles. As shown in the first part of the paper, in the case of ultrasonication (but this could be true also for the other techniques), the time of operation is essential to optimize the properties of the resultant nanofluid, but also the power and frequency of sonication, i.e., the intensity of the action on the nanoparticles, is important to obtain the final result. However, it is evident from Table 1 that information on these two parameters was quite scarce. Less than half of the papers reported the time duration for which ultrasonication or other techniques were applied and only two papers [28,58] reported the power (or the rpm in case of stirring).
3.1.6. pH and Zeta Potential
The analysis performed in the first part of the paper has shown how these parameters influence surface charge states and the resultant surface potential, influencing the stability of nanofluids and also their thermophysical properties. Moreover, they are connected to each other and at least one of them should be known to properly prepare and define the nanofluid. In eight papers [27,44,51,54,62,63,64,65] the pH of the solution was declared: in three cases [27,62,63] the values were low, attesting a more or less acid solution; in two cases [44,64] the pH was high, attesting a basic solution; in the remaining three cases [51,54,65] the pH was close to 7, attesting a neutral solution. Zeta potential was measured in five papers [27,28,44,59,62], but only three [27,44,62] that also reported pH. This means that a total of 11 papers out of 20 reported information on pH and Zeta potential, while almost half of the papers did not report any data on these parameters. In all the cases where Zeta potential was measured, its absolute value was always higher than 30 mV, attesting the good stability of the nanofluids. In some cases, the behavior of pH in relation to other parameters was described. [63] analyzed the change in pH (always below 5) as a function of temperature and volumetric fraction of nanoparticles with two different dispersants, showing it was relatively insensitive to temperature, while it decreased with the increase of solid volume fraction. [64] adjusted the pH till the optimum value of 10, observing the stability over 5–6 days. [27] analyzed experimentally the relation between pH and Zeta potential. To maximize the stability, they selected pH = 3, as far as possible from the isoelectrical point, corresponding to pH = 6.5.
3.1.7. Discussion
The description of the information reported in the literature highlighted that, despite the importance of each parameter, in many cases this information was lacking or insufficient. With reference to nanoparticles, the volumetric/mass fraction was always reported, but morphological information was frequently insufficient. Only around 50% of the papers reported the shape of the particle and just as many evaluated the size distribution of clusters of nanoparticles in dispersion which was a more realistic description than the nanoparticle nominal size reported by practically all the papers. As far as the dispersion methodology was concerned, the type of surfactant used was generally declared, but in a few cases without information on the concentration that directly influenced both the stability and thermophysical properties of nanofluids. The dispersion technique was generally ultrasonication, but information about the time of sonication was reported only by half of the papers and power of sonication only in a couple of cases. pH was also reported by around half of the papers, while its optimization was performed in a few cases only. Zeta potential was reported by only five papers.
At best, 9 out of 11 parameters were reported, but by only two papers [27,54]. In the majority of cases, three to five parameters were not reported, while three papers reported five or less parameters [56,57,60]. Moreover, in many cases it was not clear if a process of optimization had been performed for the various parameters. This suggested that the information reported was frequently not sufficient to completely understand the modality of preparation or the characteristics of the nanofluid and, as seen, to evaluate the effectiveness of the method applied. Moreover, this scarcity of information inhibits the possibility to replicate the nanofluid and confirm the results obtained.


Table 1.
Information available in the literature on the preparation parameters for the TiO2-water nanofluids.
Table 1.
Information available in the literature on the preparation parameters for the TiO2-water nanofluids.
| TiO2 Nanoparticles | Dispersant | Dispersion Methodology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Concentration | Shape | Nominal Size (nm) | Cluster Size (nm) | Particle/Cluster monitoring | Type | Concentration | Technique | Time | Power/Frequency | pH | Zeta Potential |
| [44] | 0.24%, 0.6%, 1.18% vf (1–2.5–4.9% wf) | ND | 20 | 500 after sonication; 120 after dyno-mill; 95 further dyno-mill) (at 0.6% vf 95 nm, 145 nm, 210 nm | X-ray; SEM; DLS nanosizer | ND | ND | Ultrasonication for breaking down agglomerates before measurement; dyno mill for breaking; further dyno-mill | 30 m (+ 30 m + several hours) | ND | 11 | −40 mV |
| [38] | 1–5% vf | spherical | 15 | ND | ND | CTAB | 0.1 mM | ultrasonic dismembrator | ND | ND | ND | ND |
| [48] | 0.05–5% vf | spherical | 33 (anatase) | ND | XRD; EDX; FESEM | No surfactant; CTAB; SDS; Span 80 | ND | magnetic stirrer + ultrasonic | 2 h | ND | ND | ND |
| [51] | 3–9%; 9%; 9% wt | spherical | SEM 30; 30; 30 | DLS 140; 200; 90 | SEM; DLS | No surfactant; polycarboxylate; trioxadecane acid; | high-energy tip sonication | 15 min | ND | 7.2; 7.5; unknown | ND | |
| [27] | 1–5% | ND | 34 | 170 nm | SEM | NO surfactant | 0 | high shear homogenizer | 50 min (optimized) | ND | 3 | Z as a function of pH |
| [28] | 0.27–1.39% | ND | 32 | 100–200 (analytical discussion) | Laser diffraction technique; SEM; XRD | ND | ND | stirred bead milling/high shear homogenizer/ultrasonication | 12 h/15 min/ND | 1440 rpm/10,000–18,000rpm/130 W–20 Hz | ND | 42 mV |
| [54] | 0.5–5% vf | rod | 10 × 40 | TEM, particle size analyzer | Oleic acid | 0.01–0.02% | ultrasonication | 8–10 h | ND | 6.8–6.2 | ND | |
| spherical | 15 | cluster analysis | CTAB | |||||||||
| [55] | 10–20–40% mf | spherical | 40 | ND | TEM | No surfactant | 0 | ultrasonication | ND | ND | ND | ND |
| [56] | 0.2–3% vf | ND | 21 | ND | ND | No surfactant | 0 | ultrasonication | ND | ND | ND | ND |
| [57] | 0.5–4% vf | ND | 26 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| [58] | 0.1–1% vf | ND | 25 | ND | TEM; XRD | No surfactant | 0 | ultrasonication | ND | 700 W/20 kHz | ND | ND |
| [59] | 0.01–1% mf | spherical | 21 | around 100 nm | DLS | PEG600 | 2 disp:1 np | high pressure homogenization | − | ND | ND | 37–43 |
| [62] | 0.5–2.5% vf | spherical | 40 | ND | TEM | No surfactant | 0 | sonication | ND | ND | ND | ND |
| [61] | 0.2–1.2% | spherical | 25 | ND | SEM, DLS | No surfactant | 0 | Ultrasonication/shear homogenizer/medium-mill | ND | ND | high | ND |
| [62] | 1–10–20–35% mf | spherical | ND | 72–76 | DLS | Acetic acid | 1–5% | commercial (dilution with ultrasonication) | ND | ND | 1.86–3.07 | 55 mV |
| [63] | 0.1–2% | ND | 10–40 | 147–207 | TEM, HRTEM; DLS | CTAB; acetic acid | 1:10 nps | Stirring + ultrasonic | 2 h + 2−3 h | ND | 2.8–3.7 (AA); 3.9–4.9 (CTAB) | ND |
| [64] | 0.99–4% | ND | 27 | ND | visual | No surfactant | 0 | high speed mixer | 2 h | ND | 10 (analytical discussion) | ND |
| [65] | 0.2–2% (40% diluted) | spherical | 21 | ND | TEM | ND | ND | stirring for dilution; ultrasonication for cluster breaking | sonication 2 h | ND | 6.5–7.5 | ND |
| [66] | 0.1–4% | ND | 10 | ND | ND | CMC | 0.5% mf | ultrasonication | 1 h | ND | ND | |
| [67] | 0.89–6% | spherical | 5/5–15/30–50 | ND | NO | No surfactant; PVP | 1% mf | ultrasonication | 2 h; ND | ND | ND | ND |
3.2. Analysis Of Experimental Thermal Conductivity Enhancement
Nanofluids can be designed for many applications in various industrial fields. Among these, nanofluids have been studied intensively as potential heat transfer fluids due to their enhanced thermal conductivity with reference to the base fluid. Here, we will discuss the results available on thermal conductivity for the TiO2-water nanofluids. The objective is to compare the results obtained by different laboratories as they are generally represented, i.e., thermal conductivity enhancement as a function of volumetric fraction or temperature, to highlight if they are consistent with each other or if there are contradictions.
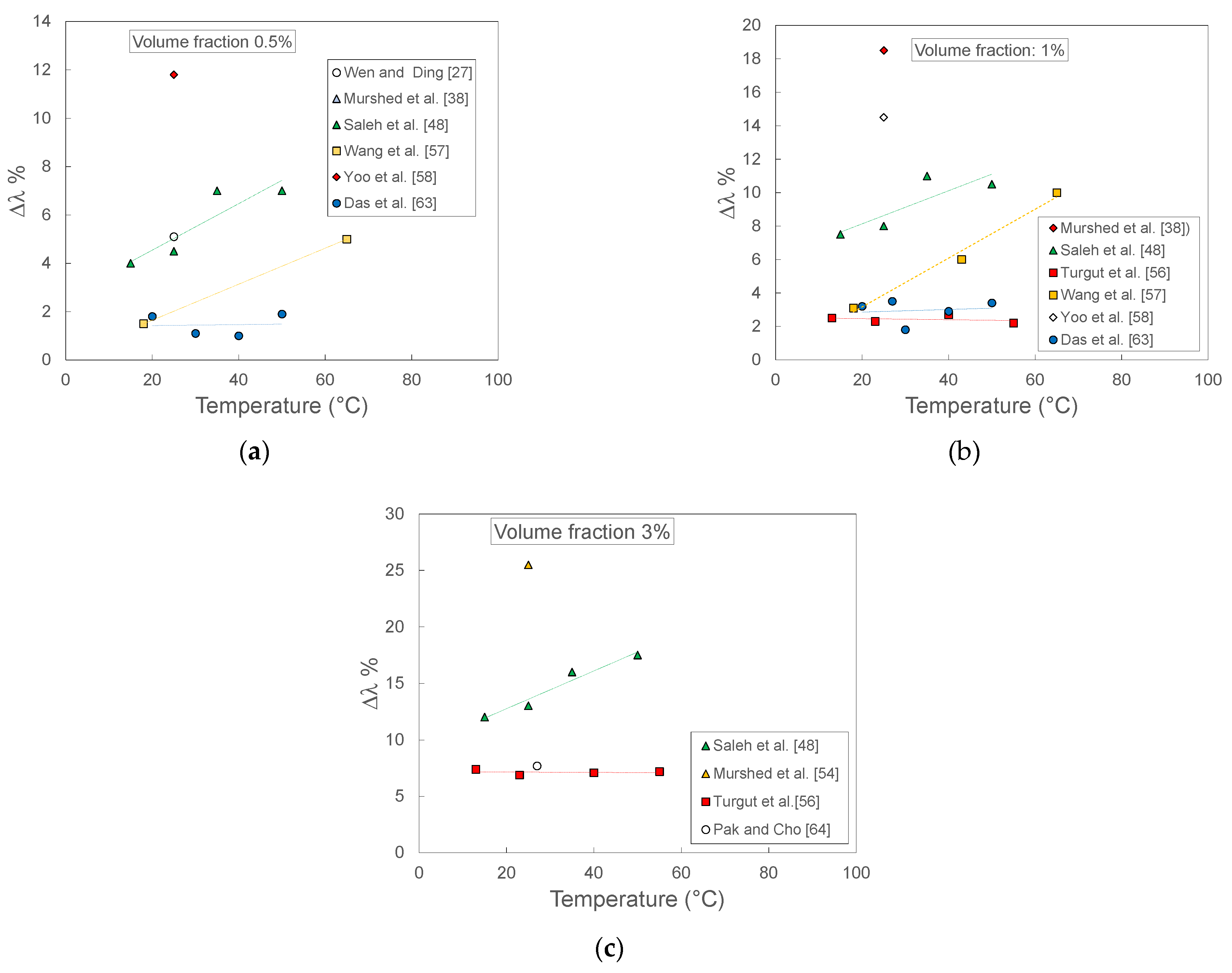
Figure 11 reports the thermal conductivity enhancement as a function of temperature at three different volumetric fractions of TiO2 nanoparticles in water. The data have been published in 11 different papers among those considered in the first part of this section. The selected volume fractions and temperatures are those for which more data are available. It is evident how the differences between different sets of data are quite significant, both in terms of enhancement at the same temperature and as trend versus the temperature at given volumetric fraction. For example, at any volume fraction, the data of Saleh et al. [48] show increasing enhancements at increasing temperatures, while other sets are almost independent of temperature (Das et al. [63], Turgut et al. [56]). The entity of enhancement is also significantly different: the enhancement shown at around 20 °C by Saleh et al. [48] data is 2–3 times higher than those of Das et al. [63] and Turgut et al. [56], and even more at higher temperatures. Single data (Murshed et al. [38] and Yoo et al. [58]) show much higher enhancements.
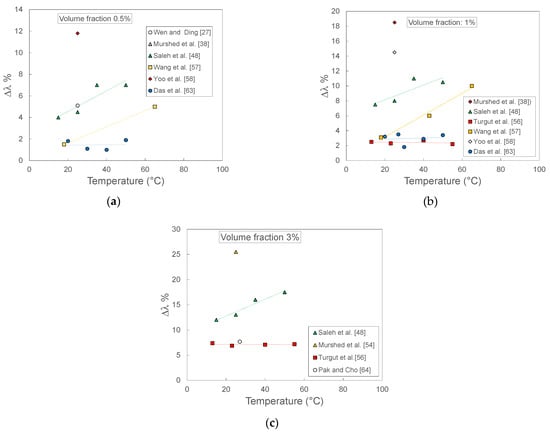
Figure 11.
Thermal conductivity enhancement for TiO2-water nanofluids measured by different laboratories as a function of temperature at three different nanoparticles volume fractions: (a) 0.5%; (b) 1%; (c) 3%.
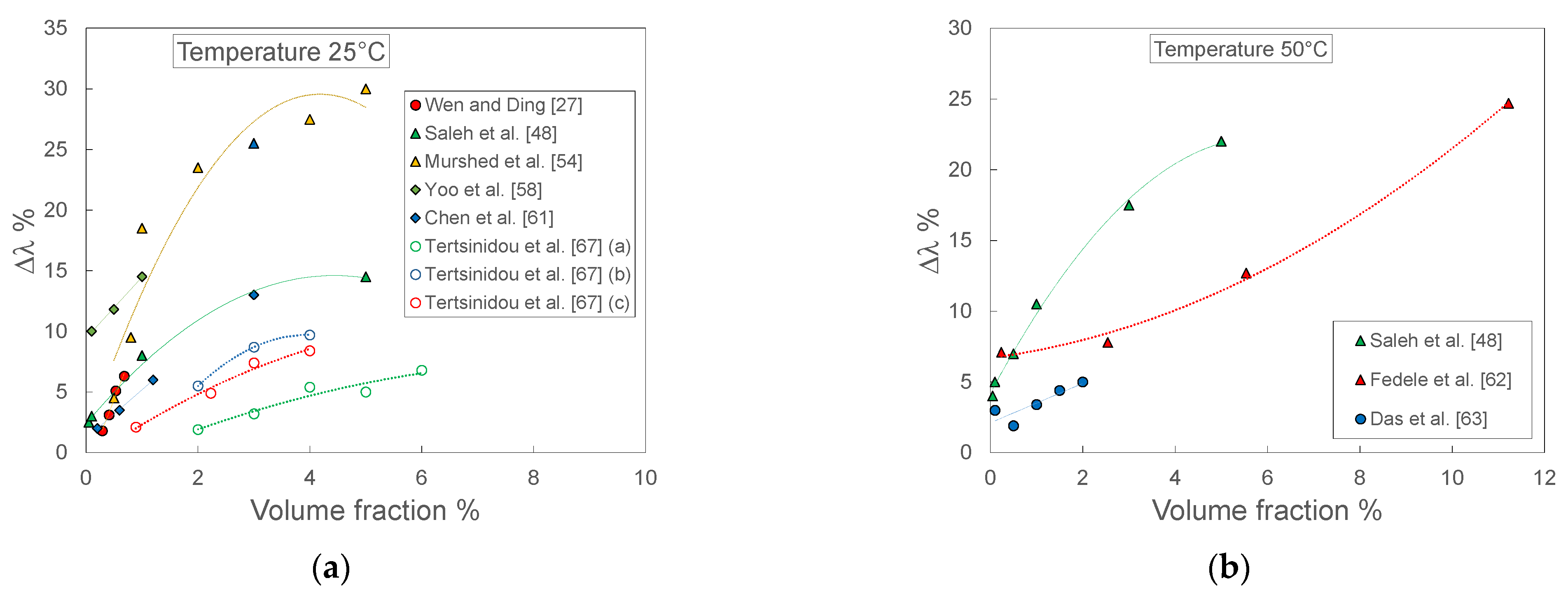
Figure 12 reports the thermal conductivity enhancement as a function of volumetric fraction at two different temperatures, namely 25 °C and 50 °C. In this case, all the sets of data show increasing enhancement at increasing volume fraction of TiO2 nanoparticles, even if with different slopes. However, there are evident differences in terms of magnitude of the enhancement. At 25 °C and low volume fractions (Figure 12a), the data of Yoo et al. [61] show 2–3 times higher enhancements than the other sets, while at higher volume fraction the enhancement of Murshed et al. [54] data is at least double that shown by Saleh et al. [48] and one order of magnitude higher than Tertsinidou et al. [67]. Similar considerations could be done also for the data at 50°C, shown in Figure 12b.
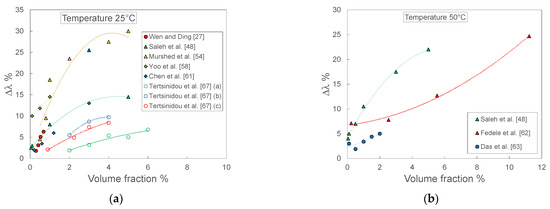
Figure 12.
Thermal conductivity enhancement for TiO2-water nanofluids measured by different laboratories as a function of volume fraction at two different temperatures: (a) 25 °C; (b) 50 °C.
By comparing the data available in the literature, it is very difficult to establish what is the actual effect of adding TiO2 nanoparticles to water. In some cases, the enhancement is quite modest and within the prediction of classical theories. In others, it is strongly higher and thus it is much more attractive in view of possible applications. What, however, are the reasons for such differences? The answer, as suggested by the analysis performed in the first part of the paper, could come from the knowledge of the parameters which characterize each nanofluid. The parameters should be, in principle, determined to give the optimal effect not only on thermal conductivity, but also on viscosity or, more precisely, with the objective to maximize the heat transfer coefficient. In this sense, every nanofluid is a complex object that should be obtained by multiparameter optimization, not only adjusting one parameter or another. However, looking at Table 1, it is clear that in several cases information about each nanofluid was not declared, as already discussed. Just as an example, in Figure 11c, the two sets of data of Saleh et al. [48] and Turgut at al. [56] showed very different behaviors, but in both cases some useful information about nanofluid preparation was missing. Saleh et al. [48] did not report the size distribution of the clusters inside the nanofluid, the pH and the Zeta potential, while Turgut et al. [56] did not report the shape of primary nanoparticles, the cluster size distribution, the time of sonication, the pH and the Zeta potential. With this situation, it is difficult to understand what the actual reasons could be for the different behaviors of the two nanofluids.
Effect of Measurement Technique
Besides the parameters here considered, another aspect of the characterization of nanofluids must be taken into consideration. Thermal conductivity and viscosity can be measured, especially the former, by different kinds of instruments. With reference to thermal conductivity, the most used measurement technique is the transient hot wire (THW), but the 3ω method, steady-state concentric cylinder technique, transient hot-disk technique, and others are also employed. However, the analysis performed in [68] highlights the importance of applying the proper techniques to obtain correct thermal conductivity measurements. Anomalous enhancements with respect to theoretical expectation (e.g., Hamilton−Crosser equation), are frequently due to either a wrong design of the instrument (in particular THW) or the intrinsic inadequacy of the measurement principle (e.g., the one applied by [68]). Accurate measurements are essential to properly highlight the actual effects induced by other parameters. It is interesting to analyze in greater detail the results reported in Figure 12a. All the measurements reported were performed at 25 °C, but the results are widespread. Following the analysis performed by [67], a large number of these differences could be explained by considering that most of the instruments used were probably not suitable for correct measurements: [56,57] applied the 3ω method, [48,63] commercial KD2 pro, [38,58] THW with a single wire. The only data obtained with a correctly designed THW sensor were those of Tertsinidou et al. [67], who studied three different sets of data characterized by different nanoparticle sizes and/or dispersant: set (a) size 5 nm, no surfactant; set (b) size 5–15 nm, PVP 1% by mass; (c) size 30–50 nm, PVP 1% by mass. Assuming the measurements were correct, as demonstrated in [59], the clear differences in the enhancement obtained with the three sets can be attributed to the different sizes of the nanoparticles and the presence or not of the dispersant. In particular, solutions (b) and (c), with PVP dispersant, showed higher enhancement than the one without dispersant (a). However, as stated by the authors, it was not possible to conclude whether the superior enhancement obtained was due to the dispersant or to the lower size of the particles, for which the dispersant was not needed. These results highlight the importance of (a) properly defining the parameters describing the nanofluid, possibly evaluating the influence of variations of each parameter in view of the optimization of the nanofluid in terms of heat exchange and (b) applying a suitable instrument and a proper measurement methodology so as not to hinder the effects of the various parameters due to inaccurate measurements. These two points could be satisfied by (a) defining a clear protocol to define and optimize the parameters characterizing the nanofluid and (b) defining a standard nanofluid to perform the reference measurements that could allow every laboratory to test the suitability of the instrumentation and methodology applied.
4. Conclusions
The article had two main objectives:
- (a)
- to highlight, through a selective analysis of the literature, the importance of the various parameters that characterize the complexity of nanofluids and their influence on thermophysical properties;
- (b)
- to evaluate, through the systematic comparison of the information reported in the literature for a case study, whether these parameters are always reported, in order to allow an adequate definition of the nanofluid and the interpretation of the results obtained from the property measurements.
The analysis performed in the first part of the paper highlighted the potential impact of the various parameters characterizing a nanofluid by its thermophysical properties, in particular, thermal conductivity and viscosity. Optimizing parameters such as size and shape of nanoparticles, material and concentration of dispersants, methodology of dispersion, pH and Zeta potential, enables a significant improvement of the performance of the nanofluid. More precisely, considering the mutual influence of several of these parameters, a multiparameter optimization should ideally be performed. In the second part of the paper, a case study was discussed with reference to the available literature on TiO2-water-based nanofluids. It has been highlighted that the information reported in the literature about the main characterizing parameters of these nanofluids was frequently lacking. Moreover, comparing the results obtained in different laboratories for thermal conductivity, it emerged that they were often contradictory and not attributable to a single interpretative model. It is quite clear that it is not enough to define the formulation of a nanofluid in relation to information such as the base fluid, the type of nanoparticle and the concentration of the nanoparticles only. The other parameters that define the nanofluid are important as well, to the point that the performance of nanofluids of the same type (in this case TiO2-water-based nanofluids) can differ significantly. However, these striking differences could be induced not only by different parameters, but also by the measurement methodology which, at least in the case of thermal conductivity, can lead to misleading results. In any case, the lack of information available on the parameters that characterize the various nanofluids makes it difficult to interpret the results, and suggests that it would be desirable to establish a protocol to define the minimum information necessary to fully characterize a nanofluid, in order to correctly interpret its properties and guarantee the reproducibility in the laboratory of the nanofluid itself. At the same time, the development of a standard nanofluid and benchmark measurements performed with certified accurate instruments would enable every laboratory to test the available instrumentation and assess the accuracy of their measurements. Accurate measurements are essential not to hinder the effects of the various parameters characterizing the nanofluid. In this way, it would be possible to develop and verify theories and interpretative models that would allow the performance of nanofluids to be correctly described and predicted.
To summarize, the article has highlighted the complexity of nanofluids given by the variety of parameters that influence their properties. At the same time, through the analysis of a case study, it has highlighted how often the available information in the literature is not sufficient to fully describe the nanofluid, making its reproducibility difficult. Furthermore, it has been seen that the measurements of the properties, at least with regards to thermal conductivity, are often contradictory to each other and how the lack of information makes it difficult to interpret these differences. However, since the variety of nanofluids is considerable, the conclusions taken for water-TiO2 nanofluids are not generalizable and therefore a similar analysis should be extended to other types of nanofluid.
Author Contributions
Conceptualization, S.B. and L.F.; methodology, O.M.; formal analysis, B.B.; data curation, S.V.; writing—original draft preparation, S.B.; writing—review and editing, S.B.; supervision, L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Benos, L.; Spyrou, L.A.; Sarris, I.E. Development of a new theoretical model for blood-CNTs effective thermal conductivity pertaining to hyperthermia therapy of glioblastoma multiform. Comput. Methods Prog. Biomed. 2019, 172, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing Efficiency of Particles in Heavy Metal Removal Processes under Various Inlet Conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Experimental comparison between ZnO and MoS2 nanoparticles as additives on performance of diesel oil-based nano lubricant. Sci. Rep. 2020, 10, 5813. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Proceedings of the International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995; pp. 99–106. [Google Scholar]
- Sidik, N.A.; Yazid, M.N.; Mamat, R. A review on the application of nanofluids in vehicle engine cooling system. Int. Commun. Heat Mass 2015, 68, 85–90. [Google Scholar] [CrossRef]
- Gómez-Villarejo, R.; Estellé, P.; Navas, J. Boron nitride nanotubes-based nanofluids with enhanced thermal properties for use as heat transfer fluids in solar thermal applications. Sol. Energy Mater. Sol. Cells 2020, 205, 110266. [Google Scholar] [CrossRef]
- Kulkarni, D.P.; Das, D.K.; Vajjha, R.S. Application of nanofluids in heating buildings and reducing pollution. Appl. Energy 2009, 86, 2566–2573. [Google Scholar] [CrossRef]
- Bhattad, A.; Sarkar, J.; Ghosh, P. Improving the performance of refrigeration systems by using nanofluids: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 3656–3669. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Electronics cooling with nanofluids: A critical review. Energy Convers. Manag. 2018, 172, 438–456. [Google Scholar] [CrossRef]
- Gkountas, A.A.; Benos, L.T.; Nikas, K.-S.; Sarris, I.E. Heat transfer improvement by an Al2O3-water nanofluid coolant in printed-circuit heat exchangers of supercritical CO2 Brayton cycle. Therm. Sci. Eng. Prog. 2020, 20, 100694. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.Y.; Mohammed, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 164, 115259. [Google Scholar] [CrossRef]
- Koca, H.D.; Doganay, S.; Turgut, A.; Tavman, I.H.; Saidur, R.; Mahbubul, I.M. Effect of particle size on the viscosity of nanofluids: A review. Renew. Sustain. Energy Rev. 2018, 82, 1664–1674. [Google Scholar] [CrossRef]
- Maheshwary, P.B.; Handa, C.C.; Nemade, K.R. A comprehensive study of effect of concentration, particle size and particle shape on thermal conductivity of titania/water based nanofluid. Appl. Therm. Eng. 2017, 119, 79–88. [Google Scholar] [CrossRef]
- Zhou, M.; Xia, G.; Li, J.; Chai, L.; Zhou, L. Analysis of factors influencing thermal conductivity and viscosity in different kinds of surfactant solutions. Exp. Therm. Fluid Sci. 2012, 36, 22–29. [Google Scholar]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, X.-F. Influence of pH on Nanofluids’ Viscosity and Thermal Conductivity. Chin. Phys. Lett. 2009, 26, 056601. [Google Scholar]
- Lee, D.; Kim, J.-W.; Kim, B.G. A new parameter to control heat transport in nanofluids: Surface charge state of the particle in suspension. J. Phys. Chem. B 2006, 110, 4323–4328. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, V.; Kumar, R.; Said, Z. A review on thermophysical properties of nanofluids and heat transfer applications. Renew. Sustain. Energy Rev. 2017, 74, 638–670. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Zyla, G.; Estellè, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkooshar, A. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state-of-the-art review. Powder Technol. 2019, 352, 209–226. [Google Scholar] [CrossRef]
- Hafiz, M.A.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation Techniques of TiO2 Nanofluids and Challenges: A Review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef]
- Saini, A.; Kaur, H.; Sharma, S.; Gangacharyulu, D. Nanofluids: A Review Preparation, Stability, Properties and Applications. Int. J. Eng. Res. Technol. 2016, 5, 11–15. [Google Scholar]
- Kwak, K.; Kim, C. Viscosity and thermal conductivity of copper oxide nanofluid dispersed in ethylene glycol. Korea-Aust. Rheol. J. 2005, 17, 35–40. [Google Scholar]
- Wen, D.; Ding, Y. Natural Convective Heat Transfer of Suspensions of Titanium Dioxide Nanoparticles (Nanofluids). IEEE Trans. Nanotechnol. 2006, 5, 220–227. [Google Scholar]
- Silambarasan, M.; Manikandan, S.; Rajan, K.S. Viscosity and thermal conductivity of dispersions of sub-micron TiO2 particles in water prepared by stirred bead milling and ultrasonication. Int. J. Heat Mass Transf. 2012, 55, 7991–8802. [Google Scholar] [CrossRef]
- Li, X.F.; Zhu, D.S.; Wang, X.J.; Wang, N.; Gao, J.W.; Li, H. Thermal conductivity enhancement dependent pH and chemical surfactant for Cu-H2O nanofluids. Thermochim. Acta 2008, 469, 98–103. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar]
- Lee, G.J.; Kim, C.K.; Lee, M.K.; Rhee, C.K. Characterization of ethylene glycol based TiO2 nanofluid prepared by pulsed wire evaporation (PWE) method. Rev. Adv. Mater. Sci. 2011, 28, 126–129. [Google Scholar]
- Lo, C.H.; Tsung, T.T.; Chen, L.C. Shape-controlled synthesis of Cu-based nanofluid using submerged arc nanoparticle synthesis system (SANSS). J. Cryst. Growth 2005, 277, 636–642. [Google Scholar] [CrossRef]
- Wu, Y.; Kao, M. Using TiO2 nanofluid additive for engine lubrication oil. Ind. Lubr. Tribol. 2011, 63, 440–445. [Google Scholar] [CrossRef]
- Huang, X.X.; Zhang, W.G. Study on successively preparation of nano-TiO2 ethanol colloids by pulsed laser ablation and fluorescence property. Appl. Surf. Sci. 2008, 254, 3403–3407. [Google Scholar] [CrossRef]
- Zhu, H.T.; Lin, Y.S.; Yin, Y.S. A novel one-step chemical method for preparation of copper nanofluids. J. Colloid Interface Sci. 2004, 277, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Prk, S.D.; Kang, S.; Bang, I.C.; Kim, J.H. Investigation of viscosity and thermal conductivity of SiC nanofluids for heat transfer applications. Int. J. Heat Mass. Transf. 2011, 54, 433–438. [Google Scholar] [CrossRef]
- Teng, T.P.; Hung, Y.H.; Teng, T.C.; Mo, H.E.; Hsu, H.G. The effect of alumina/water nanofluid particle size on thermal conductivity. Appl. Therm. Eng. 2010, 30, 2213–2218. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int. Commun. Heat Mass 2013, 44, 7–14. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Smith, D.S.; Yo, W.; France, D.M.; Singh, D.; Routbort, J.L. Particle size and interfacial effects on thermo-physical and heat transfer characteristics of water-based α-SiC nanofluids. Nanotechnology 2010, 21, 215703. [Google Scholar] [CrossRef]
- Paul, G.; Pal, T.; Manna, I. Thermo-physical property measurement of nano-gold dispersed water based nanofluids prepared by chemical precipitation technique. J. Colloid Interface Sci. 2010, 349, 434–437. [Google Scholar] [CrossRef]
- Kwek, D.; Crivol, A.; Duan, F. Effects of Temperature and Particle Size on the Thermal Property Measurements of Al2O3-Water Nanofluids. J. Chem. Eng. Data 2010, 55, 5690–5695. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.R.; Choi, S.U.S.; Eastman, J.A. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass 2002, 45, 855–863. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass 2007, 50, 2272–2281. [Google Scholar] [CrossRef]
- Shalkevich, N.; Escher, W.; Burgi, T.; Michel, B.; Si-Ahmed, L.; Poulikakos, D. On the thermal conductivity of gold nanoparticle colloids. Langmuir 2010, 26, 663–670. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Marè, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids—Hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Mirmohammadi, S.A.; Behi, M.; Gan, Y.; Shen, L. Particle-shape-, temperature-, and concentration-dependent thermal conductivity and viscosity of nanofluids. Phys. Rev. E 2019, 99, 043109. [Google Scholar] [CrossRef]
- Saleh, R.; Putra, N.; Wibowo, R.E.; Septiadi, W.N.; Prakoso, S.P. Titanium dioxide nanofluids for heat transfer applications. Exp. Therm. Fluid Sci. 2014, 52, 19–29. [Google Scholar] [CrossRef]
- Hu, H.; Peng, H.; Ding, G. Nucleate pool boiling heat transfer characteristics of refrigerant/nanolubricant mixture with surfactant. Int. J. Refrig. 2013, 36, 1045–1055. [Google Scholar] [CrossRef]
- Alphonse, P.; Bleta, R.; Soules, R. Effect of PEG on rheology and stability of nanocrystalline titania hydrosols. J. Colloid Interface Sci. 2009, 337, 81–87. [Google Scholar] [CrossRef]
- Jarahnejad, M.; Haghighi, E.B.; Saleemi, M.; NIkkam, N.; Khodabandeh, R.; Palm, B.; Toprak, M.S.; Muhammed, M. Experimental investigation on viscosity of water-based Al2O3 and TiO2 nanofluids. Rheol. Acta 2015, 54, 411–422. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S.; Agresti, F. Experimental stability analysis of different water based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-C.; Li, F.-C.; Zhou, W.-W.; He, Y.-R.; Jiang, C.-B. Experimental investigation on the thermal conductivity and shear viscosity of viscoelastic-fluid-based nanofluids. Int. J. Heat Mass Transf. 2012, 55, 3160–3166. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2—water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Experimental Study on the Effective Thermal Conductivity and Thermal Diffusivity of Nanofluids. Int. J. Thermophys. 2006, 27, 569–580. [Google Scholar] [CrossRef]
- Turgut, A.; Tavman, I.; Chirtoc, M.; Schuchmann, H.P.; Sauter, C.; Tavman, S. Thermal Conductivity and Viscosity Measurements of Water-Based TiO2 Nanofluids. Int. J. Thermophys. 2009, 30, 1213–1226. [Google Scholar] [CrossRef]
- Wang, Z.I.; Tang, D.W.; Liu, S.; Zheng, X.H.; Araki, N. Thermal-Conductivity and Thermal-Diffusivity Measurements of Nanofluids by 3ω Method and Mechanism Analysis of Heat Transport. Int. J. Thermophys. 2007, 28, 1255–1268. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Hong, K.S.; Yang, H.-S. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar] [CrossRef]
- Bobbo, S.; Fedele, L.; Benetti, A.; Colla, L.; Fabrizio, M.; Pagura, C.; Barison, S. Viscosity of water based SWCNH and TiO2 nanofluids. Exp. Therm. Fluid Sci. 2012, 36, 65–71. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. Exp. Therm. Fluid Sci. 2007, 31, 593–599. [Google Scholar] [CrossRef]
- Chen, H.; Witharana, S.; Jin, Y.; Kim, C.; Ding, Y. Predicting thermal conductivity of liquid suspensions of nanoparticles (nanofluids) based on rheology. Particuology 2009, 7, 151–157. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. [Google Scholar] [CrossRef]
- Das, P.; Mallik, A.K.; Ganguly, R.; Santra, A.K. Synthesis and characterization of TiO2-water nanofluids with different surfactants. Int. Commun. Heat Mass 2016, 75, 341–348. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 2007, 11, 151–170. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Hojjat, M.; Etemad, S.G.; Bagheri, R.; Thibault, J. Thermal conductivity of non-Newtonian nanofluids: Experimental data and modeling using neural network. Int. J. Heat Mass Trans. 2011, 54, 1017–1023. [Google Scholar] [CrossRef]
- Tertsinidou, G.J.; Tsolakidou, C.M.; Pantzali, M.; Assael, M.J.; Colla, L.; Fedele, L.; Bobbo, S.; Wakeham, W.A. New Measurements of the Apparent Thermal Conductivity of Nanofluids and Investigation of Their Heat Transfer Capabilities. J. Chem. Eng. Data 2017, 62, 491–507. [Google Scholar] [CrossRef]
- Tertsinidou, G.; Assael, M.J.; Wakeham, W.A. The Apparent Thermal Conductivity of Liquids Containing Solid Particles of Nanometer Dimensions: A Critique. Int. J. Thermophys. 2015, 36, 1367–1395. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).