Antibiotic Activity Screened by the Rheology of S. aureus Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Rheology
- (i)
- flow curve: a steel cone and plate geometry, with diameter 40 mm, angle 2° and gap 55 µm for the steady-state shear flow tests, which were performed at 20 °C. Each step was acquired assuring that a minimum of 1500 units of deformation was imposed to the sample. Using a CP geometry with a very small gap, sample ejection, due to centrifuge effect, did not occur within the range of shear rate values considered;
- (ii)
- viscosity growth curve: a steel plate-plate geometry, with diameter 40 mm and gap of 2000 µm (to ensure a good signal) for the steady shear viscosity measurement, imposing a constant shear rate of 10 s−1, during time and at 37 °C to allow optimal growth conditions.
3. Results and Discussion
3.1. Antibiotic Effect on S. aureus Growth
3.2. Steady State Shear Flow Assays
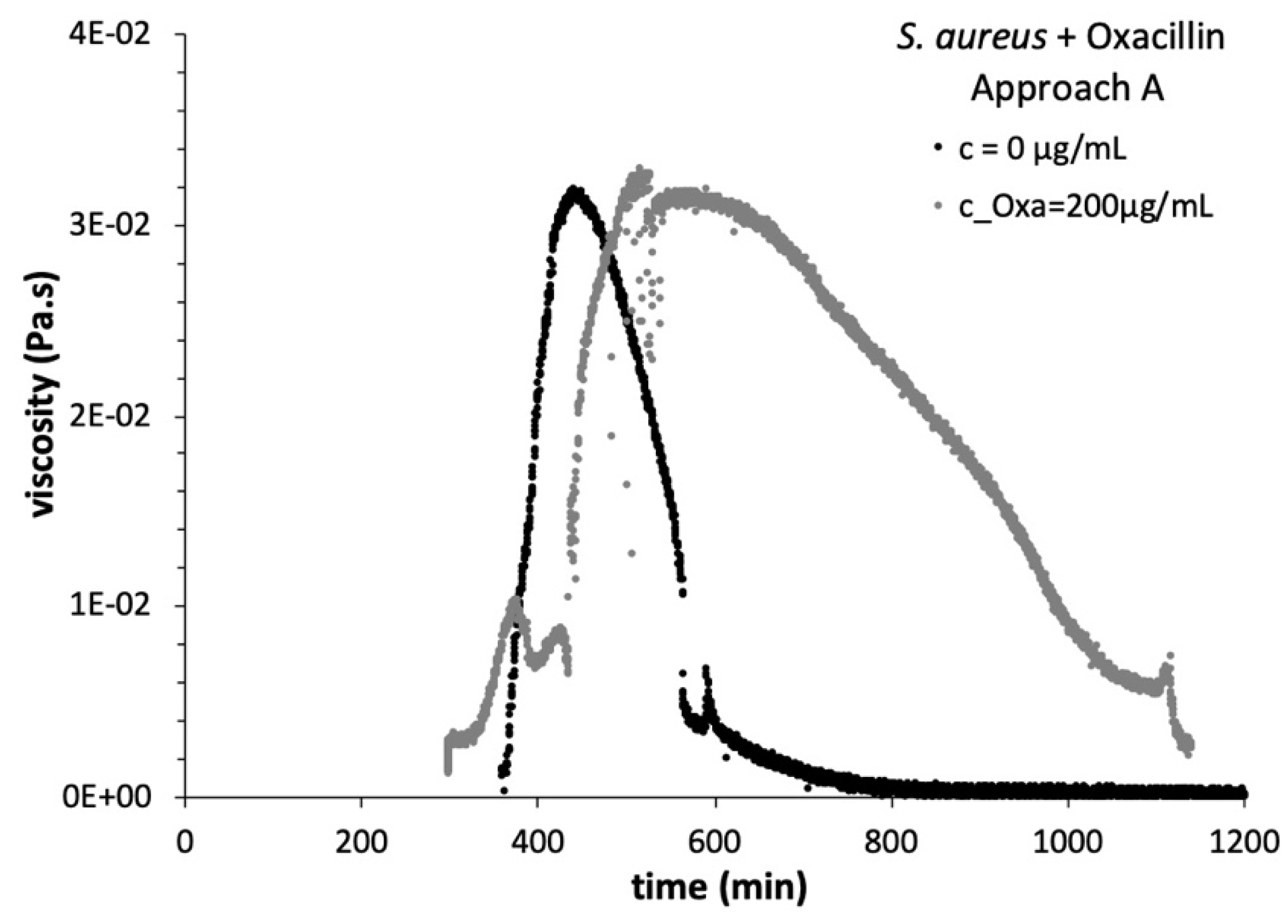
3.3. Viscosity Growth Curves
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peterson, B.W.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; van Winkelhoff, A.J.; Neut, D.; Stoodley, P.; van der Mei, H.C.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234. [Google Scholar] [CrossRef] [PubMed]
- Klapper, I.; Rupp, C.J.; Cargo, R.; Purevdorj, B.; Stoodley, P. Viscoelastic fluid description of bacterial biofilm material properties. Biotech. Bioeng. 2002, 80, 289. [Google Scholar] [CrossRef] [PubMed]
- Towler, B.W.; Rupp, C.J.; Cunningham, A.B.; Stoodley, P. Viscoelastic properties of a mixed culture biofilm from rheometer creep analysis. Biofouling 2003, 19, 279. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.S.; van der Walle, C.; Waigh, T.A. Microrheology of Bacterial Biofilms In Vitro: Staphylococcus aureus and Pseudomonas aeruginosa. Langmuir 2008, 24, 13549. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, Y.D.; Wilms, T.; Vanbever, R.; Bambekea, F.V. Activity of antibiotics against Staphylococcus aureus in an in vitro model of biofilms in the context of cystic fibrosis: Influence of the culture medium. Antimicrob. Agents Chemother. 2019, 63, e00602. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Almeida, P.L.; Patrício, P.; Cidade, T.; Sobral, R.G.; Leal, C.R. Real-time rheology of actively growing bacteria. Phys. Rev. E 2013, 87, 030701(R). [Google Scholar] [CrossRef]
- Patrício, P.; Almeida, P.; Portela, R.; Sobral, R.; Grilo, I.; Cidade, T.; Leal, C.R. Living bacteria rheology: Population growth, aggregation patterns, and collective behavior under different shear flows. Phys. Rev. E 2014, 90, 022720. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Patrício, P.; Almeida, P.L.; Sobral, R.G.; Franco, J.M.; Leal, C.R. Rotational tumbling of Escherichia coli aggregates under shear. Phys. Rev. E 2016, 94, 062402. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Almeida, P.L.; Sobral, R.G.; Leal, C.R. Motility and cell shape roles in the rheology of growing bacteria cultures. Eur. Phys. J. E 2019, 42, 26. [Google Scholar] [CrossRef]
- Franco, J.M.; Patrício, P.; Almeida, P.; Portela, R.; Sobral, R.G.; Leal, C.R. Cell necklaces behave as a soft glassy material. In Proceedings of the Ibereo 2015 Conference—Challenges in Rheology and Product Development, Coimbra, Portugal, 7–9 September 2015; Rasteiro, M.G., Ed.; pp. 118–121, ISBN 978-989-26-1056-6. [Google Scholar]
- Sollich, P.; Lequeux, F.; Hebraud, P.; Cates, M.E. Rheology of Soft Glassy Materials. Phys. Rev. Lett. 1997, 78, 2020. [Google Scholar] [CrossRef]
- Jevons, M.P. “Celbenin” - resistant Staphylococci. Br. Med. J. 1961, 1, 124–125. [Google Scholar] [CrossRef]
- Ghuysen, J.M. Molecular structures of penicillin-binding proteins and β-lactamases. Trends Microbiol. 1994, 2, 372–380. [Google Scholar] [CrossRef]
- Miragaia, M. Factors Contributing to the Evolution of mecA-Mediated β-lactam Resistance in Staphylococci: Update and New Insights from Whole Genome Sequencing (WGS). Front. Microbiol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Fuda, C.; Suvorov, M.; Vakulenko, S.B.; Mobashery, S. The Basis for Resistance to b--Lactam Antibiotics by Penicillinbinding Protein 2a of Methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2004, 279, 40802–40806. [Google Scholar] [CrossRef] [PubMed]
- Raynor, R.H.; Scott, D.F.; Best, G.K. Oxacillin-induced lysis of Staphylococcus aureus. Antimicrob Agents Chemother. 1979, 16, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sobral, R.G.; Ludovice, A.M.; Gardete, S.; Tabei, K.; De Lencastre, H.; Tomasz, A. Normally functioning murF is essential for the optimal expression of methicillin resistance in Staphylococcus aureus. Microb. Drug Resist. 2003, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Paszkowiak, J.J.; Dardik, A. Arterial wall shear stress: Observations from the bench to the bedside. Vasc. Endovasc. Surg. 2003, 37, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Fouts, D.E.; Archer, G.L.; Mongodin, E.F.; DeBoy, R.T.; Ravel, J.; Paulsen, I.T.; Kolonay, J.F.; Brinkac, L.; Beanan, M.; et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 2005, 187, 2426–2438. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portela, R.; Valcovo, F.; Almeida, P.L.; Sobral, R.G.; Leal, C.R. Antibiotic Activity Screened by the Rheology of S. aureus Cultures. Fluids 2020, 5, 76. https://doi.org/10.3390/fluids5020076
Portela R, Valcovo F, Almeida PL, Sobral RG, Leal CR. Antibiotic Activity Screened by the Rheology of S. aureus Cultures. Fluids. 2020; 5(2):76. https://doi.org/10.3390/fluids5020076
Chicago/Turabian StylePortela, Raquel, Filipe Valcovo, Pedro L. Almeida, Rita G. Sobral, and Catarina R. Leal. 2020. "Antibiotic Activity Screened by the Rheology of S. aureus Cultures" Fluids 5, no. 2: 76. https://doi.org/10.3390/fluids5020076
APA StylePortela, R., Valcovo, F., Almeida, P. L., Sobral, R. G., & Leal, C. R. (2020). Antibiotic Activity Screened by the Rheology of S. aureus Cultures. Fluids, 5(2), 76. https://doi.org/10.3390/fluids5020076






