Advancing Chemical Risk Assessment through Human Physiology-Based Biochemical Process Modeling
Abstract
:1. Introduction
2. Materials and Methods
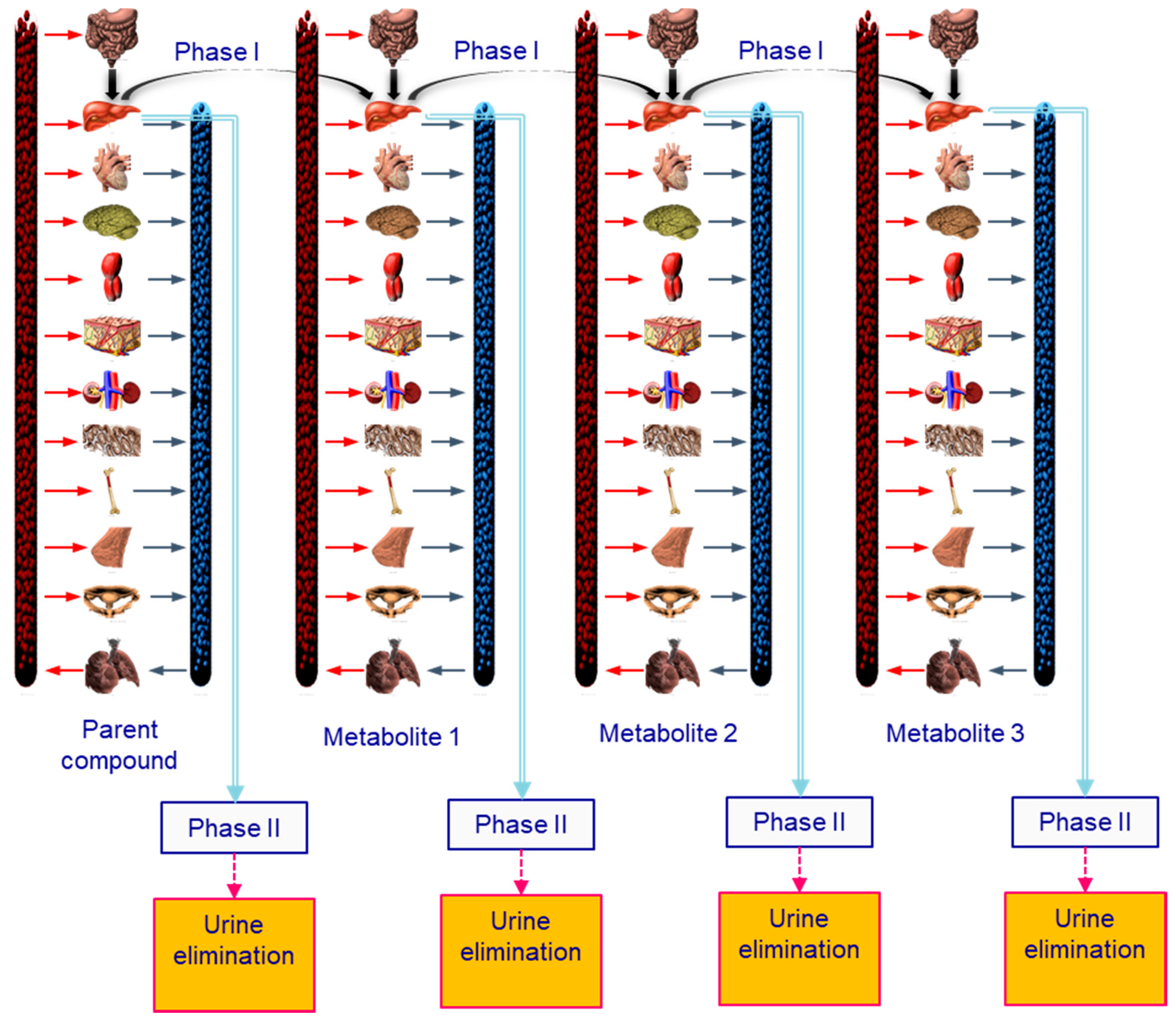
2.1. Development of the Generic Lifetime PBPK Model
- -
- excess molar refraction; a property that can be determined if the compound refractive index is known,
- -
- compound dipolarity/polarizability,
- -
- solute effective or summation hydrogen-bond acidity,
- -
- solute effective or summation hydrogen-bond basicity, and
- -
- McGowan characteristic volume that can be calculated based on the molecular structure of the solute.
2.2. BPA Toxicokinetic Considerations
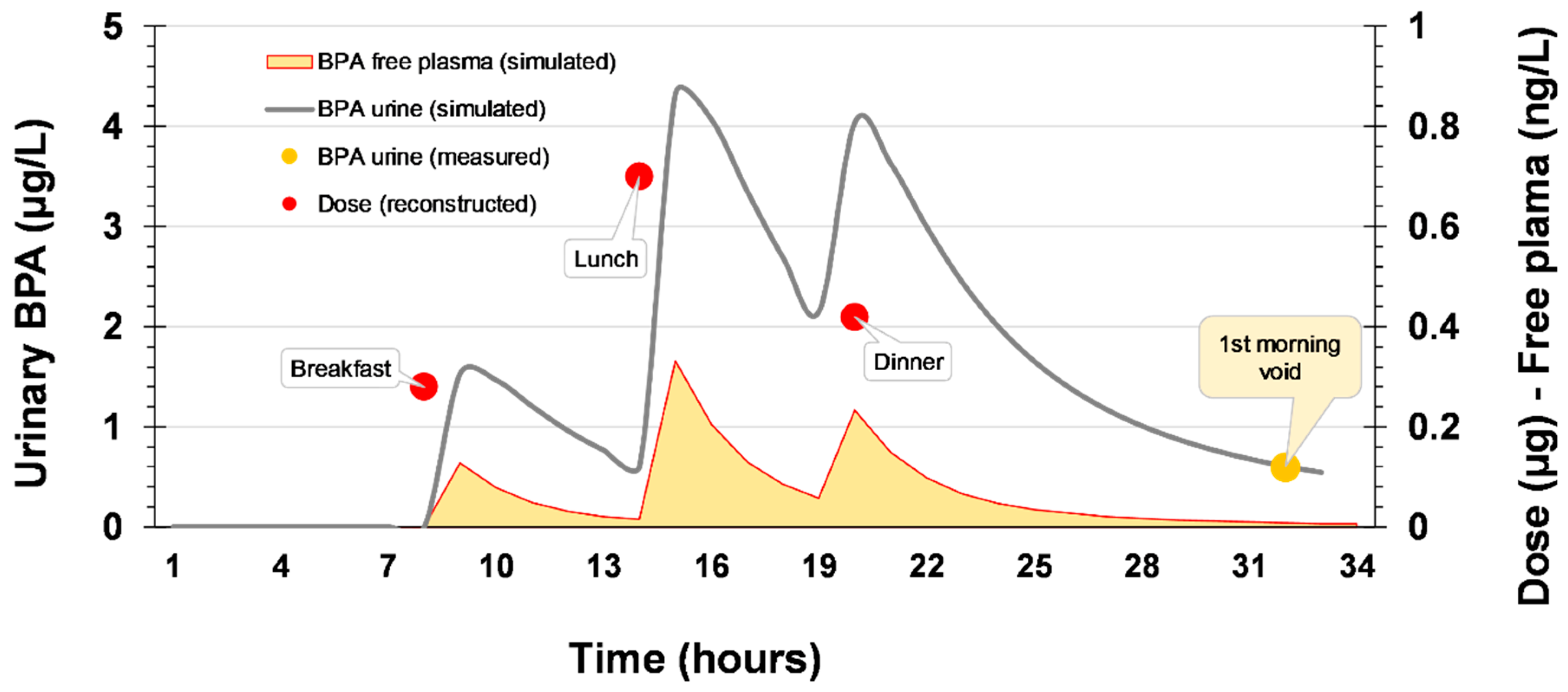
2.3. Exposure Reconstruction Starting from Human Biomonitoring (HBM) Data
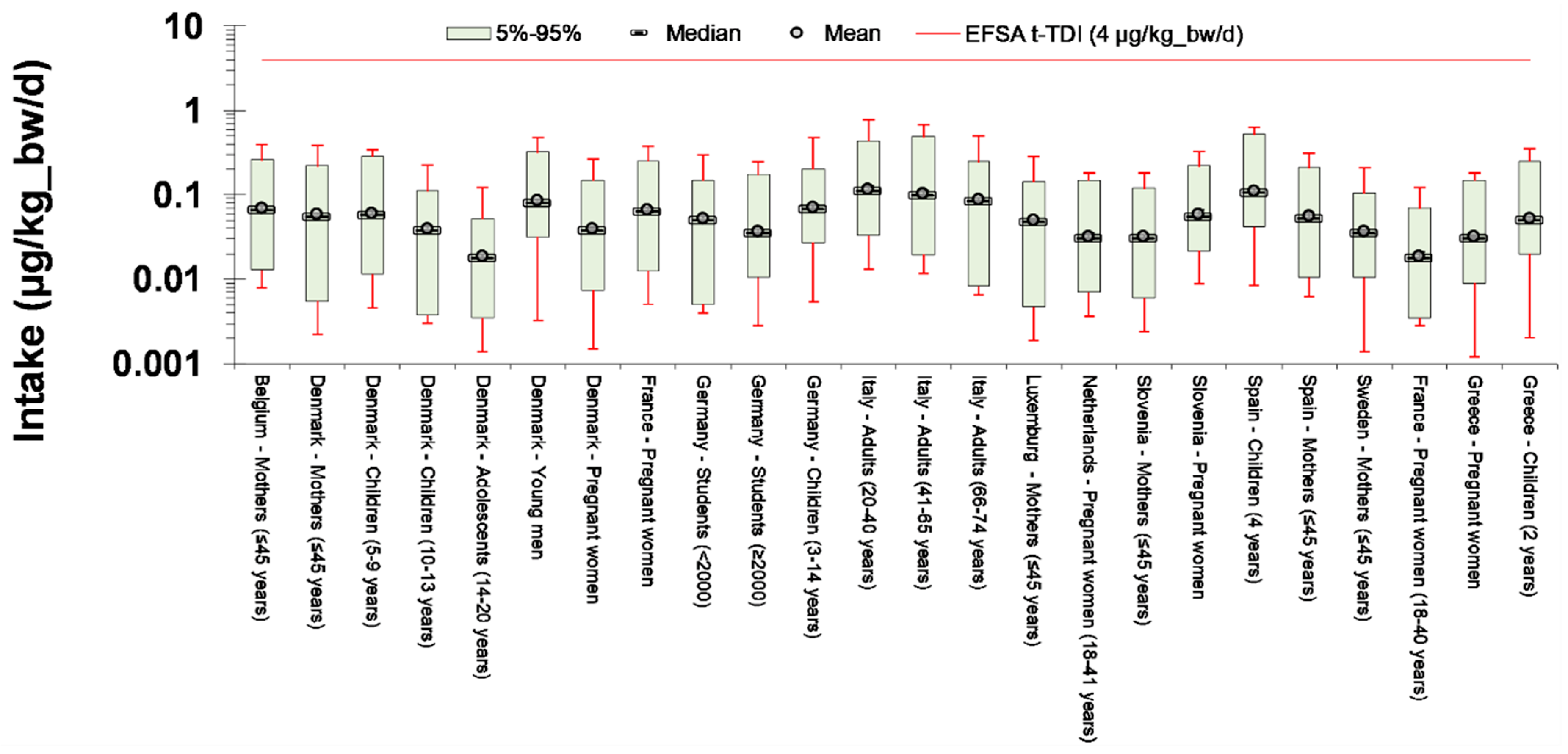
2.4. Exposure Assessment
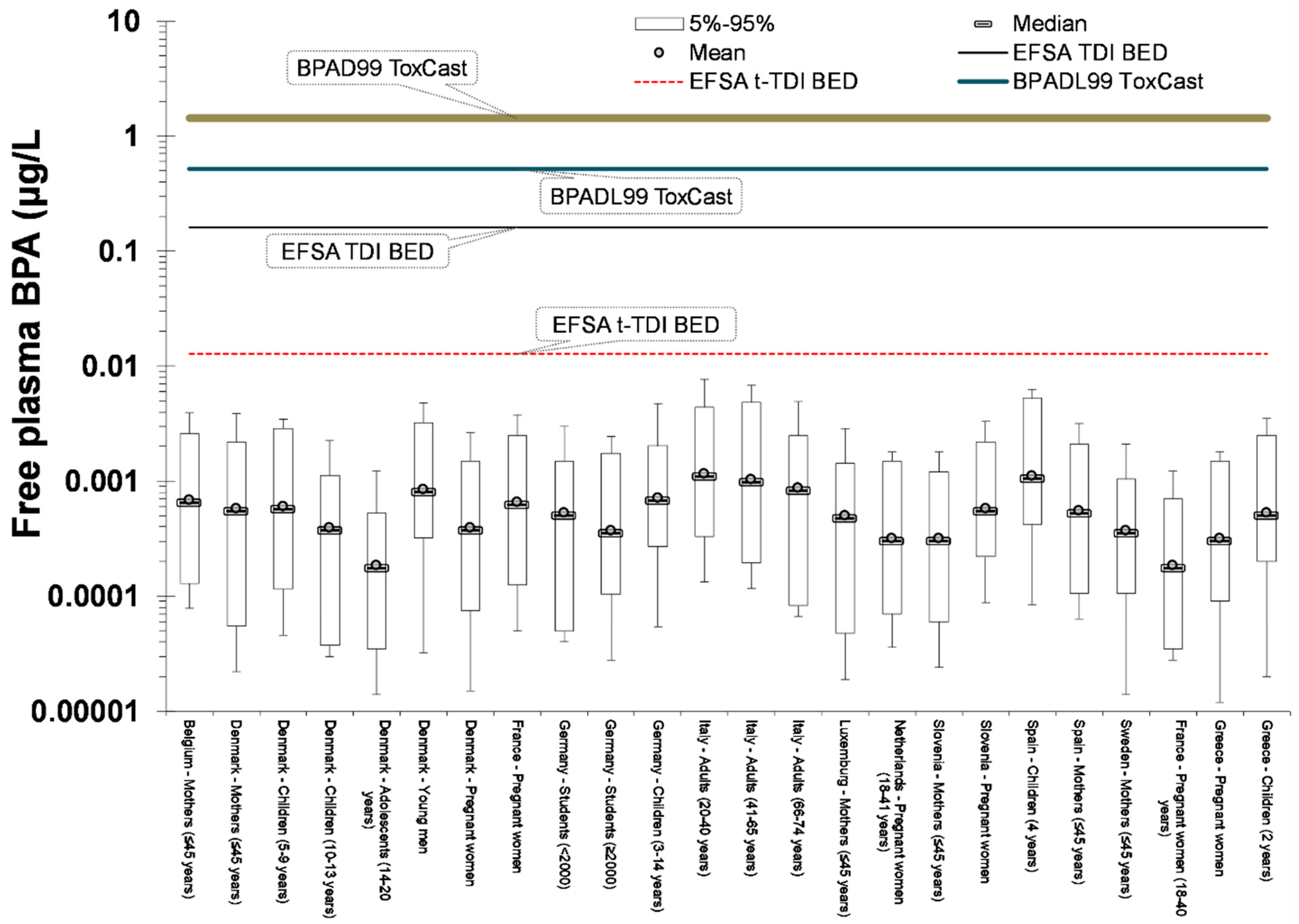
2.5. Risk Assessment
- Direct comparison of exposure reconstruction intake estimates to EFSA t-TDI of 4 μg/kg_bw/day.
- Use of a biomonitoring equivalent (BE) value for urinary data. An original BE for BPA has been derived by Krishnan et al. [50] equal to 2000 μg/L, on the basis of the old EFSA TDI (equal to 50 μg/kg_bw/day), following the original BE concept initially proposed by Hays et al. [51] and further expanded by Aylward [52]. The reference dose for deriving the BE value was the EFSA t-TDI of 4 μg/kg_bw/day. It was assumed that this dose is given orally to an adult of 70 kg body weight at a constant rate during the day. After that, this intake was fed to the PBBK model resulting to urinary BPA-Glu concentration of 280 μg/L.
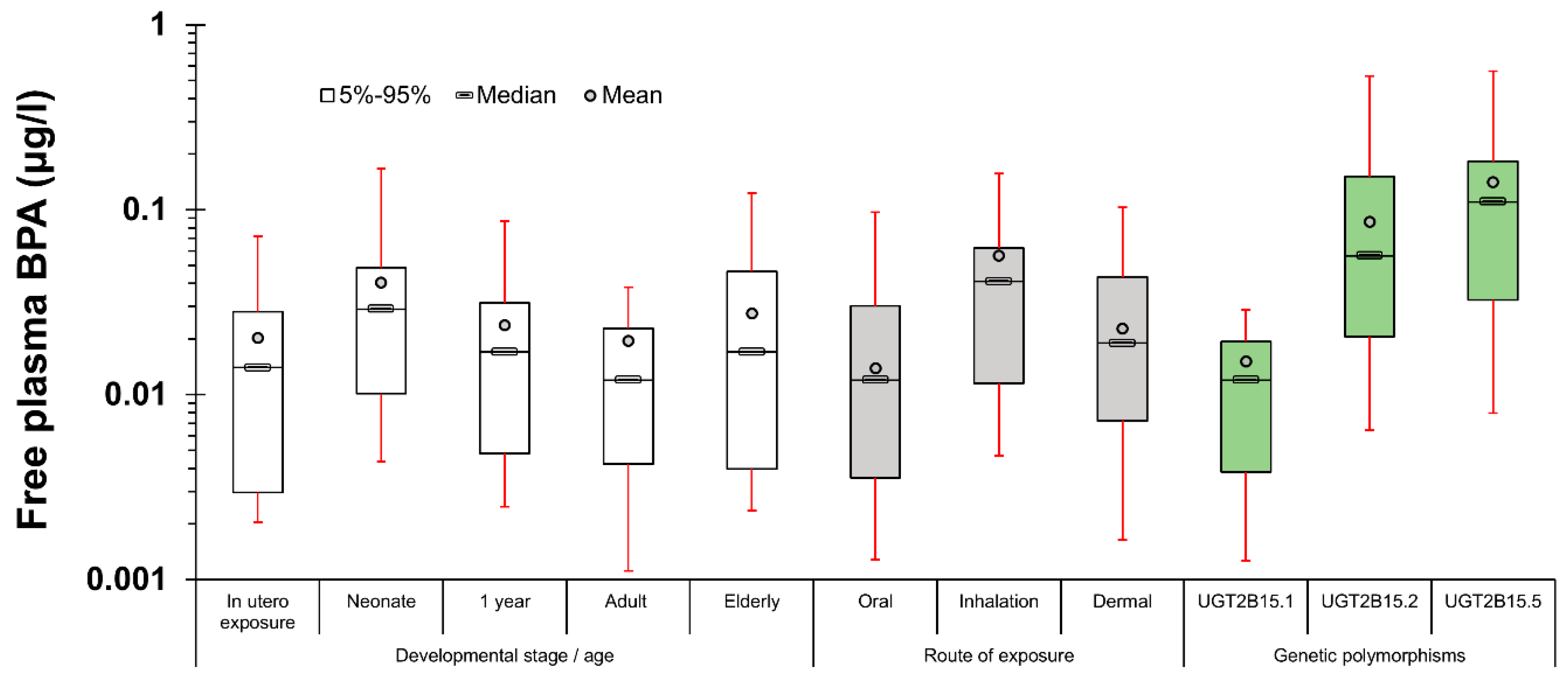
- Given the limitations of exposure back-calculation based on urinary BPA-Glu levels, the use of another exposure metric more relevant to where the xenobiotics exert their toxicity has to be considered. Towards this aim, free plasma BPA was selected as a descriptive metric linked to the biologically effective dose (BED). The use of this internal exposure metric, allows us to further differentiate internal and external exposure as a result of bioavailability differences related to developmental stage, point of entrance and eventually genetics. As a result, the calculated area under the curve (AUC) for 24 h, equals 0.312 μg 24 h/L (for one hour time interval) [25].
3. Results
3.1. Exposure Reconstruction based on HBM Data
3.2. Risk Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sarigiannis, D.A.; Karakitsios, S.P. A dynamic physiology based pharmacokinetic model for assessing lifelong internal dose. In Proceedings of the AIChE 2012, Pittsburgh, PA, USA, 28 October–2 November 2012. [Google Scholar]
- Sarigiannis, D.A.; Gotti, A. Biology-based dose-response models for health risk assessment of chemical mixtures. Fres. Environ. Bull. 2008, 17, 1439–1451. [Google Scholar]
- Georgopoulos, P.G.; Sasso, A.F.; Isukapalli, S.S.; Lioy, P.J.; Vallero, D.A.; Okino, M.; Reiter, L. Reconstructing population exposures to environmental chemicals from biomarkers: Challenges and opportunities. J. Expo. Sci. Envion. Epidemiol. 2008, 19, 149–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andra, S.S.; Charisiadis, P.; Karakitsios, S.; Sarigiannis, D.A.; Makris, K.C. Passive exposures of children to volatile trihalomethanes during domestic cleaning activities of their parents. Environ. Res. 2015, 136, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.M.; Liao, K.; Conolly, R.; Blount, B.; Mason, A.; Clewell, H. Use of a physiologically based pharmacokinetic model to identify exposures consistent with human biomonitoring data for chloroform. J. Toxicol. Environ. Health Part A: Curr. Issues 2006, 69, 1727–1756. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.S.; Kavlock, R.J.; Setzer, R.W.; Cohen Hubal, E.A.; Martin, M.T.; Knudsen, T.B.; Houck, K.A.; Thomas, R.S.; Wetmore, B.A.; Dix, D.J. Estimating toxicity-related biological pathway altering doses for high-throughput chemical risk assessment. Chem. Res. Toxicol. 2011, 24, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Eissing, T.; Kuepfer, L.; Becker, C.; Block, M.; Coboeken, K.; Gaub, T.; Goerlitz, L.; Jaeger, J.; Loosen, R.; Ludewig, B.; et al. A computational systems biology software platform for multiscale modeling and simulation: Integrating whole-body physiology, disease biology, and molecular reaction networks. Front. Phys. 2011, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Krauss, M.; Schaller, S.; Borchers, S.; Findeisen, R.; Lippert, J.; Kuepfer, L. Integrating Cellular Metabolism into a Multiscale Whole-Body Model. PLoS Comp. Biol. 2012, 8, e1002750. [Google Scholar] [CrossRef]
- Morck, T. Chapter 3G Bisphenol A. In Biomarkers and Human Biomonitoring; The Royal Society of Chemistry: London, UK, 2012; Volume 1, pp. 360–380. [Google Scholar]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Thomas, B.F.; Keimowitz, A.R.; Brine, D.R.; Veselica, M.M.; Fail, P.A.; Chang, T.Y.; Seely, J.C.; et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002, 68, 121–146. [Google Scholar] [CrossRef]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Sloan, C.S.; Castillo, N.P.; Veselica, M.M.; Seely, J.C.; Dimond, S.S.; Van Miller, J.P.; Shiotsuka, R.N.; et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (swiss) mice. Toxicol. Sci. 2008, 104, 362–384. [Google Scholar] [CrossRef]
- Ferguson, S.A.; Law, C.D., Jr.; Abshire, J.S. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci. 2011, 124, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Delclos, K.B.; Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Latendresse, J.R.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Gamboa da Costa, G.; Woodling, K.A.; et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 2014, 139, 174–197. [Google Scholar] [CrossRef]
- Edginton, A.N.; Ritter, L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ. Health Perspect. 2009, 117, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, G.; Rice, D.C. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 2009, 117, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D.; Karakitsios, S.; Gotti, A.; Loizou, G.; Cherrie, J.; Smolders, R.; De Brouwere, K.; Galea, K.; Jones, K.; Handakas, E.; et al. Integra: From global scale contamination to tissue dose. In Proceedings of the 7th International Congress on Environmental Modelling and Software: Bold Visions for Environmental Modeling, San Diego, CA, USA, 15–19 June 2014; pp. 1001–1008. [Google Scholar]
- Sarigiannis, D.A.; Papadaki, K.; Kontoroupis, P.; Karakitsios, S.P. Development of QSARs for parameterizing Physiology Based ToxicoKinetic models. Food Chem. Toxicol. 2017, 106, 114–124. [Google Scholar] [CrossRef]
- Papadaki, K.C.; Karakitsios, S.P.; Sarigiannis, D.A. Modeling of adipose/blood partition coefficient for environmental chemicals. Food Chem. Toxicol. 2017, 110, 274–285. [Google Scholar] [CrossRef]
- Beaudouin, R.; Micallef, S.; Brochot, C. A stochastic whole-body physiologically based pharmacokinetic model to assess the impact of inter-individual variability on tissue dosimetry over the human lifespan. Regul. Toxicol. Pharmacol. 2010, 57, 103–116. [Google Scholar] [CrossRef]
- Lee, S.K.; Ou, Y.C.; Andersen, M.E.; Yang, R.S.H. A physiologically based pharmacokinetic model for lactational transfer of PCB 153 with or without PCB 126 in mice. Arch. Toxicol. 2007, 81, 101–111. [Google Scholar] [CrossRef]
- Verner, M.A.; Charbonneau, M.; Lopez-Carrillo, L.; Haddad, S. Physiologically based pharmacokinetic modeling of persistent organic pollutants for lifetime exposure assessment: A new tool in breast cancer epidemiologic studies. Environ. Health Perspect. 2008, 116, 886–892. [Google Scholar] [CrossRef]
- Touitou, E. Drug delivery across the skin. Expert Opin. Biol. Ther. 2002, 2, 723–733. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anissimov, Y.G.; Bunge, A.L.; Frasch, H.F.; Guy, R.H.; Hadgraft, J.; Kasting, G.B.; Lane, M.E.; Roberts, M.S. Mathematical models of skin permeability: An overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarigiannis, D.; Karakitsios, S.; Handakas, E.; Simou, K.; Solomou, E.; Gotti, A. Integrated exposure and risk characterization of bisphenol-A in Europe. Food Chem. Toxicol. 2016, 98, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Schmitt, W.; Voith, B.; Willmann, S. A mechanistic approach for the scaling of clearance in children. Clin. Pharmacokinet. 2006, 45, 683–704. [Google Scholar] [CrossRef] [PubMed]
- Leeder, J.S. Developmental pharmacogenetics: A general paradigm for application to neonatal pharmacology and toxicology. Clin. Pharmacol. Ther. 2009, 86, 678–682. [Google Scholar] [CrossRef]
- Court, M.H.; Zhang, X.; Ding, X.; Yee, K.K.; Hesse, L.M.; Finel, M. Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica 2012, 42, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Teeguarden, J.G.; Twaddle, N.C.; Churchwell, M.I.; Yang, X.; Fisher, J.W.; Seryak, L.M.; Doerge, D.R. 24-hour human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicol. Appl. Pharmacol. 2015, 288, 131–142. [Google Scholar] [CrossRef]
- Yang, X.; Doerge, D.R.; Teeguarden, J.G.; Fisher, J.W. Development of a physiologically based pharmacokinetic model for assessment of human exposure to bisphenol A. Toxicol. Appl. Pharmacol. 2015, 289, 442–456. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values; ICRP: Ottawa, ON, Canada, 2002. [Google Scholar]
- Sarigiannis, D.; Karakitsios, S.; Gotti, A.; Handakas, E. Life cycle-based health risk assessment of plastic waste. In Proceedings of the 5th International Conference on Sustainable Solid Waste Manage, Athens, Greece, 21–24 June 2017. [Google Scholar]
- Gilks, W.R.; Roberts, G.O. Strategies for improving MCMC. In Markov Chain Monte Carlo in Practice; Springer: Berlin, Germany, 1996; pp. 89–114. [Google Scholar] [CrossRef]
- Haario, H.; Laine, M.; Mira, A.; Saksman, E. DRAM: Efficient adaptive MCMC. Stat. Comp. 2006, 16, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Ter Braak, C.J. A Markov Chain Monte Carlo version of the genetic algorithm Differential Evolution: Easy Bayesian computing for real parameter spaces. Stat. Comp. 2006, 16, 239–249. [Google Scholar] [CrossRef]
- Vandentorren, S.; Zeman, F.; Morin, L.; Sarter, H.; Bidondo, M.L.; Oleko, A.; Leridon, H. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: Implications for large-scale biomonitoring studies. Environ. Res. 2011, 111, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Federal Environment Agency (UBA). The German Environment Specimen Bank. Available online: http://www.umweltprobenbank.de (accessed on 17 April 2017).
- DEMOCOPHES. DEMOCOPHES Layman’s Report—Human Biomonitoring on a European Scale. Available online: http://www.eu-hbm.info/euresult/layman-report (accessed on 17 April 2017).
- Covaci, A.; Hond, E.D.; Geens, T.; Govarts, E.; Koppen, G.; Frederiksen, H.; Knudsen, L.E.; Mørck, T.A.; Gutleb, A.C.; Guignard, C.; et al. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environ. Res. 2015, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Jensen, T.K.; Jørgensen, N.; Kyhl, H.B.; Husby, S.; Skakkebæk, N.E.; Main, K.M.; Juul, A.; Andersson, A. Human urinary excretion of non-persistent environmental chemicals: An overview of Danish data collected between 2006 and 2012. Reproduction 2014, 147, 555–565. [Google Scholar] [CrossRef]
- Becker, K.; Göen, T.; Seiwert, M.; Conrad, A.; Pick-Fuß, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.; Cipelli, R.; Guralnik, J.; Ferrucci, L.; Bandinelli, S.; Corsi, A.M.; Money, C.; McCormack, P.; Melzer, D. Daily bisphenol a excretion and associations with sex hormone concentrations: Results from the InCHIANTI adult population study. Environ. Health Perspect. 2010, 118, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pierik, F.H.; Hauser, R.; Duty, S.; Angerer, J.; Park, M.M.; Burdorf, A.; Hofman, A.; Jaddoe, V.W.V.; Mackenbach, J.P.; et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environ. Res. 2008, 108, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, L.; Fernandez, M.F.; Llop, S.; Guxens, M.; Ballester, F.; Olea, N.; Irurzun, M.B.; Rodriguez, L.S.; Riano, I.; Tardon, A.; et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ. Int. 2011, 37, 858–866. [Google Scholar] [CrossRef]
- Dereumeaux, C.; Fillol, C.; Charles, M.-A.; Denys, S. The French human biomonitoring program: First lessons from the perinatal component and future needs. Int. J. Hyg. Environ. Health 2017, 220, 64–70. [Google Scholar] [CrossRef]
- Myridakis, A.; Fthenou, E.; Balaska, E.; Vakinti, M.; Kogevinas, M.; Stephanou, E.G. Phthalate esters, parabens and bisphenol-A exposure among mothers and their children in Greece (Rhea cohort). Environ. Int. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Krishnan, K.; Gagné, M.; Nong, A.; Aylward, L.L.; Hays, S.M. Biomonitoring Equivalents for bisphenol A (BPA). Regul. Toxicol. Pharmacol. 2010, 58, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.M.; Becker, R.A.; Leung, H.W.; Aylward, L.L.; Pyatt, D.W. Biomonitoring equivalents: A screening approach for interpreting biomonitoring results from a public health risk perspective. Regul. Toxicol. Pharmacol. 2007, 47, 96–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aylward, L.L. Integration of biomonitoring data into risk assessment. Curr. Opin. Toxicol. 2018, 9, 14–20. [Google Scholar] [CrossRef]
- Geens, T.; Apelbaum, T.Z.; Goeyens, L.; Neels, H.; Covaci, A. Intake of bisphenol A from canned beverages and foods on the Belgian market. Food Addit. Contam.: Part A 2010, 27, 1627–1637. [Google Scholar] [CrossRef]
- Sakhi, A.K.; Lillegaard, I.T.; Voorspoels, S.; Carlsen, M.H.; Loken, E.B.; Brantsaeter, A.L.; Haugen, M.; Meltzer, H.M.; Thomsen, C. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ. Int. 2014, 73, 259–269. [Google Scholar] [CrossRef]
- von Goetz, N.; Wormuth, M.; Scheringer, M.; Hungerbuhler, K. Bisphenol A: How the most relevant exposure sources contribute to total consumer exposure. Risk Anal. Int. J. 2010, 30, 473–487. [Google Scholar] [CrossRef]
- Cao, X.L.; Perez-Locas, C.; Dufresne, G.; Clement, G.; Popovic, S.; Beraldin, F.; Dabeka, R.W.; Feeley, M. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit. Contam. 2011, 28, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Lorber, M.; Schecter, A.; Paepke, O.; Shropshire, W.; Christensen, K.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 2015, 77, 55–62. [Google Scholar] [CrossRef] [Green Version]
- LaKind, J.S.; Naiman, D.Q. Bisphenol A (BPA) daily intakes in the United States: Estimates from the 2003–2004 NHANES urinary BPA data. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 608–615. [Google Scholar] [CrossRef] [Green Version]
- LaKind, J.S.; Naiman, D.Q. Temporal trends in bisphenol A exposure in the United States from 2003–2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation. Environ. Res. 2015, 142, 84–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, J.W.; Twaddle, N.C.; Vanlandingham, M.; Doerge, D.R. Pharmacokinetic modeling: Prediction and evaluation of route dependent dosimetry of bisphenol A in monkeys with extrapolation to humans. Toxicol. Appl. Pharmacol. 2011, 257, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, N.; Oka, H.; Nagaoka, K.; Ikushiro, S.; Narimatsu, S. Effect of UDP-glucuronosyltransferase 2B15 polymorphism on bisphenol A glucuronidation. Arch. Toxicol. 2011, 85, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Partosch, F.; Mielke, H.; Gundert-Remy, U. Functional UDP-glucuronyltransferase 2B15 polymorphism and bisphenol A concentrations in blood: Results from physiologically based kinetic modelling. Arch. Toxicol. 2013, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.; Hanson-Drury, S.; Fisher, J.W.; Doerge, D.R. Are typical human serum BPA concentrations measurable and sufficient to be estrogenic in the general population? Food Chem. Toxicol. 2013, 62, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Calafat, A.M.; Ye, X.; Doerge, D.R.; Churchwell, M.I.; Gunawan, R.; Graham, M.K. Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure. Toxicol. Sci. 2011, 123, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Twaddle, N.C.; Churchwell, M.I.; Doerge, D.R. Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women. Food Chem. Toxicol. 2016, 92, 129–142. [Google Scholar] [CrossRef]
- Manrai, A.K.; Cui, Y.; Bushel, P.R.; Hall, M.; Karakitsios, S.; Mattingly, C.; Ritchie, M.; Schmitt, C.; Sarigiannis, D.A.; Thomas, D.C.; et al. Informatics and Data Analytics to Support Exposome-Based Discovery for Public Health. Ann. Rev. Public Health 2016. [Google Scholar] [CrossRef]
| Country—Study Name | Population Group | Mean | Median | Reference |
|---|---|---|---|---|
| Belgium—Democophes | Mothers (≤45 years) | 2.6 | [41] | |
| Denmark—Democophes | Mothers (≤45 years) | 2.2 | ||
| Denmark—Copenhagen Puberty Study | Children and adolescents (5–9 years) | 2.3 | [42] | |
| Children and adolescents (10–13 years) | 1.5 | |||
| Children and adolescents (14–20 years) | 0.7 | |||
| Denmark—Copenhagen Study on Male Reproductive Health | Young men | 3.2 | ||
| Denmark—Odense Child Cohort | Pregnant women | 1.5 | ||
| France—ELFE | Pregnant women | 2.5 | 2 | [38] |
| Germany—ESB | Students (<2000)—Münster | 2.0 | [39] | |
| Students (≥2000)—Münster | 1.4 | |||
| Germany—GerES | 3–14 years | 2.7 | 2.7 | [43] |
| 3–5 years | 3.5 | 3.6 | ||
| 6–8 years | 2.8 | 2.7 | ||
| 9–11 years | 2.1 | 2.2 | ||
| 12–14 years | 2.6 | 2.4 | ||
| Italy—InCHIANTI | 20–40 years | 4.4 | 4.3 | [44] |
| 41–65 years | 3.9 | 3.7 | ||
| 66–74 years | 3.3 | 3.2 | ||
| Luxembourg—Democophes | Mothers (≤45 years) | 1.9 | [41] | |
| Netherlands—Generation R | Pregnant women (18–41 years) | 1.2 | 1.1 | [45] |
| Slovenia—Democophes | Mothers (≤45 years) | 1.2 | [41] | |
| Spain—INMA | Pregnant women | 2.2 | [46] | |
| Spain—INMA | Children (4 years) | 4.2 | ||
| Spain—Democophes | Mothers (≤45 years) | 2.1 | [41] | |
| Sweden—Democophes | Mothers (≤45 years) | 1.4 | ||
| France—ELFE | Pregnant women (18–40 years) | 0.7 | [47] | |
| Greece—Rhea | Pregnant women | 1.2 | 1.2 | [48] |
| Children (2 years) | 2.0 | 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarigiannis, D.; Karakitsios, S. Advancing Chemical Risk Assessment through Human Physiology-Based Biochemical Process Modeling. Fluids 2019, 4, 4. https://doi.org/10.3390/fluids4010004
Sarigiannis D, Karakitsios S. Advancing Chemical Risk Assessment through Human Physiology-Based Biochemical Process Modeling. Fluids. 2019; 4(1):4. https://doi.org/10.3390/fluids4010004
Chicago/Turabian StyleSarigiannis, Dimosthenis, and Spyros Karakitsios. 2019. "Advancing Chemical Risk Assessment through Human Physiology-Based Biochemical Process Modeling" Fluids 4, no. 1: 4. https://doi.org/10.3390/fluids4010004
APA StyleSarigiannis, D., & Karakitsios, S. (2019). Advancing Chemical Risk Assessment through Human Physiology-Based Biochemical Process Modeling. Fluids, 4(1), 4. https://doi.org/10.3390/fluids4010004





