1. Introduction
The rheological properties of macromolecular solutions are important in many industrial fields, such as the food industry, pharmaceutical and cosmetics, in the paper industry, in enhanced oil recovery (EOR), in water treatment, etc. Water-soluble polymers are used in these fields as thickeners, gelling agents, texture modifiers, stabilizers, etc. [
1,
2]. In the particular case of EOR, they are added to control mobility and reduce the permeability of the tank by increasing the viscosity of the injected water.
Hence, an important requirement for a potential polymer to be used in EOR is that the polymer-water solution has favorable rheological behavior. Gum diutan and rhamsan gum belong to that group of water-soluble polymers of high molecular weight. They are exopolysaccharides (EPS) belonging to the sphingans, as well as gellan gum or wellan gum [
1,
3,
4]. The basic structure of all these polysaccharides is constituted by a tetrasaccharide repeat unit based on β-
d-glucose, β-
d-glucuronic acid, and α-
l-rhamnose. Gum diutan, obtained by aerobic fermentation of Sphingomonas S.PATCC53159, has this basic structure, and each glucosidic residue that is next to a rhamnosil residue is substituted by two units of
l-rhamnose. Rhamsan gum differs from gum diutan in the side chain substituents, which in this case is a disaccharide chain of β-
d-glucopyranosyl or α-
d-glucopyranosyl [
5,
6]. Both gums are non-gelling polysaccharides, unlike gellan gum, which belongs to the same family. It is precisely the difference in the side-chains responsible for it, namely the occurrence of different side-chains’ influences on the behavior in aqueous media. Several studies reveal that native diutan and rhamsan gum exhibit a double helical conformation, which is maintained with the temperature as a consequence of their chains’ strength [
6,
7].
Regarding applications, rhamsan gum has wider applications in the food industry compared to other sphingans, since it can tolerate high concentrations of sodium chloride and phosphates [
8]. In general, they are used not only in the food industry but also in personal hygiene products, in construction materials, in oil operations, etc.
Nowadays, it is not only important to know the rheological properties of these gums in solution but also the modifications that these properties undergo when the temperature varies. This information is of great interest in numerous industrial processes and in the conditions of use and application of them. For example, in the food industry, sterilization, drying, extrusion, etc., are processes in which temperature is of vital importance. In this work, the influence of temperature on the viscoelastic properties and flow properties of aqueous solutions containing 1 wt % diutan gum or rhamsan gum were studied in detail, establishing a comparison between both gums. In order to reach this objective, stress and frequency sweep measurements and steady state tests were performed.
2. Materials and Methods
2.1. Materials
Diutan gum (CP Kelco 1A9722A) and rhamsan gum (OL9478A) used were cordially provided by CP-Kelco (San Diego, CA, USA). Both gums exhibit high molecular weight. Their values and the intrinsic viscosity values can be obtained from works published [
6,
7]. Sodium azide supplied by Panreac was utilized as preservative. Distilled water was used as solvent.
The sample composition is shown in
Table 1.
2.2. Gum Aqueous Solutions’ Preparation
Batches of 250 g were prepared. In order to obtain the sample, the gum amount necessary was added on the water containing sodium azide. The samples were homogenized by means of Ika-Visc MR-D1 (Ika, Staufen, Germany), at 1000 rpm for 3 h. The samples were kept refrigerated at 5 °C for some hours, and then they were stored at 25 °C.
2.3. Rheological Characterization.
The rheological tests were carried out by means of a controlled stress rheometer, Haake RS100 (Haake Thermo Scientific, Karlsruhe, Germany), using a serrated parallel plate geometry of 60 mm of diameter to avoid wall slip effects and using a measuring gap of 1 mm. To control the temperature (10 °C, 25 °C, 40 °C, and 60 °C), a Julabo circulator thermostat was utilized.
In order to prevent the mechanical history effects due to the sample placing in the sensor system, the samples were maintained at rest for 10 min before starting the measurements. To determine this equilibration time, time sweeps were performed.
All tests were carried out at least three times. Fresh sample were used in each measurement.
2.3.1. Stress Sweep Tests
To determine the linear viscoelastic region (LVR), stress sweep tests were carried out ranging from 1.0 Pa to 50 Pa, at 1 Hz.
2.3.2. Mechanical Spectra
The frequency sweep tests were performed from 0.01 to 10 Hz (0.0628 to 62.83 rad/s) at fixed shear stress within the linear viscoelastic region.
2.3.3. Steady State Tests
The flow curves were carried out in the 10–40 Pa shear stress range using a step-wise method to obtain the steady-state regime.
3. Results and Discussion
3.1. Influence of Temperature on the Linear Viscoelastic Region.
In order to study the viscoelastic behavior of gums studied, the linear viscoelastic regions were determined.
Figure 1a,b show the stress sweep results of diutan gum and rhamsan gum, respectively, at the different studied temperatures (10 °C, 25 °C, 40 °C, and 60 °C). By increasing the stress, two regions can be clearly differentiated: (a) the so-called linear viscoelastic region, in which G’ (storage or elastic modulus) and G’’ (loss or viscous modulus) remain practically constant, and (b) the non-linear viscoelastic region in which viscoelastic functions begin to decrease. It must be noted there was an increase of G’’ values before its decrease. This effect was observed in other systems and it was attributed to the reorganization of the gum solution structure before the output of the linear viscoelastic region [
9,
10].
The shear stress from which the modules cease to be constant is called critical stress (τ
c), and it corresponds to stress that leads to the first non-linear changes in the structure. As can be observed in
Figure 1, the G’’ values cease to be steady at smaller shear stress values than G’. For this reason, it has been considered that the onset of the non-linear viscoelastic region begins when G’’ ceases to be constant, particularly when the deviation of loss modulus becomes more than 5%. From
Figure 1, it can be stated that there is no influence of the temperature on critical stress values of diutan and rhamsan gum aqueous solutions, being that these values are similar for both gums at 11.83 Pa and 11.68 Pa, respectively. Furthermore, it should be noted the broad extension of the linear viscoelastic zone, which indicated a wide zone where the sample is not damaged, namely, high stability to shear.
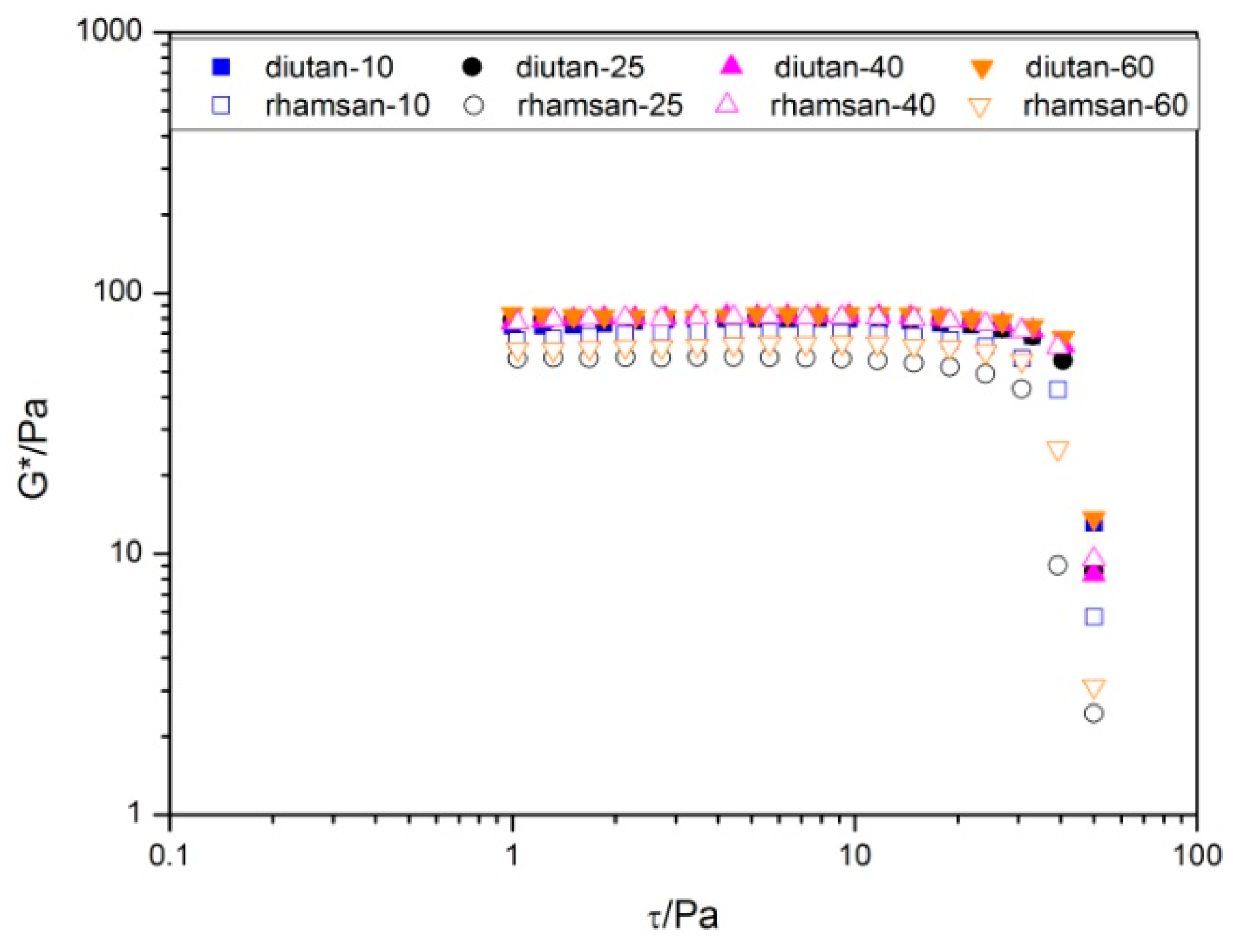
Regardless of temperature studied, G’ is greater than G’’ within LVR, which indicates that diutan and rhamsan gum dispersions are more elastic than viscous. The complex module (G*) as a function of the shear stress is shown in
Figure 2 in order to better compare the level of viscoelasticity of both polysaccharides. As can be observed, the values of G* were similar for both gums, however, they were slightly greater for the diutan gum solutions.
3.2. Influence of Temperature on the Mechanical Spectra
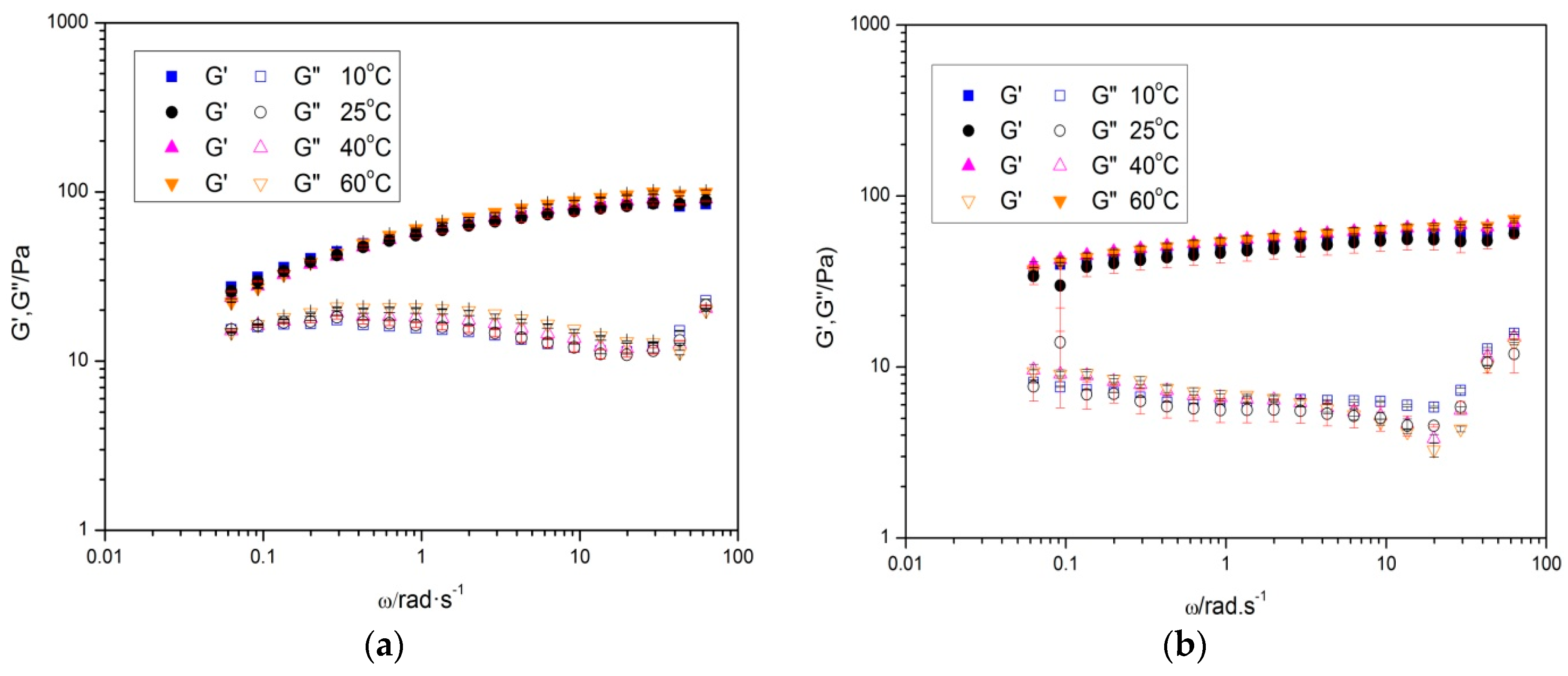
Frequency sweep tests have been carried out at a fixed shear stress within the linear viscoelastic region, so that the sample structure is not damaged by the stress imposed during test.
Figure 3 shows the influence of the temperature on the mechanical spectra of diutan and rhamsan gums, respectively. Both gums (
Figure 3) exhibited storage module values (G’) higher than those of loss module (G’’) in the whole studied frequency range, with a small frequency dependence. According to bibliography [
11,
12], the shape of these mechanical spectra makes clarification of the diutan and rhamsan gum aqueous solutions as weak gels possible. In addition, no crossover point occurred. For this reason, it can be affirmed the solid character is higher than liquid character in aqueous solutions of these gums. This rheological behavior is characteristic of systems with a high degree of internal structuring, and coincides with that presented by other hydrocolloids, such as Sterculia apetala or striata [
12,
13]. Regarding temperature,
Figure 3 illustrates the high thermal stability exhibited for both diutan and rhamsan gum.
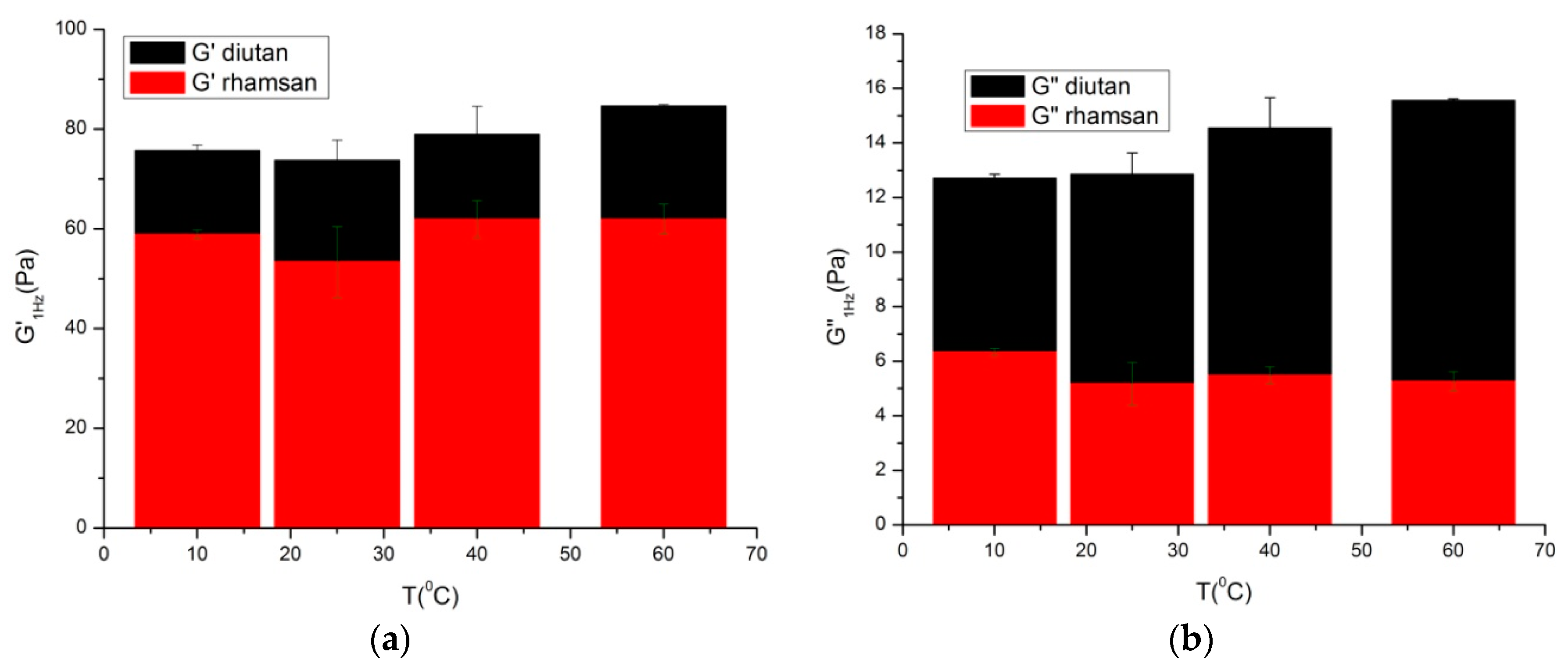
In order to compare the results obtained for both gums, by way of example in
Figure 4, the G’ and G’’ values at 1 Hz against temperature for diutan and rhamsan gums are shown. As can be observed, the elastic module of diutan gum solutions was slightly higher than that of rhamsan gum solutions, while the viscous module was clearly greater. Additionally, it is important to note that there was no significant dependence on temperature in these results.
3.3. Steady State Measurements
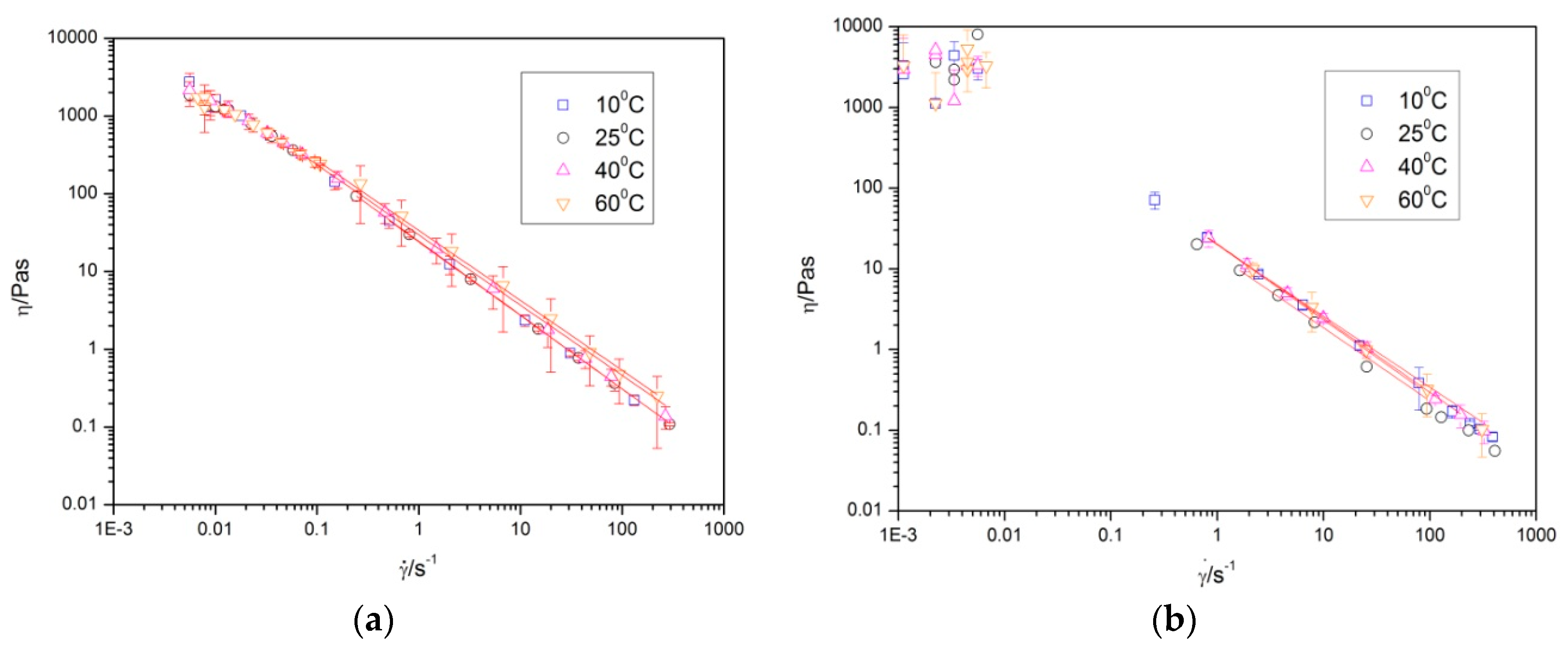
The flow behavior of both gums is illustrated in
Figure 5. These results pointed out the aqueous solution containing 1 wt % of gum exhibited a shear thinning behavior, which was distinguished by a fast decrease of the viscosity as shear rate increased. In addition, these samples exhibited a Newtonian region at low shear rate, so-called zero shear viscosity, η
0. This result was due to the fact that at low shear rate, polymeric molecules are disordered and partially aligned in the direction of the flow, resulting in a greater interaction between them, and therefore, a higher viscosity. By increasing the shear rate, the molecules were easily aligned, decreasing the physical interactions that occur between adjacent polymer chains. A marked shear thinning behavior is very interesting, since it facilitates pumping and imparts a finer consistency during swallowing if it is a food product [
14]. The flow curves were fitted to the Ostwald-de-Waele Equation with a high degree of satisfaction.
where η is the viscosity, η
1 is viscosity value at 1 s
−1,
γ is the shear rate, and n is the index flow.
The fitting parameters revealed a tendency to increase the viscosity at 1 s
−1 for diutan gum solutions, while this parameter remained unaltered for rhamsan gum solutions. Additionally, this viscosity illustrated that aqueous solutions of diutan gum were more viscous than rhamsan gum solutions. In both gums, the flow index was independent of the temperature in the range of 10 °C to 60 °C, and its value revealed the occurrence of a marked non-Newtonian character, which was maintained in both cases when the temperature increased (
Table 2).
3.4. Cox-Merz Rule
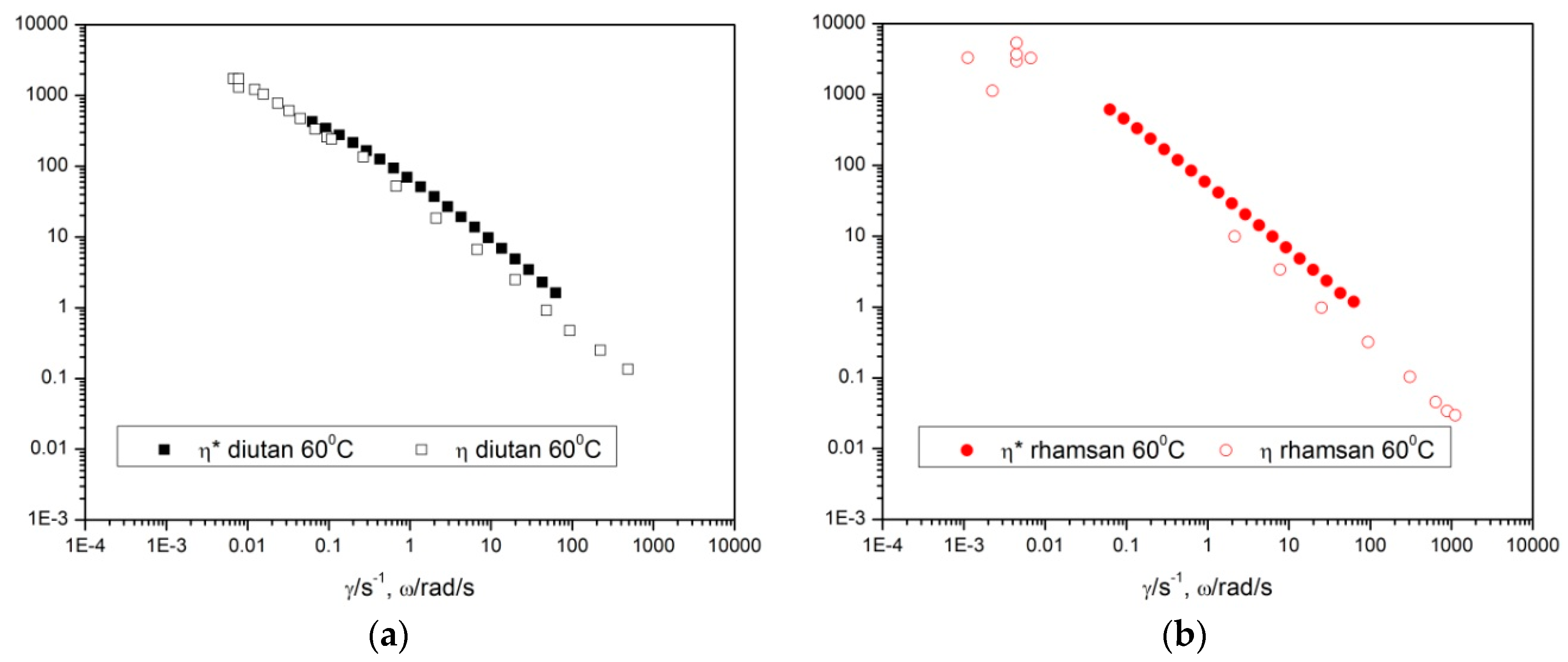
The steady state properties have been compared with dynamic properties using the Cox-Merz rule. The apparent viscosity obtained from the flow curve, η, and the complex viscosity obtained from low amplitude oscillatory tests, |η*|, have been plotted against the frequency and the shear rate. By way of example, the results are shown at a temperature of 60 °C, but similar results are obtained at all the temperatures studied.
For both diutan gum and rhamsan gum (
Figure 6a,b), |η*| was slightly greater than η. Therefore, it can be deduced that the Cox-Merz rule is not met, even though the differences found between both viscosities are very small. This means that the application of shear did not produce a significant destruction of the structure.
It is important to mention that these relationships are valid only for some types of materials or some ranges of conditions. It has been verified that it is satisfactorily fulfilled for polymers of homogeneous solutions and molten polymers, and that it failed in some other cases, such as linear and branched polyethylenes, block copolymers, and rigid molecules [
15].
Other microbial polysaccharides, such as Aeromonas gum [
16] and xanthan gum [
17] and aqueous dispersions of exudates of gums [
18], also demonstrated deviations from this rule. Clasen and Kulicke [
19] proposed that deviations from this rule arise from the formation of associations in the relaxed state. In a shear test, the imposed shear stress destroys the intermolecular associations formed when the sample is relaxed. In contrast, in an oscillatory test, the gum solution is subjected to a sinusoidal shear stress within the linear viscoelastic range small enough so that these molecular associations are not destroyed. For this reason, the apparent viscosity calculated from a destructive test showed a lower value than the complex viscosity obtained from a non-destructive oscillatory test.
A comparison of results obtained for both gums does not provide additional information to that obtained in previous sections, namely the aqueous solutions of diutan gum are slightly more viscous and viscoelastic than those of rhamsan gum, and both solutions exhibit high thermal stability within the temperature range studied (10–60 °C).
4. Conclusions
The stress sweep measurements carried out to determine the linear viscoelastic region for both gums demonstrated the diutan and rhamsan gum aqueous solutions presented a high resistance to the shear, since its linear viscoelastic region was wide. In addition, this region remained practically unaltered, with temperature increase in the range of temperature studied (10–60 °C) being the critical stress similar for both gum solutions.
In both cases, the mechanical spectra showed a typical behavior of weak gel, whose structure remained thermally stable, although this stability was greater for the diutan gum than for the rhamsan gum. The flow curves exhibited a strongly non-Newtonian behavior, whose pseudoplasticity was maintained even at high temperatures. The viscosities at 1s−1 of the diutan gum solutions showed a slight tendency to grow with the temperature, while rhamsan gum solutions showed values of η1 practically independent of temperature. On the other hand, diutan gum solutions presented higher values of viscosity.
Cox-Merz rule was not met. The viscous and elastic properties of diutan gum were higher than those of rhamsan gum, although both properties were very stable against temperature.
The rheological properties showed by both biopolymers and their high stability with temperature mean these gums have a great practical interest at the industrial level.
Author Contributions
Experimental data acquisition and calculations were obtained by M.d.S.C.G. Methodology was selected by J.M.G. Experimental design was carried out by M.-C.A.-R. Data analyses were performed by M.-C.A.-R. and M.C.G.G. The manuscript was written by M.-C.A.-R. and M.C.G.G. with comments from J.M.G.
Funding
This research received no external funding.
Acknowledgments
The financial support received from the Spanish Ministerio de Economía y Competitividad (MINECO) and FEDER, UE is kindly acknowledged (project CTQ2015-70700-P).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fialho, A.M.; Moreira, L.M.; Granja, A.T.; Popescu, A.O.; Hoffmann, K.; Sá-Correia, I. Occurrence, production, and applications of gellan: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 79, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, I.B.; Survase, S.A.; Saudagar, P.S.; Singhal, R.S. Gellan Gum: Fermentative Production, Downstream Processing and Applications. Food Technol. Biotechnol. 2007, 45, 341–354. [Google Scholar]
- Schmid, J.; Sperl, N.; Sieber, V. A comparison of genes involved in sphingan biosynthesis brought up to date. Appl. Microbiol. Biotechnol. 2014, 98, 7719–7733. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Bera, M.B.; Panesar, P.S.; Kumar, H.; Kennedy, J.F. Welan gum: Microbial production, characterization, and applications. Int. J. Biol. Macromol. 2014, 65, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.A.; Lindberg, B.; Lindquist, U.; Baird, J. Structural studies of an extracellular polysaccharide, S-657, elaborated by Xanthomonas ATCC 53159. Carbohydr. Res. 1987, 164, 117–122. [Google Scholar] [CrossRef]
- Campana, S.; Ganter, J.; Milas, M.; Rinaudo, M. On the solution properties of bacterial polysaccharides of the gellan family. Carbohydr. Res. 1992, 231, 31–38. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Zhang, L. Rheological behaviour of Aeromonas gum in aqueous solutions. Food Hydrocolloids 2006, 20, 723–729. [Google Scholar] [CrossRef]
- Kang, K.S.; Pettitt, D.J. In Industrial Gums: Polysaccharides and Their Derivatives; Academic Press: San Diego, CA, USA, 1993; pp. 341–397. [Google Scholar]
- Alemzadeh, T.; Mohammadifar, M.A.; Azizi, M.H.; Ghanati, K. Effect of two different species of Iranian gum tragacanth on the rheoligical properties of mayonnaise sauce. J. Food Sci. Technol. 2010, 7, 127–141. [Google Scholar]
- Rincón, F.; Muñoz, J.; Ramírez, P.; Galán, H.; Alfaro, M.C. Physicochemical and rheological characterization of Prosopis juliflora seed gum aqueous dispersions. Food Hydrocolloids 2014, 35, 348–357. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Ali, M.; Koocheky, A.; Razavi, S.M. Dynamic rheological properties of Lepidium perfoliatum seed gum: Effect of concentration, temperature and heating/cooling rate. Food Hydrocolloids 2014, 35, 583–589. [Google Scholar]
- Pérez, L.M.; Ramírez, P.; Alfaro, M.C.; Rincón, F.; Muñoz, J. Surface properties and bulk rheology of Sterculia apetala gum exudate dispersions. Food Hydrocolloids 2013, 32, 440–446. [Google Scholar] [CrossRef]
- Brito, A.C.F.; Sierakowski, M.R.; Reicher, F.; Feitosa, J.P.A.; de Paula, R.C.M. Dynamic rheological study of Sterculia striata and karaya poly-saccharides in aqueous solution. Food Hydrocolloids 2005, 19, 861–867. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Ikeda, S. Isolation and Characterization of Hydrocolloids from Monoi (Cissampelos pareira) Leaves. Food Hydrocolloids 2006, 20, 885–891. [Google Scholar] [CrossRef]
- Morrison, F.A. Understanding Rheology; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Rochefort, W.E.; Middleman, S. Rheology of xanthan gum: Salt, temperature, and strain effects in oscillatory and steady shear experiments. J. Rheol. 1987, 31, 337–369. [Google Scholar] [CrossRef]
- Rincón, F.; Muñoz, J.; De Pinto, G.L.; Alfaro, M.C.; Calero, N. Rheological properties of Cedrela odorata gum exudate aqueous dispersions. Food Hydrocolloids 2009, 23, 1031–1037. [Google Scholar] [CrossRef]
- Clasen, C.; Kulicke, W.M. A convenient way of interpreting steady shear rheo-optical data of semi-dilute polymer solution. Rheol. Acta 2001, 40, 74–85. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).