Cardiac Triangle Mapping: A New Systems Approach for Noninvasive Evaluation of Left Ventricular End Diastolic Pressure
Abstract
1. Introduction
2. Theory and Methods
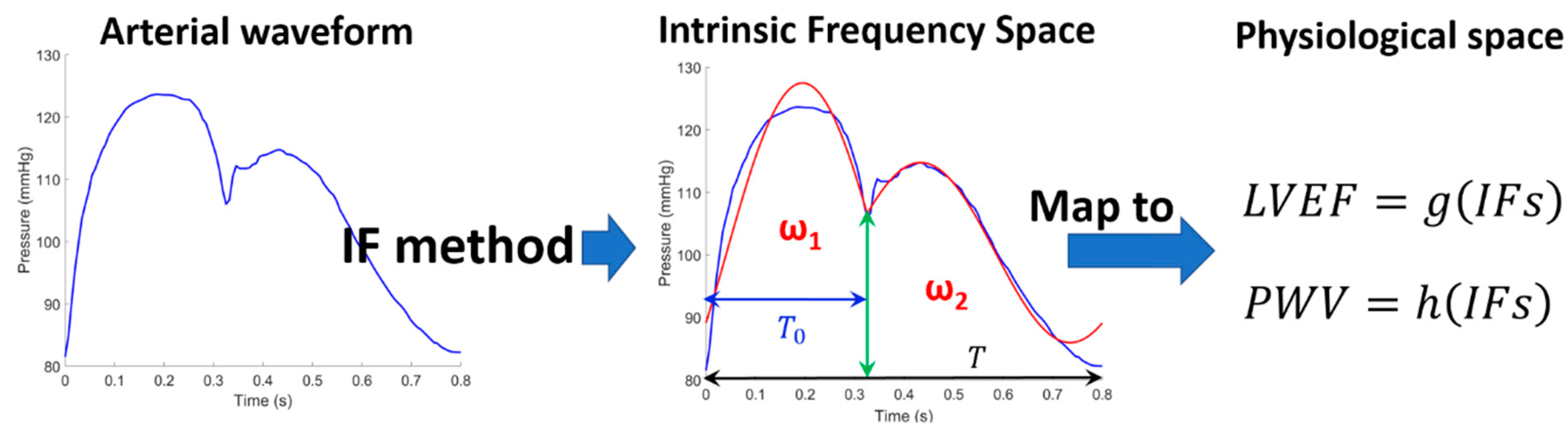
2.1. Intrinsic Frequency Method
2.2. Pre-Ejection Period
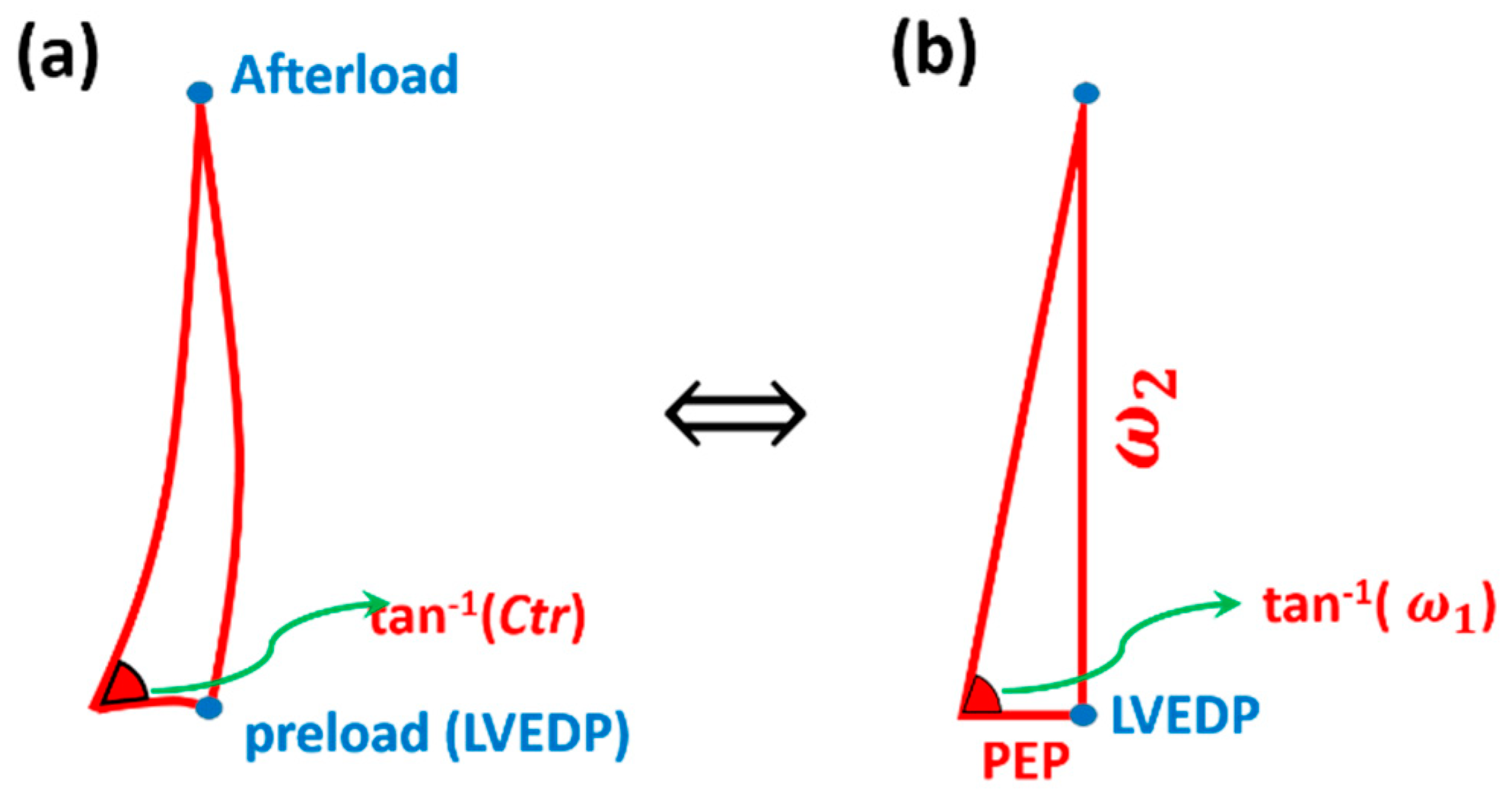
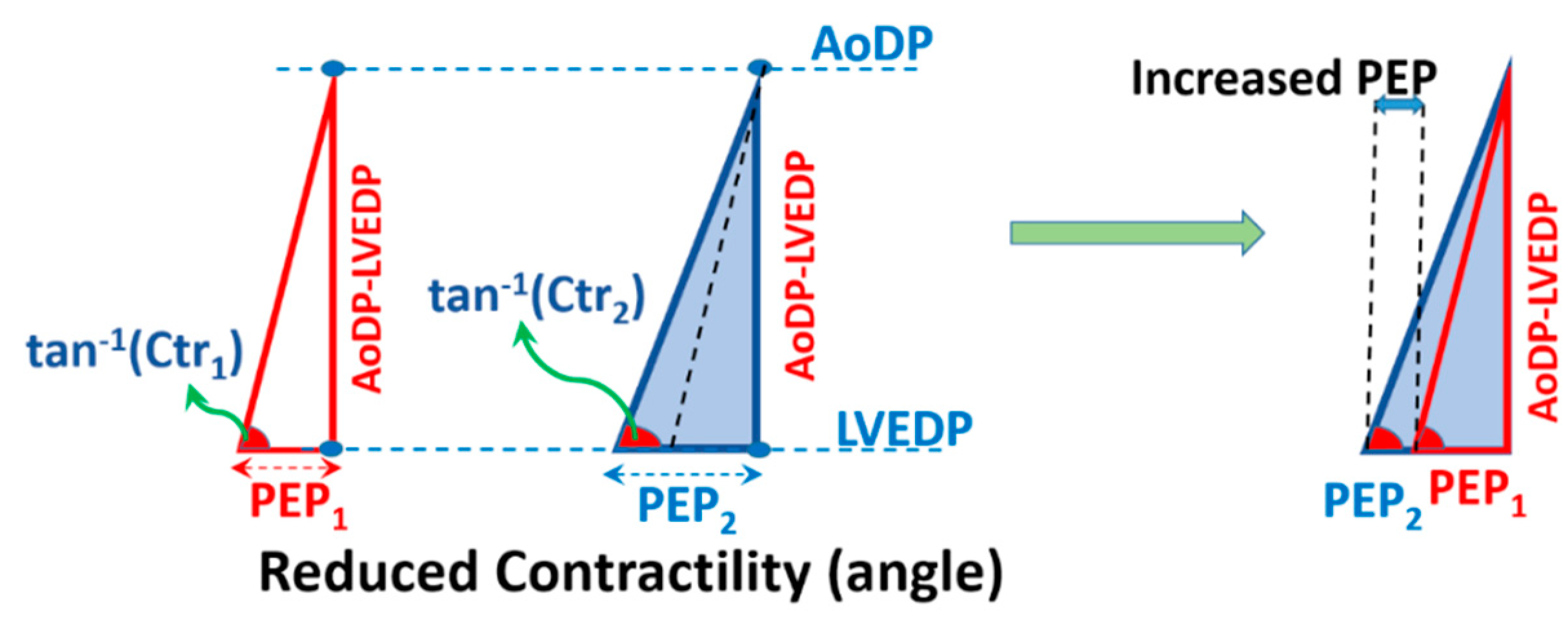
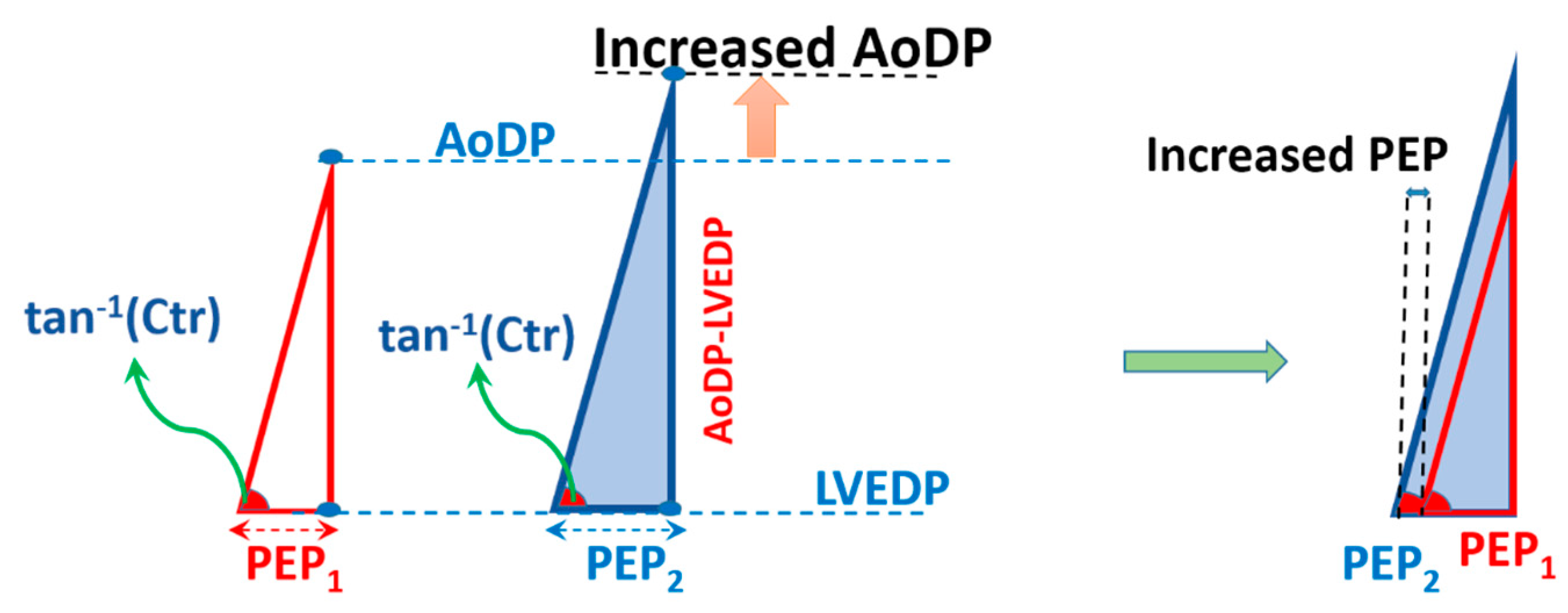
2.3. Cardiac Triangle Mapping Hypothesis
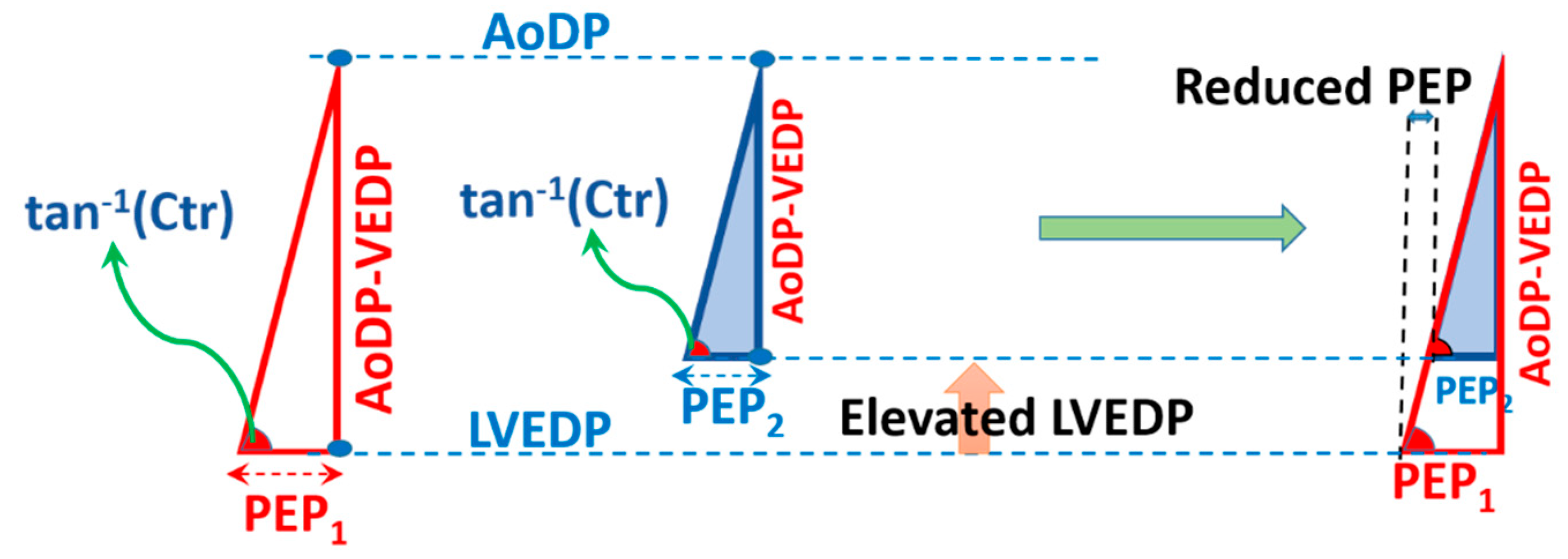
2.4. Compatibility of CTM with Hemodynamical Changes
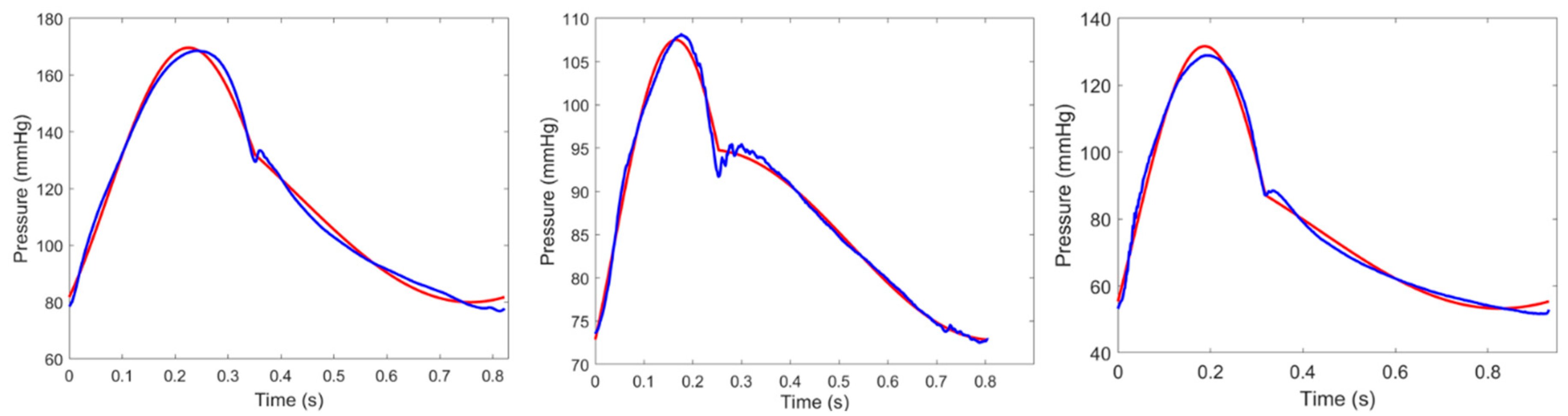
2.5. Testing CTM Using Clinical Retrospective Data
3. Results
3.1. Compatibility of CTM with Hemodynamical Changes
3.1.1. Elevation of LVEDP at Constant Contractility and AoDP
3.1.2. Changing Contractility at Constant LVEDP and AoDP
3.1.3. Elevation of AoDP at Constant Contractility and LVEDP
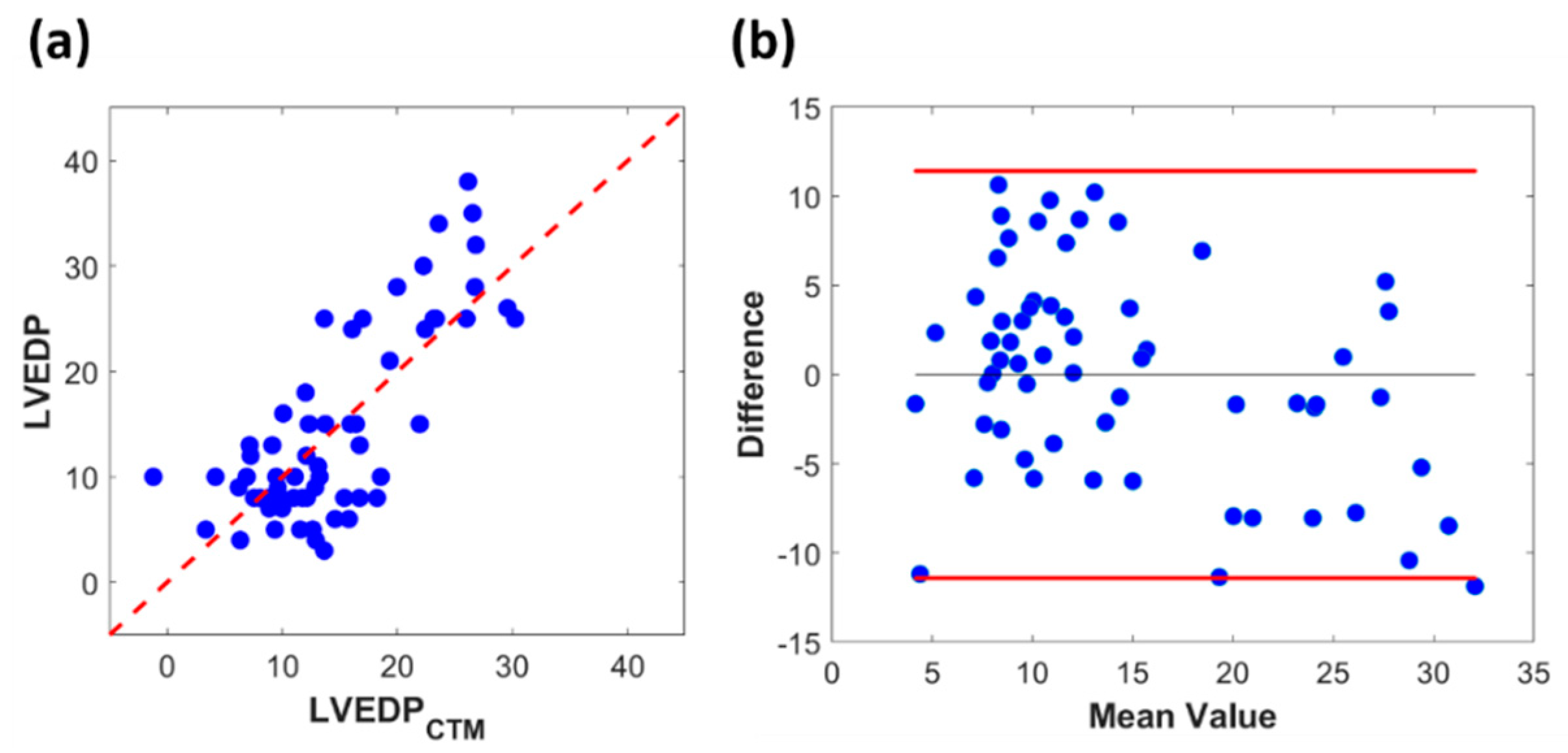
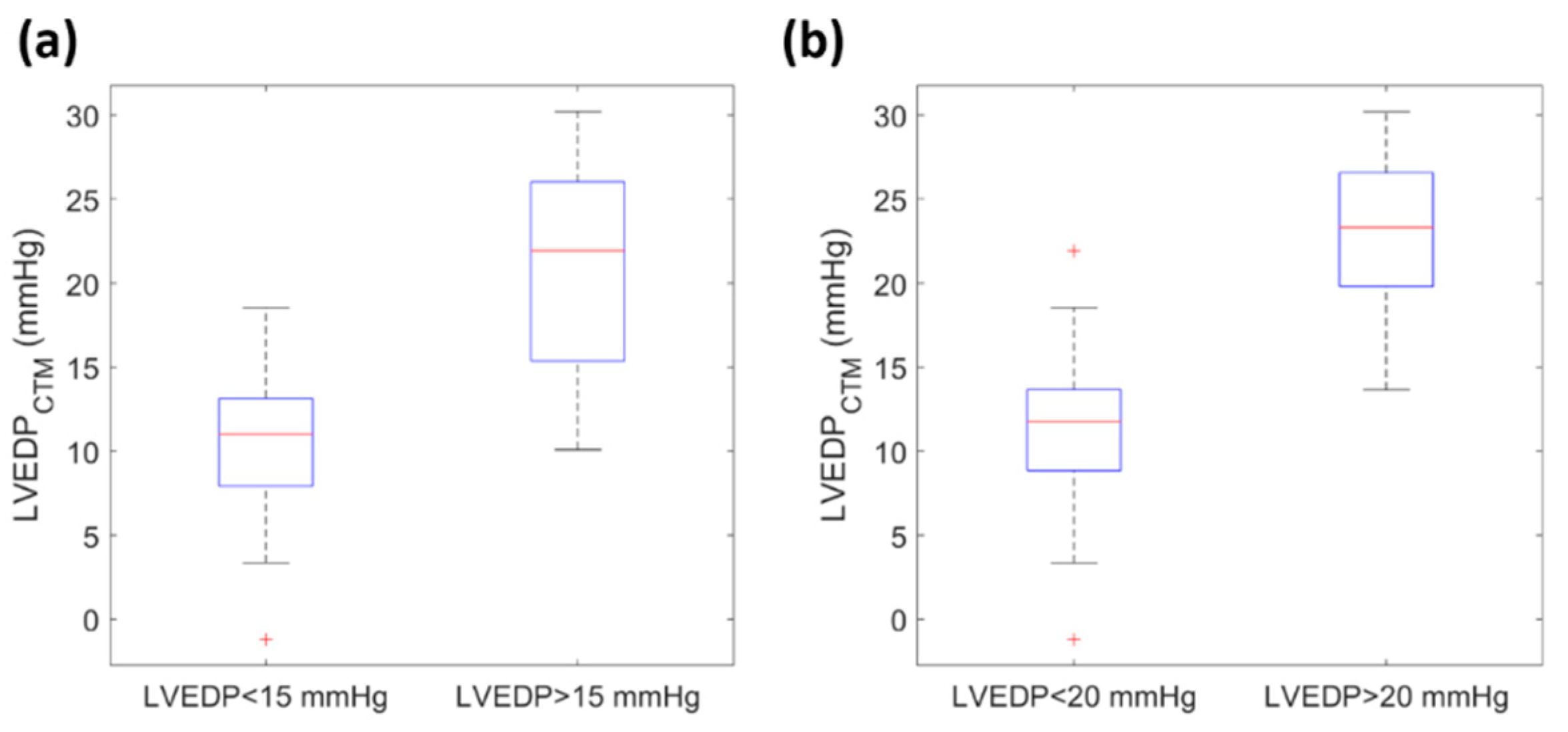
3.2. Clinical Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Larson, M.G.; Leip, E.P.; Beiser, A.; D’Agostino, R.B.; Kannel, W.B.; Murabito, J.M.; Vasan, R.S.; Benjamin, E.J.; Levy, D. Lifetime Risk for Developing Congestive Heart Failure: The Framingham Heart Study. Circulation 2002, 106, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Iskandrian, A.; Segal, B.; Hamid HAKKI, A. Left ventricular end-diastolic pressure in evaluating left ventricular function. Clin. Cardiol. 1981, 4, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Filippatos, G.; De Luca, L.; Burnett, J. Congestion in Acute Heart Failure Syndromes: An Essential Target of Evaluation and Treatment. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Sharma, G.; Woods, P.A.; Lindsey, N.; O’Connell, C.; Connolly, L.; Joseph, J.; McIntyre, K.M. Noninvasive monitoring of left ventricular end-diastolic pressure reduces rehospitalization rates in patients hospitalized for heart failure: A randomized controlled trial. J. Card. Fail. 2011, 17, 718–725. [Google Scholar] [CrossRef]
- Pahlevan, N.M.; Rinderknecht, D.; Tavallali, P.; Razavi, M.; Tran, T.T.; Fong, M.; Kloner, R.A.; Csete, M.; Gharib, M. A New Noninvasive iPhone Application to Monitor Left Ventricle Ejection Fraction in Heart Failure Patients. Circulation 2016, 134, A17227. [Google Scholar]
- Pahlevan, N.M.; Rinderknecht, D.G.; Tavallali, P.; Razavi, M.; Tran, T.T.; Fong, M.W.; Kloner, R.A.; Csete, M.; Gharib, M. Noninvasive iPhone Measurement of Left Ventricular Ejection Fraction Using Intrinsic Frequency Methodology. Crit. Care Med. 2017, 45, 1115–1120. [Google Scholar]
- Pahlevan, N.M.; Tavallali, P.; Rinderknecht, D.G.; Petrasek, D.; Matthews, R.V.; Hou, T.Y.; Gharib, M. Intrinsic frequency for a systems approach to haemodynamic waveform analysis with clinical applications. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef]
- Petrasek, D.; Pahlevan, N.M.; Tavallali, P.; Rinderknecht, D.G.; Gharib, M. Intrinsic Frequency and the Single Wave Biopsy: Implications for Insulin Resistance. J. Diabetes Sci. Technol. 2015, 9, 1246–1252. [Google Scholar] [CrossRef]
- Hou, T.Y.; Shi, Z. Adaptive data analysis via sparse time-frequency representation. Adv. Adapt. Data Anal. 2011, 3, 1–28. [Google Scholar] [CrossRef]
- Tavallali, P.; Razavi, M.; Pahlevan, N.M. Artificial Intelligence Estimation of Carotid-Femoral Pulse Wave Velocity using Carotid Waveform. Sci. Rep. 2018, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Rinderknecht, D.; Au, K.; Lindenfeld, L.; Mills, G.; Siyahian, A.; Herrera, C.; Wilson, K.; Venkataraman, K.; Mascarenhas, K. Accuracy of a novel handheld wireless platform for detection of cardiac dysfunction in anthracycline-exposed survivors of childhood cancer. Clin. Cancer Res. 2018, 24, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Pahlevan, N.M.; Petrasek, D.; Rinderknecht, D.G.; Tavallali, P.; Gharib, M. Calculating Pulse Wave Velocity from a Single Pressure Waveform Using the Intrinsic Frequency Method. Hypertension 2014, 64, A355. [Google Scholar]
- Tavallali, P.; Hou, T.Y.; Rinderknecht, D.G.; Pahlevan, N.M. On the convergence and accuracy of the cardiovascular intrinsic frequency method. R. Soc. Open Sci. 2015, 2. [Google Scholar] [CrossRef]
- Pahlevan, N.M. A Systems Approach to Cardiovascular Health and Disease with a Focus on Aortic Wave Dynamics. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 2013. [Google Scholar]
- Pahlevan, N. MRI-based Measures of Left Ventricle Contractility and Intrinsic Frequency. In Proceedings of the IEEE-Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018. [Google Scholar]
- Weissler, A.M.; Harris, W.S.; Schoenfeld, C.D. Systolic time intervals in heart failure in man. Circulation 1968, 37, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Barden, J.; List, W.F.; Gravenstein, J.S.; Spodick, D.H. Systolic Time Intervals: International Symposium, Graz, Austria September 1–2, 1978; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar]
- Talley, R.C.; Meyer, J.F.; McNay, J.L. Evaluation of the pre-ejection period as an estimate of myocardial contractility in dogs. Am. J. Cardiol. 1971, 27, 384–391. [Google Scholar] [CrossRef]
- Hosenpud, J.D.; Greenberg, B.H. Congestive Heart Failure; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- York, D. Least-squares fitting of a straight line. Can. J. Phys. 1966, 44, 1079–1086. [Google Scholar] [CrossRef]
- Garrard, C.L.; Weissler, A.M.; Dodge, H.T. The Relationship of Alterations in Systolic Time Intervals to Ejection Fraction in Patients with Cardiac Disease. Circulation 1970, 42, 455–462. [Google Scholar] [CrossRef]
- Halpern, S.D.; Taichman, D.B. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest 2009, 136, 37–43. [Google Scholar] [CrossRef]
- Bitar, A.; Selej, M.; Bolad, I.; Lahm, T. Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. PLoS ONE 2014, 9, e87304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Woods, P.A.; Lambrew, C.T.; Berg, C.M.; Pietro, D.A.; Rocco, T.P.; Welt, F.W.; Sacchetti, P.; McIntyre, K.M. Evaluation of a noninvasive system for determining left ventricular filling pressure. Arch. Intern. Med. 2002, 162, 2084–2088. [Google Scholar] [CrossRef]
- Silber, H.A.; Trost, J.C.; Johnston, P.V.; Maughan, W.L.; Wang, N.-Y.; Kasper, E.K.; Aversano, T.R.; Bush, D.E. Finger photoplethysmography during the Valsalva maneuver reflects left ventricular filling pressure. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2043–H2047. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.H.; Gorlin, R.; Storey, C.F. Clinical test for pulmonary congestion with use of the Valsalva maneuver. J. Am. Med. Assoc. 1956, 160, 44–48. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, K.M.; Vita, J.A.; Lambrew, C.T.; Freeman, J.; Loscalzo, J. A Noninvasive Method of Predicting Pulmonary-Capillary Wedge Pressure. N. Engl. J. Med. 1992, 327, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Sharpey-Schafer, E. Effects of Valsalva’s manoeuvre on the normal and failing circulation. Br. Med. J. 1955, 1, 693. [Google Scholar] [CrossRef] [PubMed]
- Galiatsatos, P.; Win, T.T.; Monti, J.; Johnston, P.V.; Herzog, W.; Trost, J.C.; Hwang, C.-W.; Fridman, G.Y.; Wang, N.-Y.; Silber, H.A. Usefulness of a Noninvasive Device to Identify Elevated Left Ventricular Filling Pressure Using Finger Photoplethysmography During a Valsalva Maneuver. Am. J. Cardiol. 2017, 119, 1053–1060. [Google Scholar] [CrossRef]
- Gilotra, N.A.; Tedford, R.J.; Wittstein, I.S.; Yenokyan, G.; Sharma, K.; Russell, S.D.; Silber, H.A. Usefulness of Pulse Amplitude Changes During the Valsalva Maneuver Measured Using Finger Photoplethysmography to Identify Elevated Pulmonary Capillary Wedge Pressure in Patients with Heart Failure. Am. J. Cardiol. 2017, 120, 966–972. [Google Scholar] [CrossRef]
- Abudiab, M.M.; Chebrolu, L.H.; Schutt, R.C.; Nagueh, S.F.; Zoghbi, W.A. Doppler Echocardiography for the Estimation of LV Filling Pressure in Patients with Mitral Annular Calcification. JACC Cardiovasc. Imaging 2017. [Google Scholar] [CrossRef]
- Dokainish, H.; Zoghbi, W.A.; Lakkis, N.M.; Al-Bakshy, F.; Dhir, M.; Quinones, M.A.; Nagueh, S.F. Optimal noninvasive assessment of left ventricular filling pressures. Circulation 2004, 109, 2432–2439. [Google Scholar] [CrossRef]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W. Estimating left ventricular filling pressure by echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Haberman, Z.C.; Jahn, R.T.; Bose, R.; Tun, H.; Shinbane, J.S.; Doshi, R.N.; Chang, P.M.; Saxon, L.A. Wireless smartphone ECG enables large-scale screening in diverse populations. J. Cardiovasc. Electrophysiol. 2015, 26, 520–526. [Google Scholar] [CrossRef] [PubMed]


| Number or Mean Value (Range) | Standard Deviation | |
|---|---|---|
| Age (years) | 46 (19–65) | 12 |
| HR (bpm) | 80 (55–114) | 15 |
| PEP (ms) | 127 (82–171) | 21.6 |
| LVEF (%) | 44 (8–84) | 18 |
| Gender (M/F) | 36/26 | NA |
| AoDP (mmHg) | 72 (50–100) | 10.6 |
| T0 (ms) | 241 (172–340) | 38 |
| LVEDP (mmHg) | 15 (3–38) | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pahlevan, N.M.; Matthews, R.V. Cardiac Triangle Mapping: A New Systems Approach for Noninvasive Evaluation of Left Ventricular End Diastolic Pressure. Fluids 2019, 4, 16. https://doi.org/10.3390/fluids4010016
Pahlevan NM, Matthews RV. Cardiac Triangle Mapping: A New Systems Approach for Noninvasive Evaluation of Left Ventricular End Diastolic Pressure. Fluids. 2019; 4(1):16. https://doi.org/10.3390/fluids4010016
Chicago/Turabian StylePahlevan, Niema M., and Ray V. Matthews. 2019. "Cardiac Triangle Mapping: A New Systems Approach for Noninvasive Evaluation of Left Ventricular End Diastolic Pressure" Fluids 4, no. 1: 16. https://doi.org/10.3390/fluids4010016
APA StylePahlevan, N. M., & Matthews, R. V. (2019). Cardiac Triangle Mapping: A New Systems Approach for Noninvasive Evaluation of Left Ventricular End Diastolic Pressure. Fluids, 4(1), 16. https://doi.org/10.3390/fluids4010016





