An Experimental Study of Pulsatile Flow in a Compliant Aortic Root Model under Varied Cardiac Outputs
Abstract
1. Introduction
2. Materials and Methods
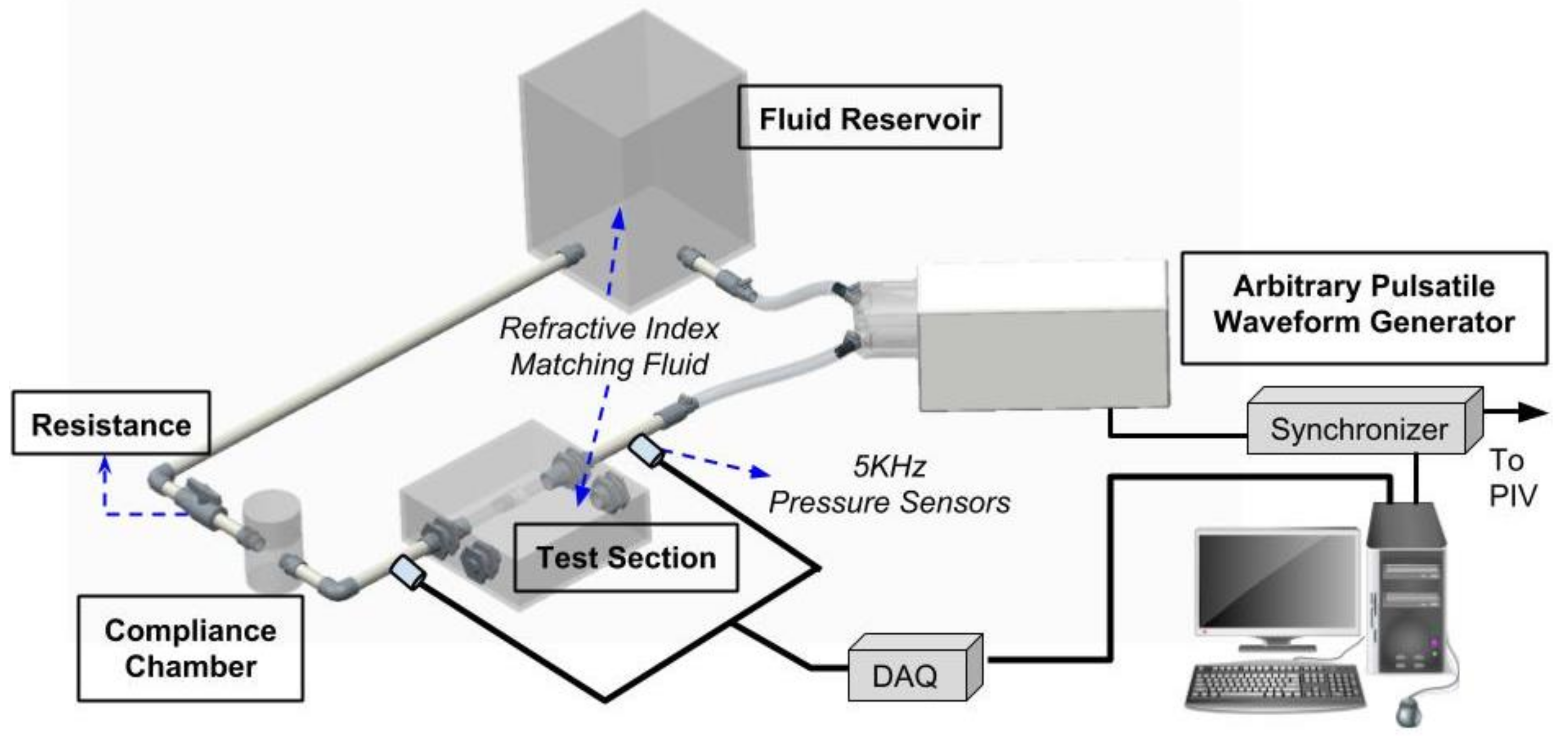
2.1. The Cardiovascular Flow Simulator
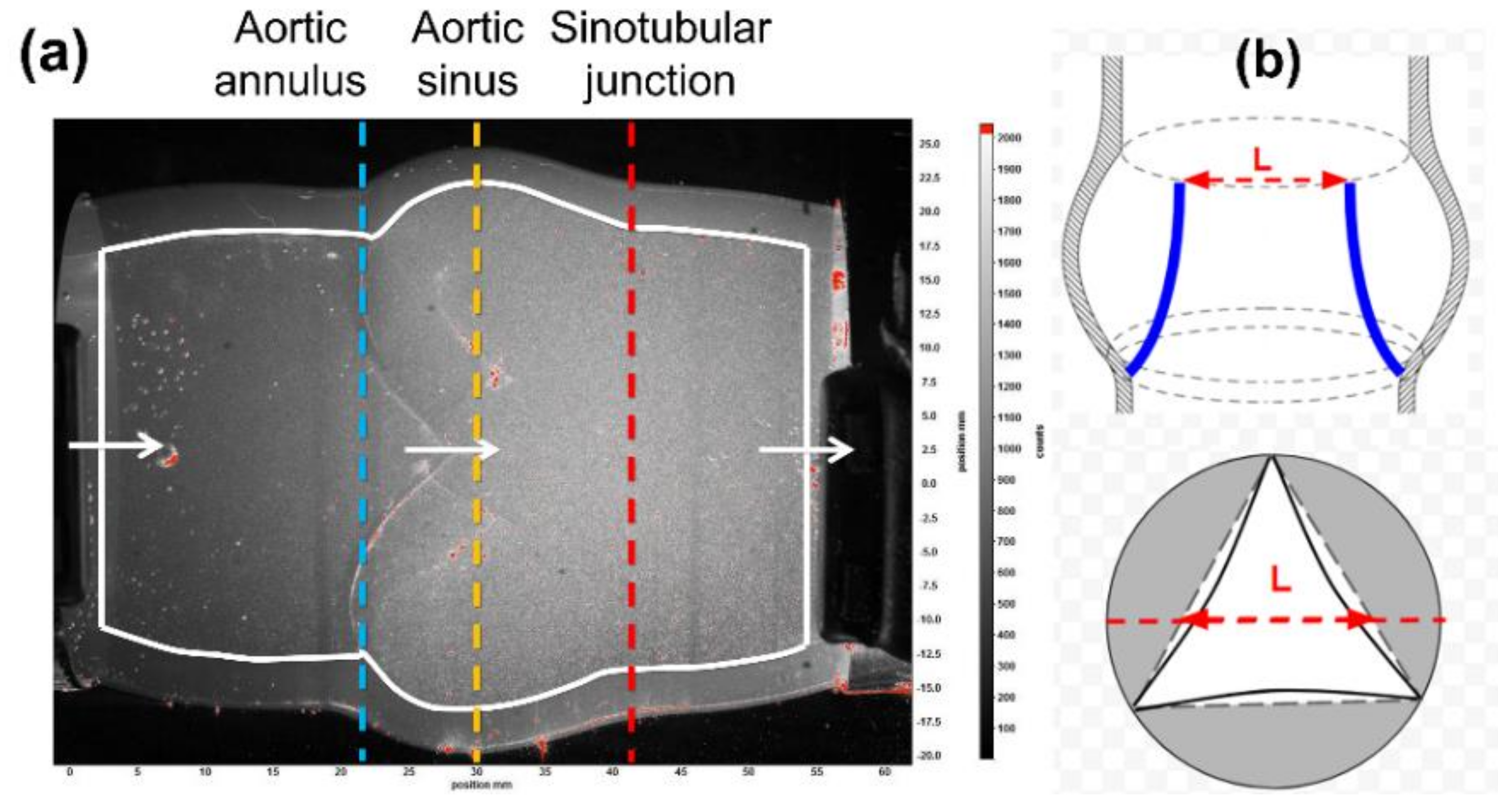
2.2. Particle Image Velocimetry (PIV)
2.3. Test Conditions
3. Results and Discussion
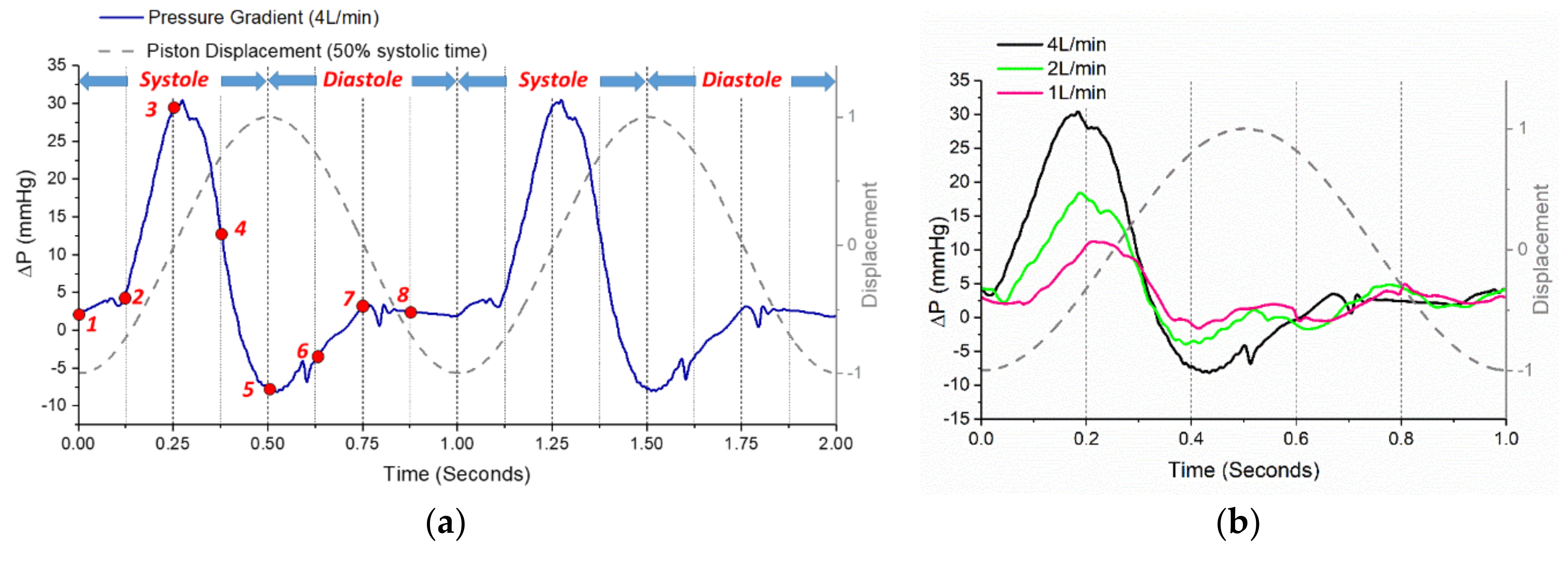
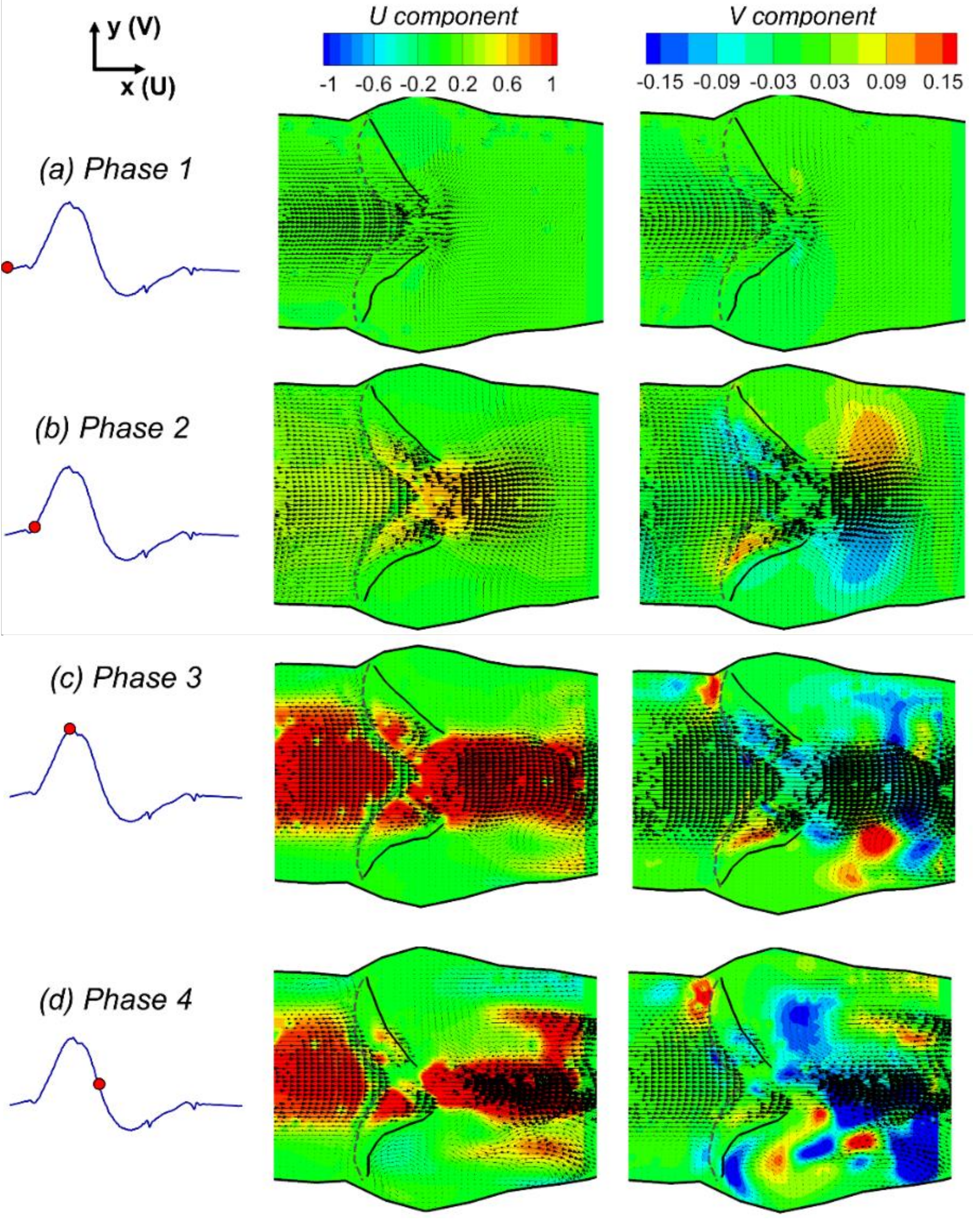
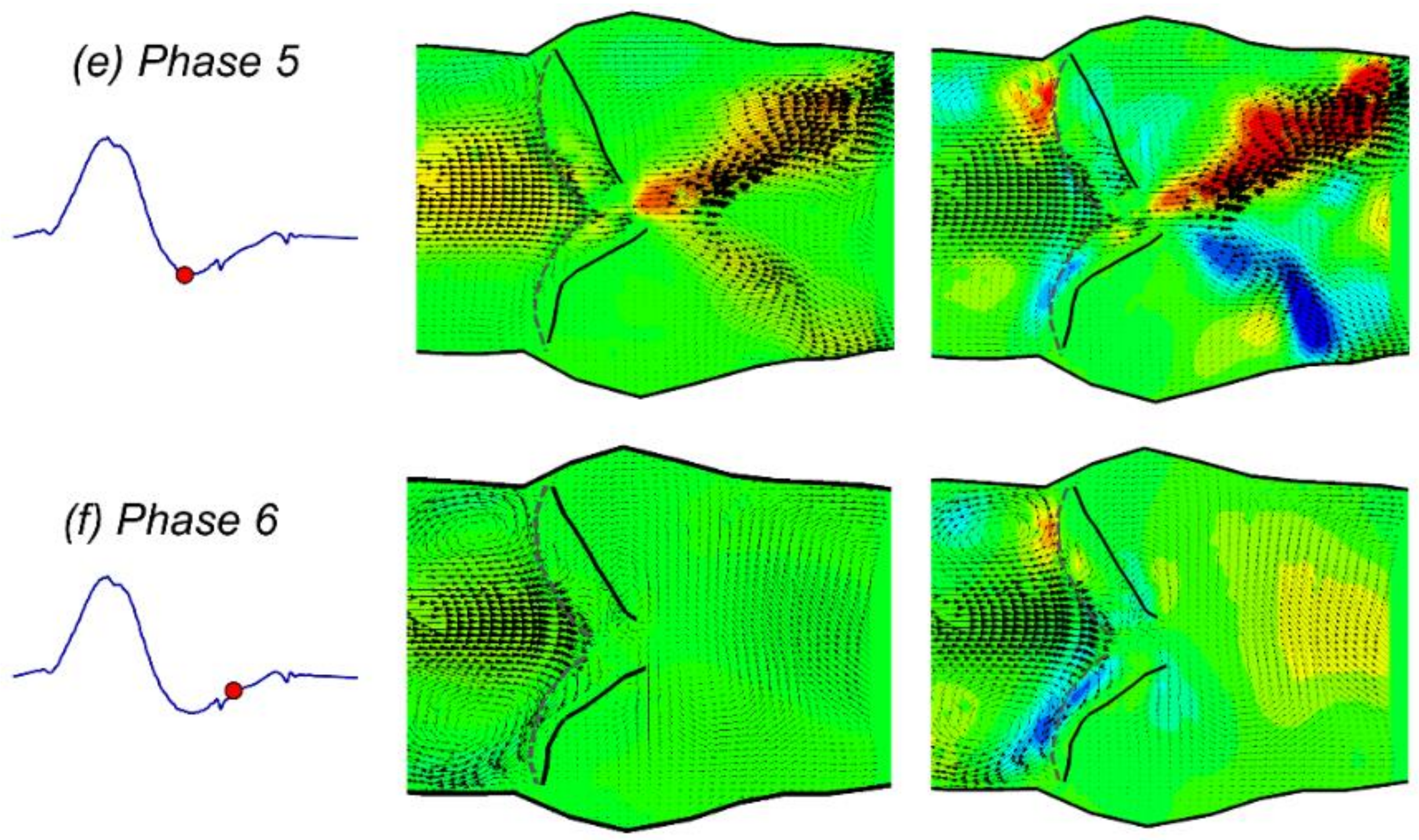
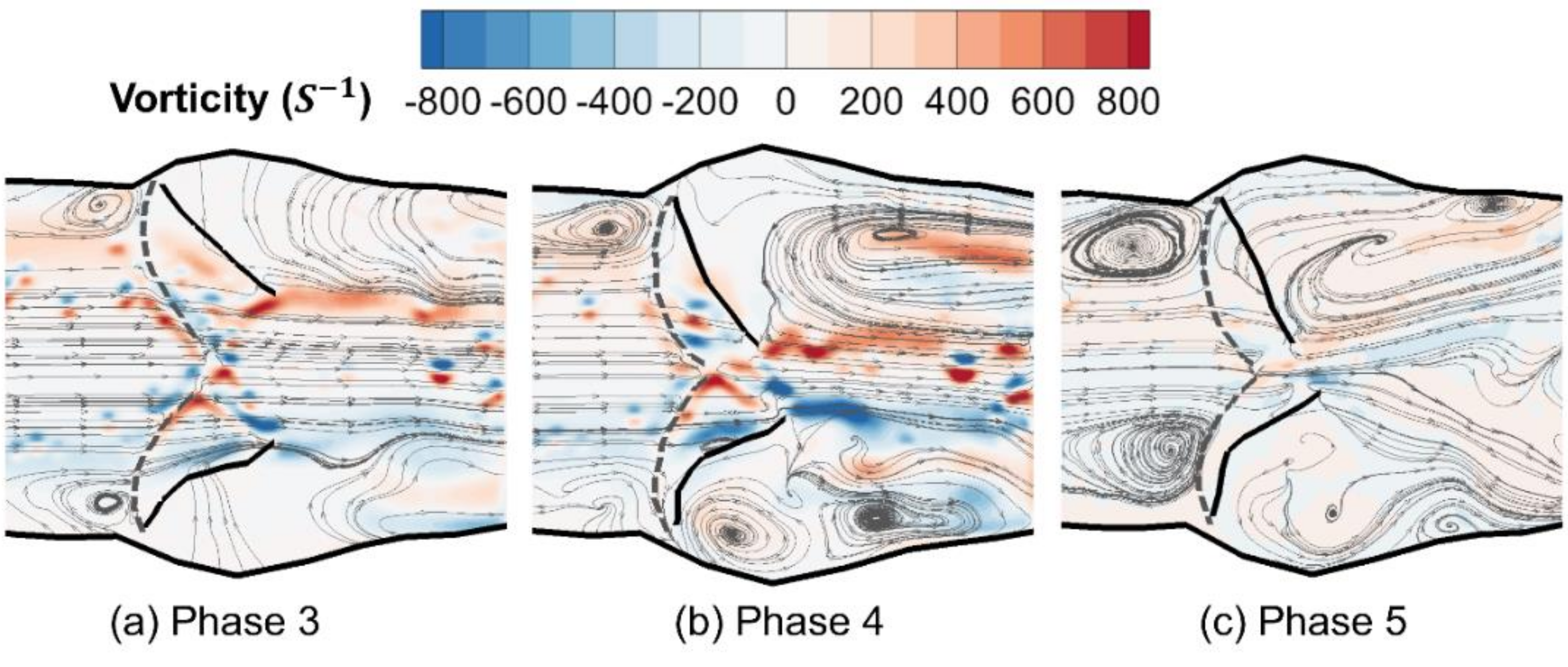
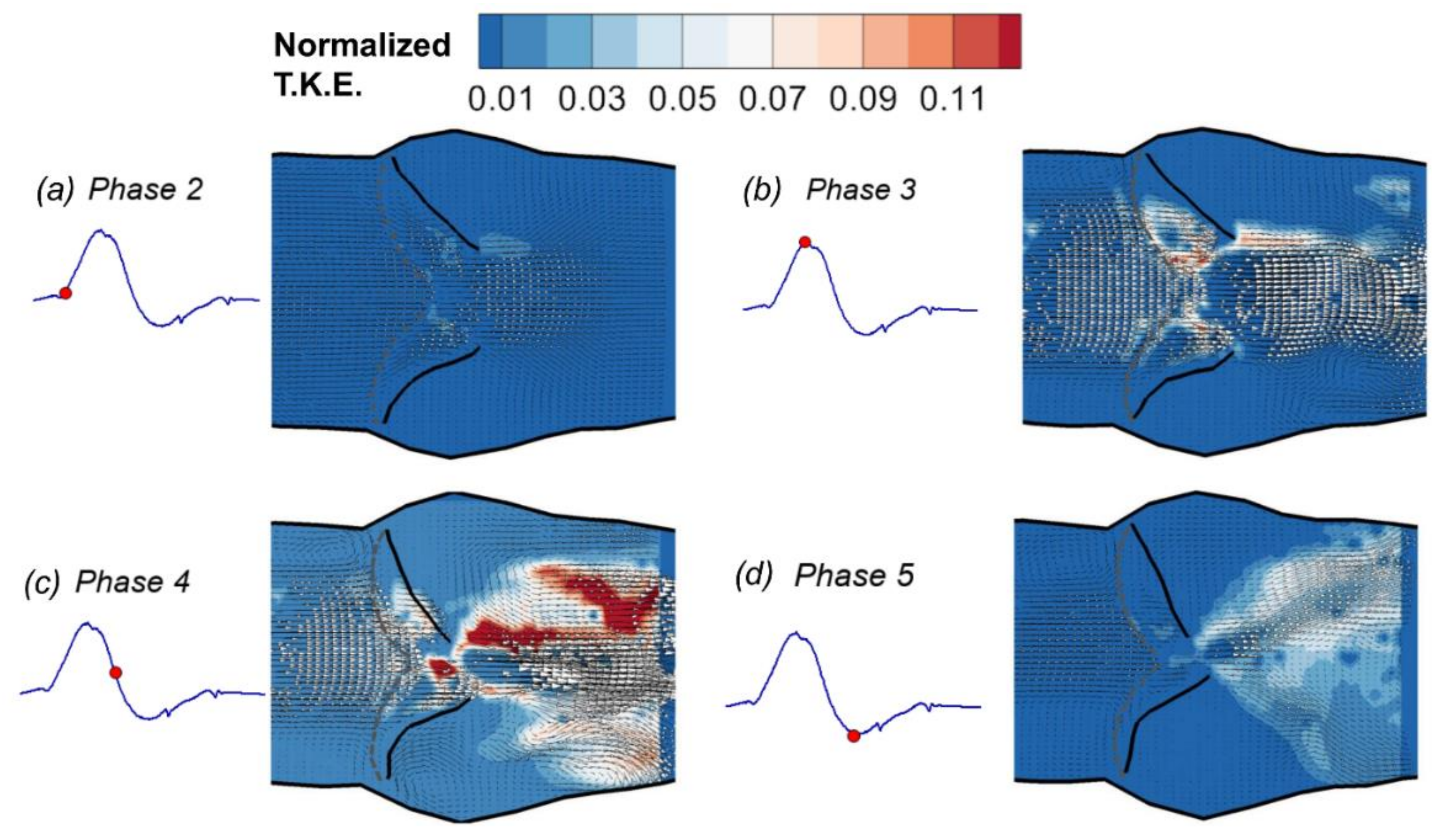
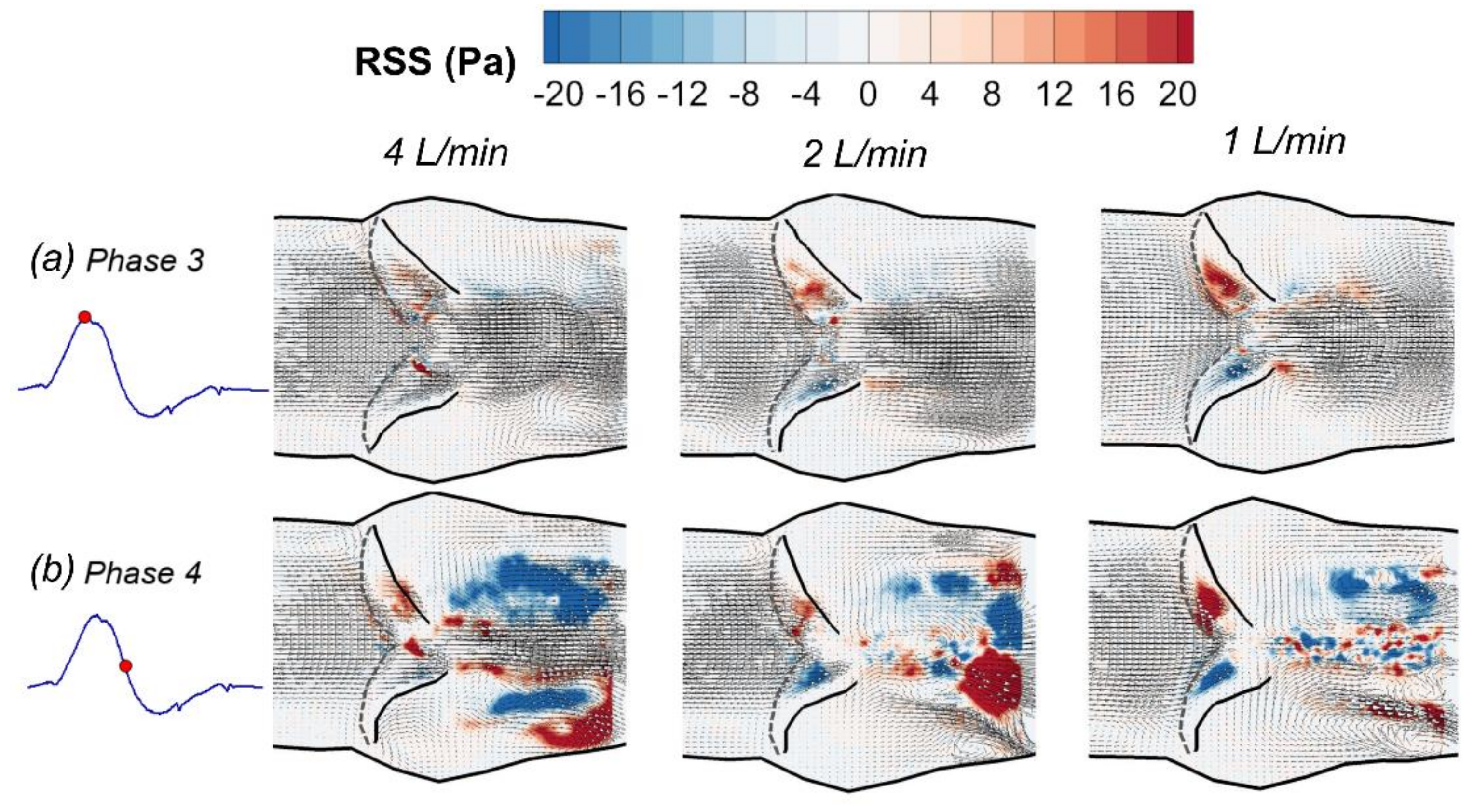
3.1. Phase-Averaged Flow Patterns in a Typical Cardiac Cycle
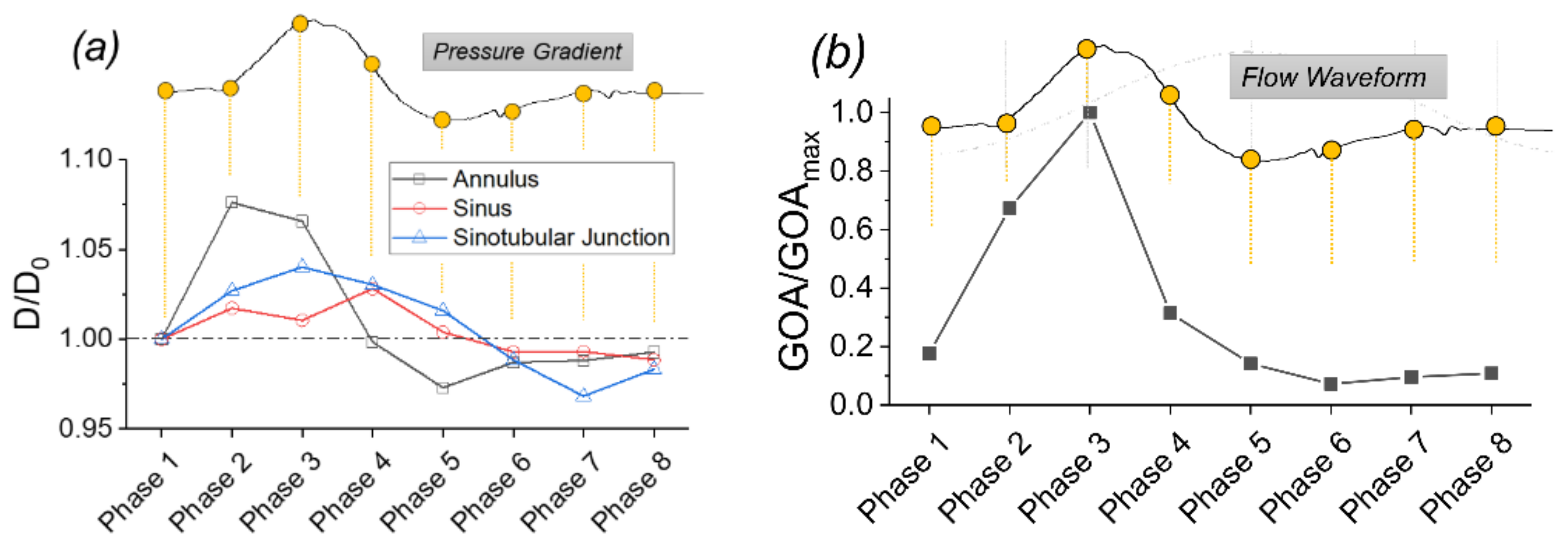
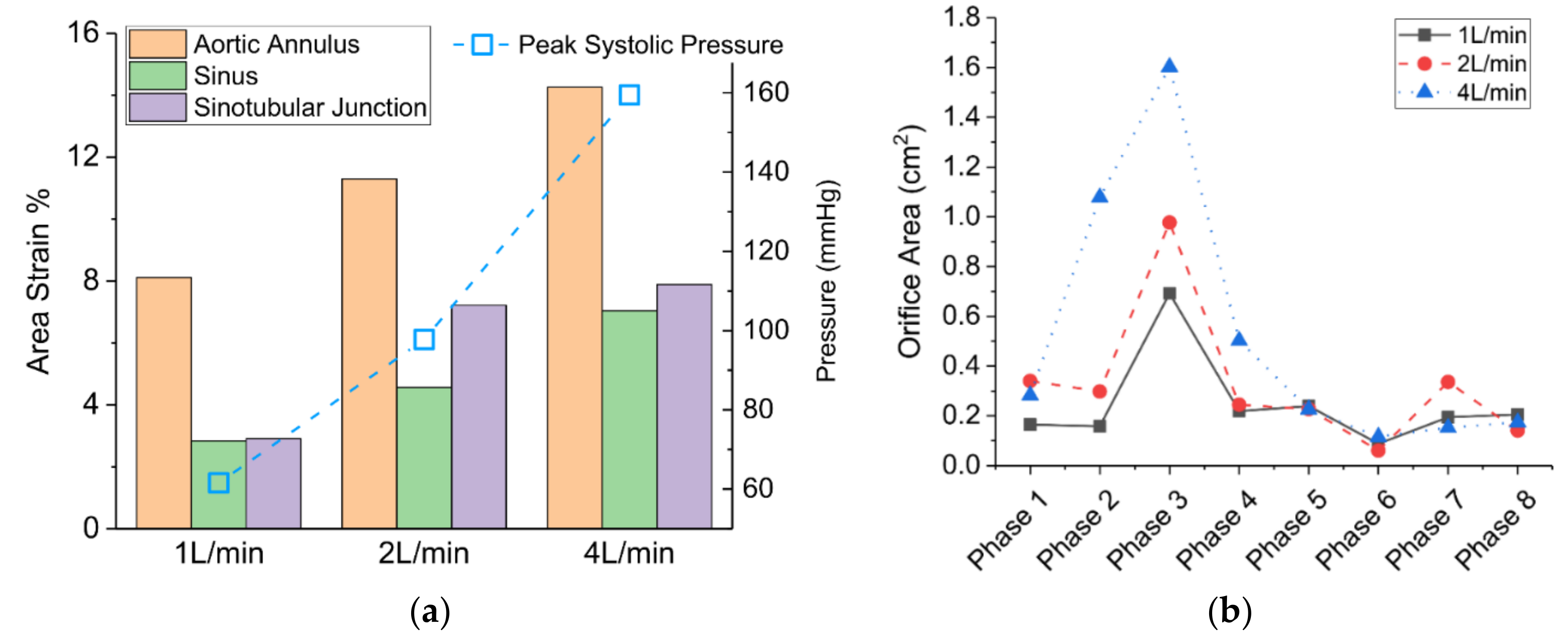
3.2. Structural Deformation in a Typical Cardiac Cycle
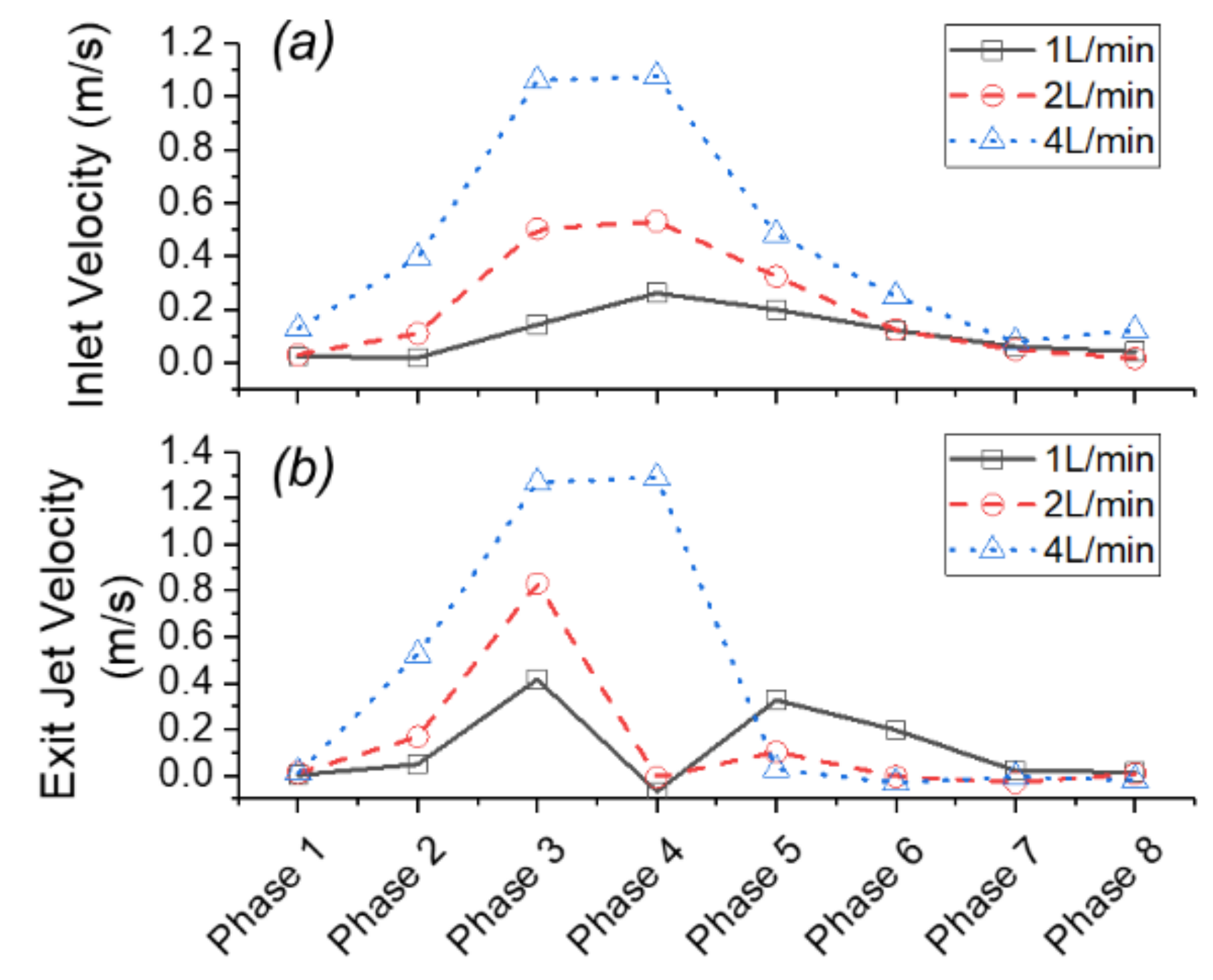
3.3. Effects of Reduced Cardiac Output
4. Conclusions
- The flow field characteristics during a typical cardiac cycle have been revealed using phase-locked PIV. Both the jet flow patterns and the turbulence kinetic energy vary significantly at different phases with changes of the pressure gradient. High TKE was observed after the peak systole when flow started to decelerate.
- The pulsatile flow caused noticeable structural deformations under normal cardiac output. The largest circumferential deformation was found at the aortic annulus upstream of the aortic valve at a phase prior to the peak systolic phase. The maximum valve orifice area was also found at a phase prior to the peak systole under the baseline 4 L/min condition.
- Cardiac output (CO) plays an important role in the hemodynamics and structural responses of the aortic valve root. A decrease of CO from 4 L/min to 2 L/min and 1 L/min resulted in a reduced exit jet flow velocity and a deceased turbulence downstream. Reduced CO also contributed to a decrease in circumferential deformation, a decrease in orifice area, and a shortened valve opening period.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Falk, E. Coronary thrombosis: Pathogenesis and clinical manifestations. Am. J. Cardiol. 1991, 68, B28–B35. [Google Scholar] [CrossRef]
- Hasan, A.; Ragaert, K.; Swieszkowski, W.; Selimović, S.; Paul, A.; Camci-Unal, G.; Mofrad, M.R.K.; Khademhosseini, A. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 47, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Leon, M.B.; Makkar, R.R.; Tuzcu, E.M.; Svensson, L.G.; Kodali, S.; Webb, J.G.; Mack, M.J.; Douglas, P.S.; Thourani, V.H.; et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2485–2491. [Google Scholar] [CrossRef]
- Wang, Y.; Joannic, D.; Patrick, J.; Keromnes, A.; Aurélien, M.; Lalande, A.; Fontaine, J.F. Comparison of Flow Measurement by 4D Flow Magnetic Resonance Imaging and by Particles Image Velocimetry on Phantom of Abdominal Aortic Aneurysm. SM Vasc. Med. 2016, 1, 1008. [Google Scholar]
- Chen, C.-Y.; Antón, R.; Hung, M.; Menon, P.; Finol, E.A.; Pekkan, K. Effects of Intraluminal Thrombus on Patient-Specific Abdominal Aortic Aneurysm Hemodynamics via Stereoscopic Particle Image Velocity and Computational Fluid Dynamics Modeling. J. Biomech. Eng. 2014, 136, 031001. [Google Scholar] [CrossRef] [PubMed]
- Knapp, Y.; Bertrand, E. Particle imaging velocimetry measurements in a heart simulator. J. Vis. 2005, 8, 217–224. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Khandheria, B.K.; Korinek, J.; Jahangir, A.; Yoshifuku, S.; Milosevic, I.; Belohlavek, M. Left ventricular isovolumic flow sequence during sinus and paced rhythms: New insights from use of high-resolution Doppler and ultrasonic digital particle imaging velocimetry. J. Am. Coll. Cardiol. 2007, 49, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-R.; Pedrizzetti, G.; Tonti, G.; Li, P.; Wei, Z.; Kim, J.K.; Baweja, A.; Liu, S.; Chung, N.; Houle, H.; et al. Characterization and Quantification of Vortex Flow in the Human Left Ventricle by Contrast Echocardiography Using Vector Particle Image Velocimetry. JACC Cardiovasc. Imaging 2008, 1, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Falahatpisheh, A.; Kheradvar, A. High-speed particle image velocimetry to assess cardiac fluid dynamics in vitro: From performance to validation. Eur. J. Mech. B Fluids 2012, 35, 2–8. [Google Scholar] [CrossRef]
- Asami, R.; Tanaka, T.; Kawabata, K.; Hashiba, K.; Okada, T.; Nishiyama, T. Accuracy and limitations of vector flow mapping: Left ventricular phantom validation using stereo particle image velocimetory. J. Echocardiogr. 2017, 15, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Day, S.W.; McDaniel, J.C.; Wood, H.G.; Allaire, P.E.; Landrot, N.; Curtas, A. Particle image velocimetry measurements of blood velocity in a continuous flow ventricular assist device. ASAIO J. 2001, 47, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Laumen, M.; Kaufmann, T.; Timms, D.; Schlanstein, P.; Jansen, S.; Gregory, S.; Wong, K.C.; Schmitz-Rode, T.; Steinseifer, U. Flow analysis of ventricular assist device inflow and outflow cannula positioning using a naturally shaped ventricle and aortic branch. Artif. Organs 2010, 34, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.L.; Chew, Y.T.; Chew, T.C.; Low, H.T. Steady flow dynamics of prosthetic aortic heart valves: A comparative evaluation with PIV techniques. J. Biomech. 1998, 31, 411–421. [Google Scholar] [CrossRef]
- Ge, L.; Leo, H.-L.; Sotiropoulos, F.; Yoganathan, A.P. Flow in a mechanical bileaflet heart valve at laminar and near-peak systole flow rates: CFD simulations and experiments. J. Biomech. Eng. 2005, 127, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Brücker, C.; Steinseifer, U.; Schröder, W.; Reul, H. Unsteady flow through a new mechanical heart valve prosthesis analysed by digital particle image velocimetry. Meas. Sci. Technol. 2002, 13, 1043. [Google Scholar] [CrossRef]
- Dasi, L.P.; Ge, L.; Simon, H.A.; Sotiropoulos, F.; Yoganathan, A.P. Vorticity dynamics of a bileaflet mechanical heart valve in an axisymmetric aorta. Phys. Fluids 2007, 19, 067105. [Google Scholar] [CrossRef]
- Bellofiore, A.; Quinlan, N.J. High-resolution measurement of the unsteady velocity field to evaluate blood damage induced by a mechanical heart valve. Ann. Biomed. Eng. 2011, 39, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Pibarot, P.; Dumesnil, J.G.; Sakr, F.; Durand, L.G. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation 2000, 101, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, R.S.; Fontaine, A.A.; Grimes, R.Y.; Sidhaye, A.; Yang, S.; Moore, K.E.; Levine, R.A.; Yoganathan, A.P. Experimental analysis of fluid mechanical energy losses in aortic valve stenosis: Importance of pressure recovery. Ann. Biomed. Eng. 1996, 24, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Kabinejadian, F.; Nguyen, Y.N.; Wui, E.T.; Kim, S.; Leo, H.L. Hemodynamic assessment of extra-cardiac tricuspid valves using particle image velocimetry. Med. Eng. Phys. 2017, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; BukaČ, M.; Sucosky, P. Three-dimensional macro-scale assessment of regional and temporal wall shear stress characteristics on aortic valve leaflets. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.D.; Peters, G.W.M.; Schreurs, P.J.G.; Baaijens, F.P.T. A three-dimensional computational analysis of fluid-structure interaction in the aortic valve. J. Biomech. 2003, 36, 103–112. [Google Scholar] [CrossRef]
- Marom, G.; Haj-Ali, R.; Raanani, E.; Schäfers, H.-J.; Rosenfeld, M. A fluid-structure interaction model of the aortic valve with coaptation and compliant aortic root. Med. Biol. Eng. Comput. 2012, 50, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lavon, K.; Halevi, R.; Marom, G.; Zekry, S.B.; Hamdan, A.; Schäfers, H.J.; Raanani, E.; Haj-Ali, R. Fluid-Structure Interaction Models of Bicuspid Aortic Valves: The Effects of Nonfused Cusp Angles. J. Biomech. Eng. 2018, 140, 031010. [Google Scholar] [CrossRef] [PubMed]
- Haj-Ali, R.; Dasi, L.P.; Kim, H.-S.; Choi, J.; Leo, H.W.; Yoganathan, A.P. Structural simulations of prosthetic tri-leaflet aortic heart valves. J. Biomech. 2008, 41, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Labrosse, M.R.; Lobo, K.; Beller, C.J. Structural analysis of the natural aortic valve in dynamics: From unpressurized to physiologically loaded. J. Biomech. 2010, 43, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Leo, H.L.; Dasi, L.P.; Carberry, J.; Simon, H.A.; Yoganathan, A.P. Fluid Dynamic Assessment of Three Polymeric Heart Valves Using Particle Image Velocimetry. Ann. Biomed. Eng. 2006, 34, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Hasler, D.; Landolt, A.; Obrist, D. Tomographic PIV behind a prosthetic heart valve. Exp. Fluids 2016, 57, 80. [Google Scholar] [CrossRef]
- Saikrishnan, N.; Yap, C.-H.; Milligan, N.C.; Vasilyev, N.V.; Yoganathan, A.P. In vitro characterization of bicuspid aortic valve hemodynamics using particle image velocimetry. Ann. Biomed. Eng. 2012, 40, 1760–1775. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Madan, A.; Sucosky, P. Morphotype-Dependent Flow Characteristics in Bicuspid Aortic Valve Ascending Aortas: A Benchtop Particle Image Velocimetry Study. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Pisani, G.; Scaffa, R.; Ieropoli, O.; Dell’Amico, E.M.; Maselli, D.; Morbiducci, U.; De Paulis, R. Role of the sinuses of Valsalva on the opening of the aortic valve. J. Thorac. Cardiovasc. Surg. 2013, 145, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Salica, A.; Pisani, G.; Morbiducci, U.; Scaffa, R.; Massai, D.; Audenino, A.; Weltert, L.; Guerrieri Wolf, L.; De Paulis, R. The combined role of sinuses of Valsalva and flow pulsatility improves energy loss of the aortic valve. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Lomivorotov, V.V.; Efremov, S.M.; Kirov, M.Y.; Fominskiy, E.V.; Karaskov, A.M. Low-Cardiac-Output Syndrome After Cardiac Surgery. J. Cardiothorac. Vasc. Anesthesia 2017, 31, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Ivanov, J.; Weisel, R.D.; Ikonomidis, J.S.; Christakis, G.T.; David, T.E. Predictors of low cardiac output syndrome after coronary artery bypass. J. Thorac. Cardiovasc. Surg. 1996, 112, 38–51. [Google Scholar] [CrossRef]
- Buchmann, N.A.; Atkinson, C.; Jeremy, M.C.; Soria, J. Tomographic particle image velocimetry investigation of the flow in a modeled human carotid artery bifurcation. Exp. Fluids 2011, 50, 1131–1151. [Google Scholar] [CrossRef]
- Bombardini, T.; Gemignani, V.; Bianchini, E.; Venneri, L.; Petersen, C.; Pasanisi, E.; Pratali, L.; Alonso-Rodriguez, D.; Pianelli, M.; Faita, F.; et al. Diastolic time–frequency relation in the stress echo lab: Filling timing and flow at different heart rates. Cardiovasc. Ultrasound 2008, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Sarnari, R.; Kamal, R.Y.; Friedberg, M.K.; Silverman, N.H. Doppler assessment of the ratio of the systolic to diastolic duration in normal children: Relation to heart rate, age and body surface area. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2009, 22, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.S.; Karamanoglu, M.; Kovács, S.J. Duration of diastole and its phases as a function of heart rate during supine bicycle exercise. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2003–H2008. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Sabbah, H.N. Measured Turbulence and Its Effect on Thrombus Formation. Circ. Res. 1974, 35, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 978-1-4757-2257-4. [Google Scholar]
- Trip, R.; Kuik, D.J.; Westerweel, J.; Poelma, C. An experimental study of transitional pulsatile pipe flow. Phys. Fluids 2012, 24, 014103. [Google Scholar] [CrossRef]
- Borse, M.; Bhushan, S.; Walters, D.K.; Burgreen, G.W. Numerical simulations of flow pattern and particle trajectories in feline aorta for hypertrophic cardiomyopathy heart conditions. Eng. Appl. Comput. Fluid Mech. 2018, 12, 57–73. [Google Scholar] [CrossRef]
- Bell, V.; Mitchell, W.A.; Sigurðsson, S.; Westenberg, J.J.M.; Gotal, J.D.; Torjesen, A.A.; Aspelund, T.; Launer, L.J.; de Roos, A.; Gudnason, V.; et al. Longitudinal and Circumferential Strain of the Proximal Aorta. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2014, 3. [Google Scholar] [CrossRef]
- Büsen, M.; Arenz, C.; Neidlin, M.; Liao, S.; Schmitz-Rode, T.; Steinseifer, U.; Sonntag, S.J. Development of an In Vitro PIV Setup for Preliminary Investigation of the Effects of Aortic Compliance on Flow Patterns and Hemodynamics. Cardiovasc. Eng. Technol. 2017, 8, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Dasi, L.P. Spatiotemporal complexity of the aortic sinus vortex. Exp. Fluids 2014, 55, 1770. [Google Scholar] [CrossRef] [PubMed]
- Lansac, E.; Lim, H.S.; Shomura, Y.; Lim, K.H.; Rice, N.T.; Goetz, W.; Acar, C.; Duran, C.M.G. A four-dimensional study of the aortic root dynamics. Eur. J. Cardio-Thorac. Surg. 2002, 22, 497–503. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Rodés-Cabau, J.; Dumont, É.; Bagur, R.; Bergeron, S.; De Larochellière, R.; Doyle, D.; Larose, É.; Dumesnil, J.G.; Pibarot, P. Validation and Characterization of Transcatheter Aortic Valve Effective Orifice Area Measured by Doppler Echocardiography. JACC Cardiovasc. Imaging 2011, 4, 1053–1062. [Google Scholar] [CrossRef] [PubMed]

| Cardiac Output | Stroke Volume | Re | Wo |
|---|---|---|---|
| [L/min] | [mL] | ||
| 1 | 16.7 | 903 | 11.7 |
| 2 | 33.3 | 2249 | |
| 4 | 66.7 | 4370 |
| Cardiac Output | 1 L/min | 2 L/min | 4 L/min | Effective Orifice Area of a Bioprosthetic Aortic Valve [48] | Area Strain (Mean) [44] |
|---|---|---|---|---|---|
| GOA (cm2) | 0.7 | 1.0 | 1.6 | 1.37–1.9 | - |
| AS (%) at Aortic Annulus | 8.1 | 11.3 | 14.3 | - | - |
| AS (%) at Aortic Sinus | 2.8 | 4.6 | 7.0 | - | - |
| AS (%) at Sinotubular Junction | 2.9 | 7.2 | 7.9 | - | 8.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhang, Y. An Experimental Study of Pulsatile Flow in a Compliant Aortic Root Model under Varied Cardiac Outputs. Fluids 2018, 3, 71. https://doi.org/10.3390/fluids3040071
Zhang R, Zhang Y. An Experimental Study of Pulsatile Flow in a Compliant Aortic Root Model under Varied Cardiac Outputs. Fluids. 2018; 3(4):71. https://doi.org/10.3390/fluids3040071
Chicago/Turabian StyleZhang, Ruihang, and Yan Zhang. 2018. "An Experimental Study of Pulsatile Flow in a Compliant Aortic Root Model under Varied Cardiac Outputs" Fluids 3, no. 4: 71. https://doi.org/10.3390/fluids3040071
APA StyleZhang, R., & Zhang, Y. (2018). An Experimental Study of Pulsatile Flow in a Compliant Aortic Root Model under Varied Cardiac Outputs. Fluids, 3(4), 71. https://doi.org/10.3390/fluids3040071






