Abstract
Liposomes are considered to be one of the most successful drug delivery systems. They apply nanotechnology to potentiate the therapeutic efficacy and reduce the toxicity of conventional medicines. Shikonin and alkannin, a pair of chiral natural naphthoquinone compounds, derived from Alkanna and Lithospermum species, are widely used due to their various pharmacological activities, mainly wound healing, antioxidant, anti-inflammatory and their recently established antitumor activity. The purpose of this study was to prepare conventional and PEGylated shikonin-loaded liposomal formulations and measure the effects of different lipids and polyethylene glycol (PEG) on parameters related to particle size distribution, the polydispersity index, the zeta potential, drug-loading efficiency and the stability of the prepared formulations. Three types of lipids were assessed (1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-distearoyl-sn-glycero-3-phospho-rac-(1-glycerol) (DSPG)), separately and in mixtures, forming anionic liposomes with good physicochemical characteristics, high entrapment efficiencies (varying from 56.5 to 89.4%), satisfactory in vitro release profiles and good physical stability. The addition of the negatively charged DSPG lipids to DOPC, led to an increment in the drug’s incorporation efficiency and reduced the particle size distribution. Furthermore, the shikonin–loaded PEGylated sample with DOPC/DSPG, demonstrated the most satisfactory characteristics. These findings are considered promising and could be used for further design and improvement of such formulations.
1. Introduction
Alkannin and shikonin (A/S; Figure 1), a chiral pair of natural naphthoquinone compounds, biosynthesized in the roots of more than 150 species of the Boraginaceae plant family (such as lithospermum, Alkanna, Anchusa and Echium) are widely used due to their various pharmacological activities. Biological investigations have established that A/S possess a wide spectrum of biological activities, such as strong wound healing, and tissue regenerative, anti-inflammatory, antioxidant and, most prominently, antitumor activity. It is worth-mentioning that alkannin and shikonin (the S- and R-isomer, respectively; Figure 1) display similar levels of pharmacological activity [1,2,3,4,5,6].
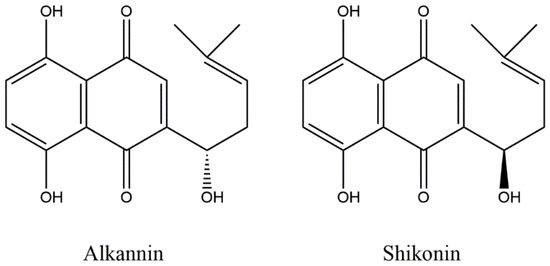
Figure 1.
The enantiomers, alkannin and shikonin.
The great scientific interest and clinical potential for the antitumor properties of A/S in the development of novel chemotherapeutics and in effective combination chemotherapy is depicted by the large number of papers (more than 140) that have appeared in the literature the last five years [7,8]. Recent studies confirmed that shikonin has the potential to induce apoptosis in a variety of human tumor cell lines, including leukemia cell lines in vitro and in vivo, with minimal or no toxicity to healthy human cells [9,10,11].
This antitumor activity of shikonin may be attributed to its accumulation in the mitochondria of cancer cells, which disrupts mitochondrial function, and eventually causes apoptosis [12]. Our group investigated the inhibition of c-MYC expression and transcriptional activity by shikonin as a novel mechanism for killing leukemia cells [9], and more recently, the cytotoxic activity of shikonin to the Huh7 cancer cell line by a metabolite profiling approach which could set a basis for the elucidation of their antitumor activity was investigated [13]. Shikonin has also been proposed as a novel dietary agent with great potential in breast cancer prevention [14] and has been found to act synergistically to potentiate doxorubicin-induced growth inhibition and apoptosis in vitro [15].
These studies prove the potent antitumor activities of shikonin in multiple tumors through targeting multiple signaling pathways, promoting the necessity of solving the problem of drug resistance. Therefore, the most promising delivery systems for A/S derivatives need to be developed and optimized to exploit and assess their anticancer properties.
Nanoscale drug carriers offer the potential to improve the therapeutic index of drug molecules by diminishing their toxicity against physiological tissues. Furthermore, they can result in controlled therapeutic levels of the drug for a prolonged time. A proper drug delivery agent could modify the solubility and improve the stability of candidate drugs, and lead to an improved ADME profile (Absorption, bioDistribution, Metabolism, and Excretion) [16,17].
Liposomes represent an advanced type of nanotechnology that has the potential to target active molecules (anticancer agents, peptide hormones, enzymes, proteins, vaccines) to the site of action, improving the therapeutic index [18,19,20]. Many clinical studies have shown that liposomes have improved the pharmacokinetics and biodistribution of therapeutic agents. Several anticancer liposomal formulations (conventional and PEGylated) have been approved and are commercially available, such as DOXIL®/Caelyx® (doxorubicin), Lipo-Dox® (doxorubicin), Myocet®/Evacet® (doxorubicin), DaunoXome® (daunorubicin), Myocet®/Evacet® (doxorubicin), Ambisome® (Amphotericin B) and Marqibo® (Vincristine) [21,22].
A significant improvement came with the incorporation of PEG-lipid leading liposomes (PEGylated or Stealth® liposomes) which remain for longer time periods in the blood circulation. The presence of PEGs on the surface of liposomes prevents their uptake by the reticuloendothelial system (RES), which is attributed to their highly hydrated surfaces due to the hydrophilic polymers that result in the inhibition of protein adsorption and opsonization of the liposomes [23]. In this way, liposomes have, to some extent, the ability to pass in and out of the liver and spleen, avoiding clearance, and thus remain in the tumor tissue due to the depleted lymphatic drainage [24,25]. In previous studies, anticancer agents that were incorporated in PEGylated liposomes displayed longer circulation times and enhanced drug delivery to tumor tissues [26].
There is great interest in exploiting the wide range of anticancer activities of the hydrophobic A/S and derivatives towards several tumors, and therefore an optimum administration system needs to be developed. The incorporation of a hydrophobic drug (such as shikonin) into liposomes improves its bioavailability and leads to increased stability and anticancer activity, along with decreased drug toxicity. Regarding shikonin, it has been recently proven that liposomes significantly decrease its toxicity in vitro and in vivo [27]. Furthermore, under the frame of developing drug delivery systems with alkannins and shikonins as bioactive molecules, we have successfully incorporated shikonin into both conventional (with lipids such as egg phosphatidylcholine (EPC), 1,2-dipalmitoylphosphatidylcholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)) [28] and stealth liposomes (DSPC-PEG2000, EPC-PEG2000, DPPC-PEG2000) [29].
In this regard, the scope of this paper was to expand our previous research by preparing and characterizing shikonin-loaded liposomes, with different types of lipids, aiming to produce an optimized formulation and to compare this with the ones already prepared. Specifically, DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) and a mixture of lipids with the charged lipid DSPG (1,2-distearoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt), like DOPC/DSPG and DSPC/DOPC, were used for the first time, for both conventional and stealth liposomes. The negatively charged lipid was used to prepare anionic liposomal formulations in order to prevent the aggregation of liposomes due to electrostatic repulsion [24,30,31]. Furthermore, it was reported that the presence of negatively charged lipids in liposomes, allows rapid uptake by the reticuloendothelial system [24], while large and positively charged liposomes induce cytokine activation and toxicity and thereby their safety for clinical use is limited [32].
Thus, three conventional and PEGylated liposomal formulations of shikonin (DOPC, DOPC/DSPG and DSPC/DSPG) were formulated and characterized with consideration of their physicochemical characteristics (particle size distribution, ζ-potential, entrapment efficiency), in vitro release profiles and physicochemical stability (4 °C for a 28 days period: drug leakage, particle size distribution, ζ-potential). The new formulations were also compared with our previously reported shikonin-loaded liposomes [28,29].
2. Materials and Methods
2.1. Materials
Shikonin was purchased from Ichimaru Pharcos Co., Ltd. (Gifu, Japan) and was used after purification through column chromatography followed by recrystallization with n-hexane, in accordance with Assimopoulou et al. [33] (purity obtained: 100% by HPLC-DAD, Agilent Technologies, Waldbronn, Germany).
1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC; MW 790.15); 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC; MW 786.11); and 1,2-distearoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt) (DSPG; MW 801.06) were generously donated by Lipoid GmbH (Ludwigshafen, Germany). Ν-(Carbonyl-methoxypolyethyleneglycol 2000)—1,2 distearoyl-en-glycero-3-phosphoethanolamine (DSPE-mPEG2000; MW 2806.0) was purchased from Genzyme Pharmaceuticals (Cambridge, MA, USA). Phosphate buffer saline of pH 7.4 (PBS), cholesterol (CHOL), sodium lauryl sulfate (SLS), Sephadex G75 and dialysis sacks (molecular weight cut off 13,000) were obtained from Sigma–Aldrich (St. Louis, MO, USA). Organic solvents used for all experiments were of analytical grade and were purchased from Sigma–Aldrich (St. Louis, MO, USA), and water was of HPLC grade.
2.2. Liposome Preparation
Shikonin-loaded liposomes were formulated using the thin-film hydration method. In brief, lipids, cholesterol and shikonin were dissolved in CHCl3/MeOH 2:1 (v/v) using the same molar ratios for all samples: lipid/CHOL (4.5:1), neutral lipid/charged lipid (9:1), lipid/DPSE-mPEG2000 (13:1) and lipid/shikonin (30:1) (see Table 1). Organic solvent was slowly removed in a rotary evaporator (EYELA N-N Series, Digital Water bath SB–651, Tokyo, Japan), forming a thin lipid film on the flask. Solvent traces were removed by leaving the flask overnight under vacuum. The lipid film was then hydrated by the addition of PBS (6.5 mL for drug-loaded liposomes and 2 mL for liposomes without drugs—“empty liposomes”) for 1.5 h in a water bath. The temperature was maintained above the main phase transition temperature (Tm) of each lipid (−20 °C for DOPC, 67 °C for DSPC and 55 °C for DSPG). Flask was vortexed in an IKA MS2 Minishaker (IKA Works Inc., Wilmington, NC, USA) at 1500 rpm for 10 min.

Table 1.
Liposome compositions used in the study.
Small unilamellar vesicles (SUVs) were obtained from the resultant multilamellar vesicles (MLVs) by probe sonication, using a Sonicator W–375 Cell Disruptor (Heat Systems–Ultrasonics Inc.) for 2 × 5 min periods, interrupted by a 5 min rest period in ice bath (amplitude 0.6; pulser 50%). Formulations were left for 30 min to anneal any structural defects.
2.3. Characterization of Shikonin-Loaded Liposomes
2.3.1. Particle Size and ζ-Potential
The size distribution and ζ-potential of the liposomal formulations were measured immediately after preparation by dynamic light scattering using a Malvern ZetaSizer Nano ZS (Malvern Instruments, Malvern, UK) at 25 °C. Prior to measurement, all samples were diluted by 60-fold in PBS (pH 7.4).
2.3.2. Entrapment Efficiency
An aliquot of freshly prepared, loaded liposomes (300 μL) was transferred to a size exclusion column (Sephadex G75) and eluted with PBS in order to separate free from entrapped shikonin and to determine the entrapment efficiency. Purified liposomes (500 μL) were diluted in methanol (2.5 mL) to destroy liposomal structure and release the drug into the organic phase. The concentration of shikonin was determined by the absorbance of the organic phase (measured with a Ultraviolet–visible spectroscopy (UV–Vis) Hitachi U1900, Hitachi High-Technologies Corporation, Tokyo, Japan) using the following calibration curve:
Shikonin Concentration (mg/mL) = 0.0316 × Absorbance − 0.00009; (R2 = 0.9999).
The entrapment efficiency was calculated as follows:
where Fi is the shikonin concentration into liposomes and Ft is the initial concentration of shikonin.
Entrapment Efficiency (%) = (Fi/Ft) × 100,
2.3.3. In Vitro Release
Briefly, 3 mL of shikonin-loaded liposomes were inserted into dialysis sacks (molecular weight cut off 13,000; Sigma–Aldrich). The sealed dialysis sacks were incubated in 20 mL of release medium (PBS + 1% SLS) at 37 °C in a water bath and stirred magnetically (RET control-visc, IKA Werke, Germany). Aliquots of release medium (3 mL) were withdrawn at specific time intervals, to determine the accumulative amount of drug released, and they were replaced with fresh release medium. The shikonin concentration in the release medium was calculated with a UV–Vis spectrometer at λmax = 516 nm with the aid of the following calibration curve (shikonin in release medium):
Shikonin Concentration (mg/mL) = 0.0538 × Absorbance − 0.0002; (R2 = 0.9999).
Release curves were drawn according to the cumulative drug release and plotted vs time (with the aid of the following equation):
% Cumulative Shikonin Releasedt = Shikonin Releasedt/Total Entrapped Shikonin × 100.
2.4. Stability
Immediately after their preparation, samples were stored in dark glass vials (in their hydrated form) at 4 °C for 28 days, in order to study their stability. Aliquots were withdrawn at specific time intervals and assessed in terms of their mean particle size, ζ-potential and drug retention.
2.5. Statistical Analysis
All results are expressed as mean values ± standard deviations of three independent experiments. Statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). In order to examine the statistical significance between samples, multiple comparisons were performed by one-way analysis of variance (ANOVA) followed by post-hoc analysis using Tukey’s test. p < 0.05 was considered statistically significant.
3. Results and Discussion
In the present study, three lipid types (DOPC, DOPC/DSPG, and DSPC/DSPG) were utilized to prepare conventional and PEGylated liposomes containing shikonin, aiming to reduce the drug’s toxicity and achieve controlled release. This is a continuation of the current authors’ active research on developing and evaluating drug delivery systems for bioactive naphthoquinones, such as alkannins and shikonins [28,29].
3.1. Liposome Characterization
The physicochemical characterization of liposomes, such as their size, shape and charge are vital parameters in the delivery of improved bio-distribution and prolonged pharmacokinetics of encapsulated cytotoxic drugs [34]. The prepared liposomal formulations (shikonin-loaded and without the drug) were characterized in terms of their particle size distribution and ζ-potential values. Furthermore, the amount of drug incorporated and the release kinetics of the drug were additionally estimated (Table 2).

Table 2.
Physicochemical characteristics of liposomal formulations.
3.1.1. Particle Size Measurement
The size of the liposomes affects their residence time in the systemic blood circulation, as well as their pathways within the body. The smaller the size, the more difficult it is for them to become detectable by the macrophages of the immune system, increasing in this way the residence time in the systemic circulation, as well as their effectiveness [35,36].
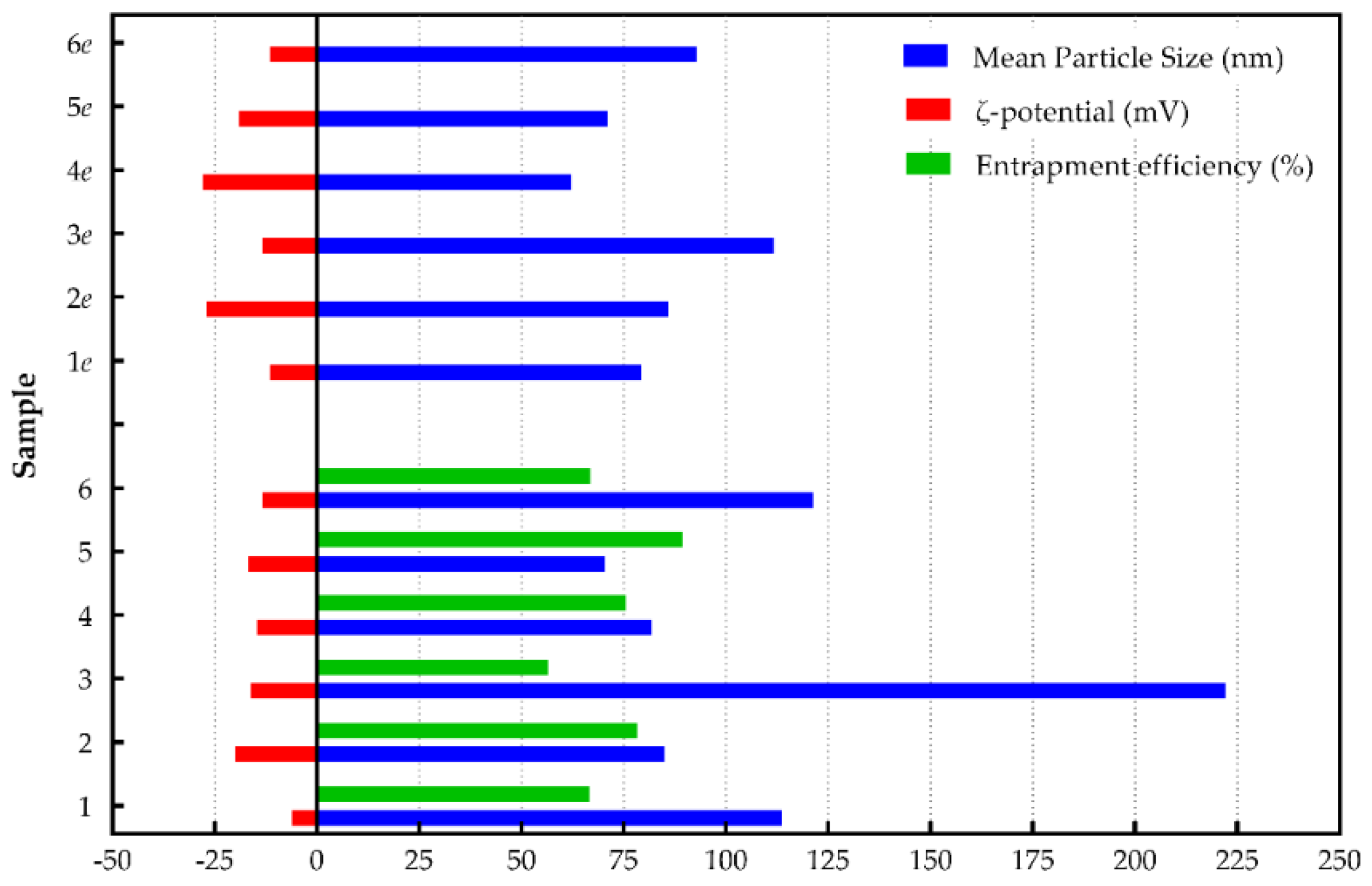
Regarding conventional formulations (samples 1, 2 and 3), the mean particle size varied from 84.9 nm to 222 nm, while for the corresponding PEGylated liposomes (samples 4, 5, and 6), sizes ranged from 70.4 nm to 121.3 nm (Table 2), giving PEGylated liposomes a significant advantage.
The lipid type significantly affected the mean particle size of the liposomal formulations. As previously reported, unsaturated fatty acids can incur oxidative reactions, altering the permeability of the liposomal bilayers [37] and if the degree of unsaturation of the fatty acid side chains is too high, there is a possibility that stable liposomes might not be formed [38]. In another study, it was reported that the average liposome size varies between 30 and 300 nm, depending on the lipid composition and ionic strength of the lipid mixture during liposome formation. Furthermore, the size effect of the charged lipids should be a factor in choosing a lipid mixture [39]. Additionally, in our previous research, it was noticed that mean particle size was affected by the lipid type as well as by the interactions between the lipid bilayer and shikonin [29].
Shikonin-loaded DOPC conventional liposomes (sample 1) presented a statistically smaller mean particle size (113.6 nm) compared to DSPC and DPPC (221.2 and 243.2 nm, respectively) and similar to EPC (144.5 nm) which were measured in our previous work [29]. The addition of a negatively charged lipid (DSPG) to DOPC (sample 2) led to a conventional formulation with a smaller mean particle size (84.9 nm) and lower polydispersity index (0.25). On the other hand, the addition of DSPG to DSPC did not significantly affect the particle size (222 nm) or polydispersity index (0.33) of DSPC formulations, resulting in liposomes with larger mean particle size compared to DOPC/DSPG ones. The addition of DSPG had different impacts on particle size distribution, depending on the type of lipid that it was combined with. Similar results arose from the corresponding formulations without the drug (samples 1e, 2e and 3e), as well as from other published studies [40]. Furthermore, it was reported that the ionic strength of the mixture during the formation of liposomes might influence their mean diameter in the presence of charged lipid components. Moreover, the ability to control the average liposome size is by varying the proportion of charged lipid components [39].
The use of DSPE-mPEG2000 resulted in PEGylated formulations (samples 4, 5, and 6) with smaller mean particle sizes and lower polydispersity indexes (Figure 2; the reduction varied between 17%–45% for drug-loaded liposomes and 17%–21% for drug-free liposomes). Briefly, PEGylated shikonin-loaded liposomes with DOPC (sample 4, 81.9 nm) had statistically smaller sizes in comparison to those in DSPC formulations (124.8 nm), and similar sizes to the EPC and DPPC liposomes (93 and 105.9 nm respectively) produced in our previous work [29]. Combining DSPG with DOPC and DSPC lipids led to PEGylated formulations with similar (sample 5; 70.4 nm) or statistically lower (sample 6; 121.3 nm) mean particle sizes compared to the conventional samples (sample 2 and 3 respectively). These results agree with the observations from the PEGylated drug-free formulations (samples 4e, 5e and 6e) as well as with similar papers in the literature [40], confirming the superiority of PEGylated formulations over conventional ones.
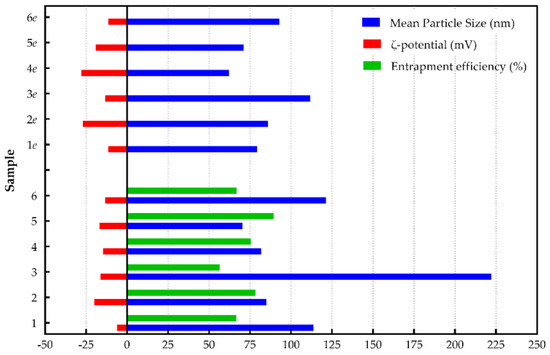
Figure 2.
Comparison of liposomal formulations.
This decrease observed in particle size could be explained by the curving of the bilayer to reduce the intensity of lateral repulsion, caused by the addition of increasing amounts of PEG in the lipid bilayer. PEGylated lipid also increases interlamellar repulsion, causing a decrease in lamellarity [41]. A large number of studies with other bioactive molecules have confirmed this trend [31,41,42].
3.1.2. ζ-Potential
The ζ-potential is the electrostatic charge of the particle surface which acts as a repulsive energy barrier controlling the stability of dispersion and opposing the aggregation of liposomes in buffer solution [43]. The charge of the phospholipids could affect the pharmacodynamic and pharmacokinetic properties of the liposomes, as well as tumor accumulation. For instance, cationic liposomes are more readily taken up by cells in comparison with anionic or neutral liposomes, due to attractive forces between the positively charged liposomes and the negatively charged outer cell membrane. However, such interactions may also damage the cell membrane, resulting in toxicity, and they have been found to cause pulmonary toxicity, due to the generation of reactive oxygen species [44].
The ζ-potential values of all samples were measured immediately after preparation. As indicated in Table 2, the ζ-potential values of conventional shikonin-loaded liposomal formulations (samples 1, 2 and 3) varied from −6.1 mV to −19.9 mV, while the ζ-potentials of the corresponding PEGylated liposomes (samples 4, 5, and 6) ranged from −13.4 mV to −16.7 mV. Similar values for other bioactive constituents have been reported in the literature [29,45,46].
Liposome’s ζ-potential values indirectly reflect the net charge of the vesicle surface. This is why the type of lipid used significantly influences the charge of the liposomal formulation [47]. Figure 2 depicts that the use of different type of lipids resulted in liposomal formulations with significant differences in ζ-potential values. More precisely, the use of DOPC lipid formed conventional liposomes (sample 1; -6.1 mV) with statistically lower ζ-potential values compared to EPC (-16.6 mV) or similar ζ-potential values compared to DSPC and DPPC lipids (-7.3 and -8.4 mV respectively) [29]. This may be attributed to interactions taking place between shikonin and the type of lipid. The addition of DSPG to DOPC and DSPC lipids formed shikonin-loaded conventional liposomes with statistically increased ζ-potential values (-19.9 and -16.2 mV, respectively). This could be due to the fact that DSPG is a negatively charged lipid, adding an additional charge in the lipid bilayer [40,48]. In addition, DOPC/DSPG liposomes (sample 2) seemed to have an advantage regarding their ζ-potential values, compared to DSPC/DSPG (sample 3). Furthermore, although in our previous work, “empty” liposomes presented decreased ζ-potential values compared to shikonin-loaded ones [29], in the present study, DOPC lipids led to the opposite trend. This trend could be attributed to the presence of shikonin, resulting in a decrease of ζ-potential values, since drug incorporation causes significant variations in the liposomal surface structure and the orientation of the phosphatidylcholine head group [49].
All drug-loaded PEGylated formulations (samples 4, 5, and 6) showed statistically increased ζ-potential values compared to the corresponding conventional liposomes (samples 1, 2 and 3). Liposomes formed with DOPC (sample 4) exhibited a ζ-potential value of -14.6 mV, which is similar to formulations previously prepared from our group with DSPC and EPC [29]. Moreover, the use of DSPG mixed with DOPC or DSPC, did not provoke any significant changes in the ζ-potential of the prepared liposomes. The same trends were obtained for the corresponding “empty” liposomes. Formulations with DSPE-PEG2000 have less negative charge compared to liposomes without DSPE-PEG2000, a fact attributed to the “masking” of some of the anionic charges of DSPG by DSPE-PEG2000, which could explain the observed trend [40,47,50].
3.1.3. Entrapment Efficiency
The entrapping efficiency of a drug into the liposome structure depends on many factors, such as the ionic strength of the buffer, the pH, the incubation time, the drug loading ratio, the lipid composition and others [51]. For the shikonin-loaded conventional liposomes (samples 1–3), the entrapment efficiency varied from 56.5 to 78.4% (as shown in Table 2), while the values of the corresponding PEGylated ones (samples 4–6) ranged from 66.9 to 89.4%.
The results of the current study showed significant variation in entrapment efficiency among samples prepared with different lipids. More precisely, the use of DOPC lipids resulted in a significant lower entrapment efficiency (66.7%) compared to similar DSPC (85.3%) and DPPC (77.9%) formulations and a higher efficiency than EPC liposomes (52.9%) [29]. The influence of the charged DSPG depended on the type of lipid that it was mixed with, improving DOPC’s performance (sample 2; 78.4%) and diminishing DSPC’s performance (sample 3; 56.5%). These results are in accordance with other published studies [47,52], and it was observed that encapsulation was affected by electrostatic interactions between the drug’s peripheral surface and the polar head groups of phospholipids. In addition, there was a linear correlation between the lipid concentration and the encapsulation efficiency [53,54].
Regardless of the type of lipid used, all PEGylated formulations (samples 4, 5 and 6) had increased drug incorporation compared to conventional ones (this increase varied from 13 to 18%). This could be due to the presence of PEG, placed on the outer surface of the lipid bilayer, causing an increase in drug entrapment within the bilayer [54,55,56]. More specifically, PEGylated liposomes with DOPC lipids showed a similar entrapment efficiency (75.6%) to previously prepared PEGylated formulations with DSPC, EPC and DPPC lipids (91.8%, 71.8%, and 84.9% respectively) [29]. These observations are in accordance with similar studies [50] and imply that shikonin incorporation could be strengthened by the presence of PEG on the outer surface of the lipid bilayer.
3.1.4. In Vitro Drug Release
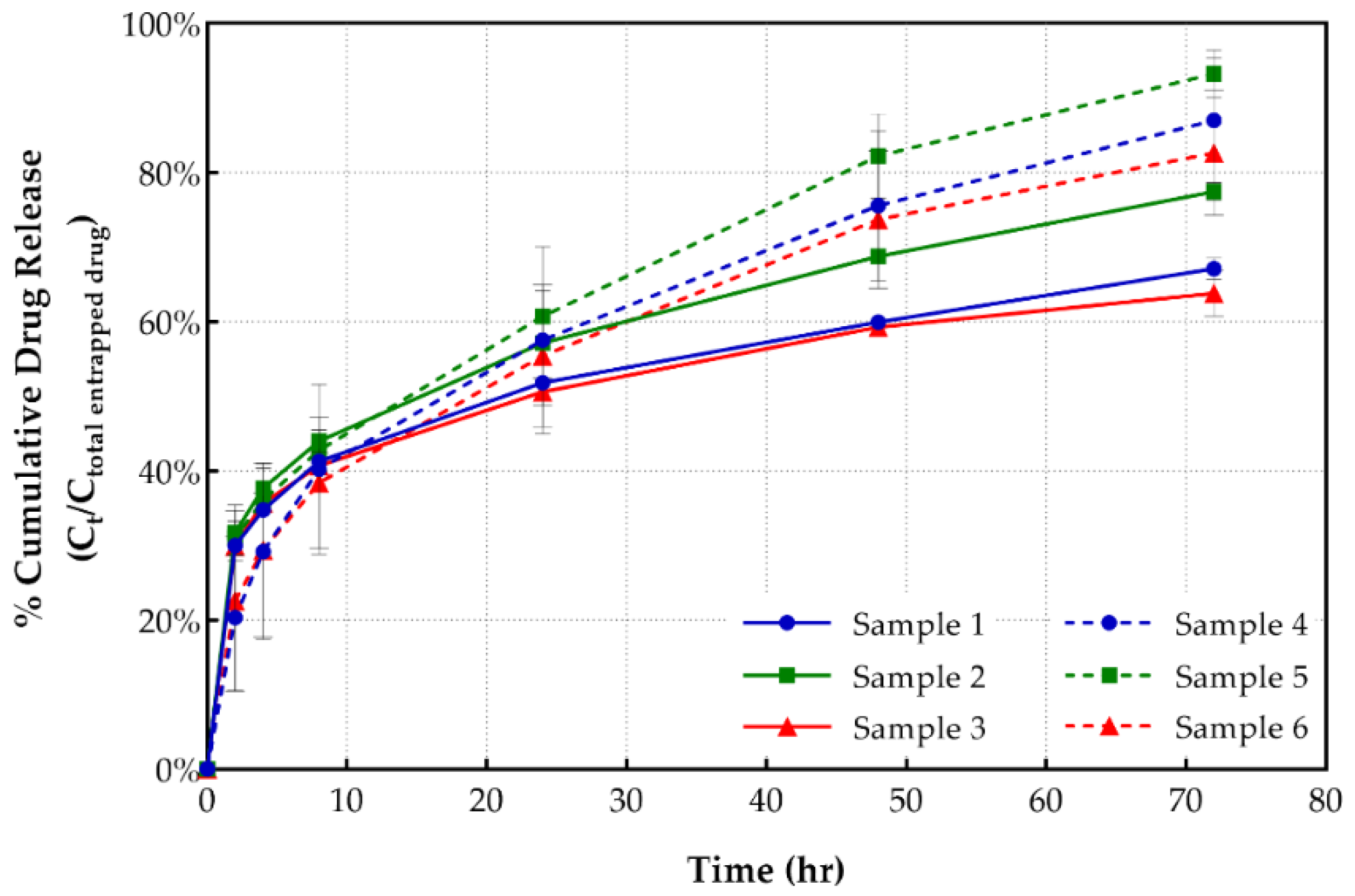
The drug release profiles of all shikonin-loaded formulations were assessed (as depicted in Figure 3). In addition, we calculated the amount (%) of shikonin released, in regard to the total entrapped drug, after 8 and 72 h, as well as the required time period to release 50% of the total released drug (t50%) (as shown in Table 3).
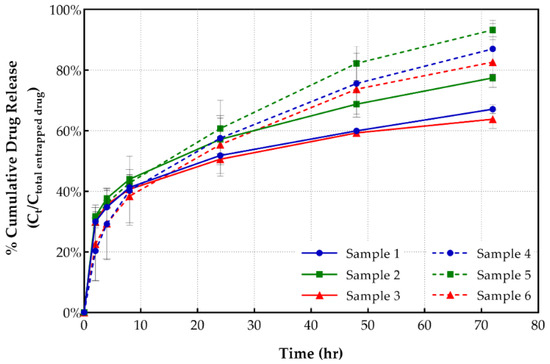
Figure 3.
Cumulative release from PEGylated and conventional liposomes vs time.

Table 3.
Release data of shikonin-loaded formulations.
As depicted in Table 3, PEGylated liposomes (samples 4–6) released approximately 20%–30% more shikonin compared to the corresponding conventional formulations (samples 1–3). In addition, Figure 3 depicts an interaction between the shikonin release rate and the type of lipid used. Briefly, samples with DOPC lipid released a statistically greater amount of drug (87%) after 72 h, in comparison to similar, previously reported shikonin-loaded liposomal formulations (60.1% for samples with EPC, 63.3% for samples with DSPC and 66.9% for samples with DPPC) [29]. The addition of DSPG seems to improve the release profile of all formulations, increasing the amount of drug released over a 72 h period.
Conventional samples, on the other hand, appear to follow the same trend, with DOPC liposomes releasing a greater amount of shikonin (67.1%) compared to other formulations with EPC (20.9% release), DSPC (51.4% release) and DPPC (47.5% release) prepared by Kontogiannopoulos et. al. [29]. Moreover, DSPG increased the amount of drug released from conventional liposomes, as well.
As shown in Table 3, the type of lipid used also influenced the required time period to release 50% of the total released drug (t50%). Briefly, the t50% for conventional samples ranged between 3.6 and 7.0 h and between 17.9 and 18.5 h for the corresponding PEGylated liposomes. In both cases, the DOPC:DSPG mixture exhibited the higher value. These observations, are in line with the report that longer alkyl chain lipids enhance the binding of the drug with the lipid bilayer, resulting in slower or sustained drug release [57].
The different behaviour in terms of the release rate between the PEGylated and conventional liposomes may be attributed to the bilayer rigidity—the more rigid the bilayer, the slower the release of the drug [50]. Thus, it can be assumed that shikonin’s release profile could be modified by the existence of PEG. Furthermore, differences among the prepared formulations could be attributed to several factors, such as lipid type and dose, interactions between the drug and lipid bilayer, as well as the lipid–PEG conjugate in the case of stealth liposomes [58]. Each type of phospholipid affects the efflux rate in a different way, e.g., a higher degree of saturation and an increased fatty acid chain-length, retarding the leakage rate of molecules from liposomes [37].
3.2. Stability Study
The stability of any pharmaceutical formulation is essential. Thus, all samples were maintained in their hydrated state for 28 days at 4 °C, while drug leakage, mean particle size, the polydispersity index (PDI) and the ζ-potential were monitored in order to assess their stability.
3.2.1. Particle Size Distribution
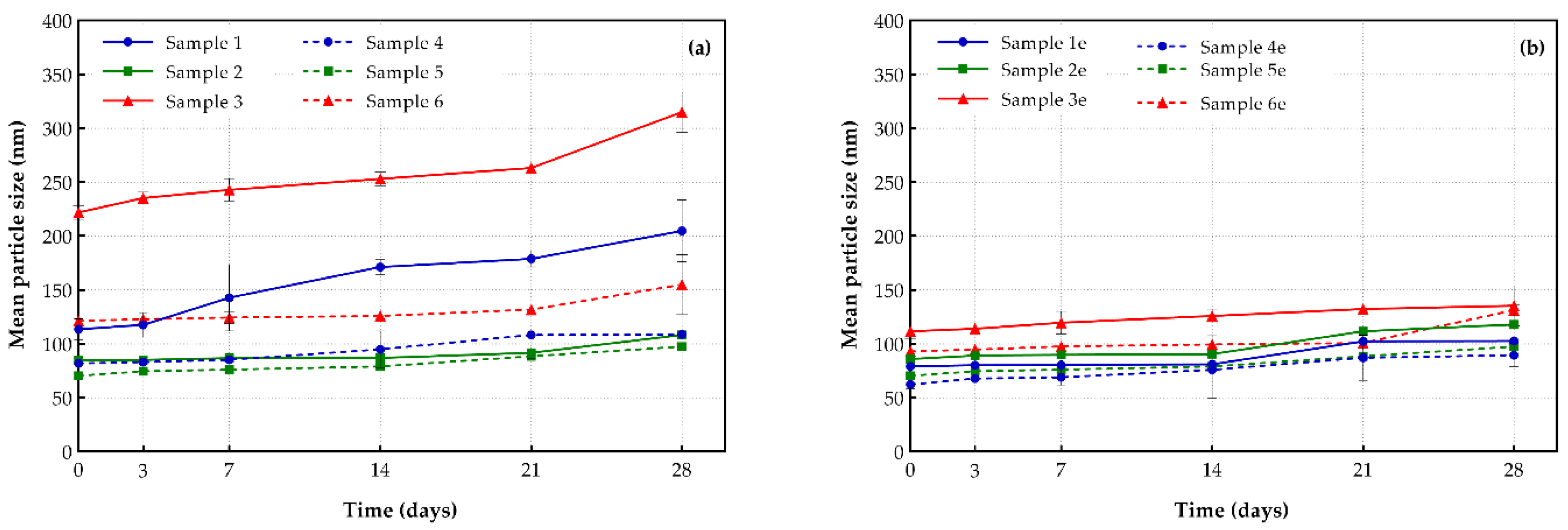
The results from the stability study depicted a significant variability in the mean particle size and PDI in the conventional shikonin-loaded formulations. On the contrary, PEGylated liposomes remained more stable after 28 days of preservation at 4 °C (Figure 4). Regarding conventional liposomes, sample 1 (prepared with DOPC) presented a size increment of 80.2%, which was higher than similar DSPC samples have shown (52.3% in particular) and lower than DPPC samples (104% increment) [29]. Moreover, when DSPG was added to DOPC (sample 2) or DSPC (sample 3) led to the formation of more stable conventional formulations (the observed size increases were 27.6% and 41.9%, respectively).
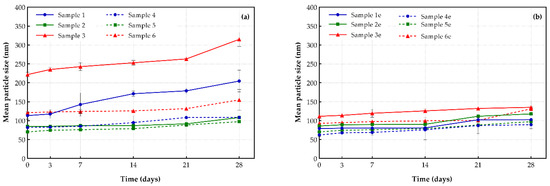
Figure 4.
Mean particle size stability of (a) drug-loaded and (b) drug-free liposomal formulations (storage conditions: 4 °C, 28 days).
Concerning the prepared PEGylated formulations, liposomes with DOPC (sample 4) were less stable (32.8% increment) than contiguous samples of DSPC, EPC and DPPC (increases of 9.5%, 2.5%, and 6%, respectively) from our previous work [29]. It is perceived that the addition of DSPG to DOPC (sample 5) or DSPC (sample 6) lipids, led to formulations with similar rates of increase in particle size (38.4% and 41.9%, respectively). It is worth mentioning that conventional shikonin-loaded liposomes prepared with DOPC/DSPG remained particularly stable in terms of their particle size distribution and PDI, with a final mean particle size (after their residence for 28 days at 4 °C) that was close enough to PEGylated systems.
Both PEGylated and conventional formulations appeared to be particularly stable until day 21, and a slight increment in their mean size was observed in the period from day 21 to day 28 (Figure 4). Finally, it was observed that the increased rate in mean particle size of “empty” DOPC/DSPG (sample 2e) and DOPC/DSPG-PEG (sample 5e) liposomes was slightly higher (38.3% and 59.5% respectively) compared to the corresponding drug-loaded systems (samples 2 and 5; 27.6% and 38.4% respectively).
These observations confirm the supremacy of PEGylated liposomes regarding their particle size distribution stability over conventional formulations.
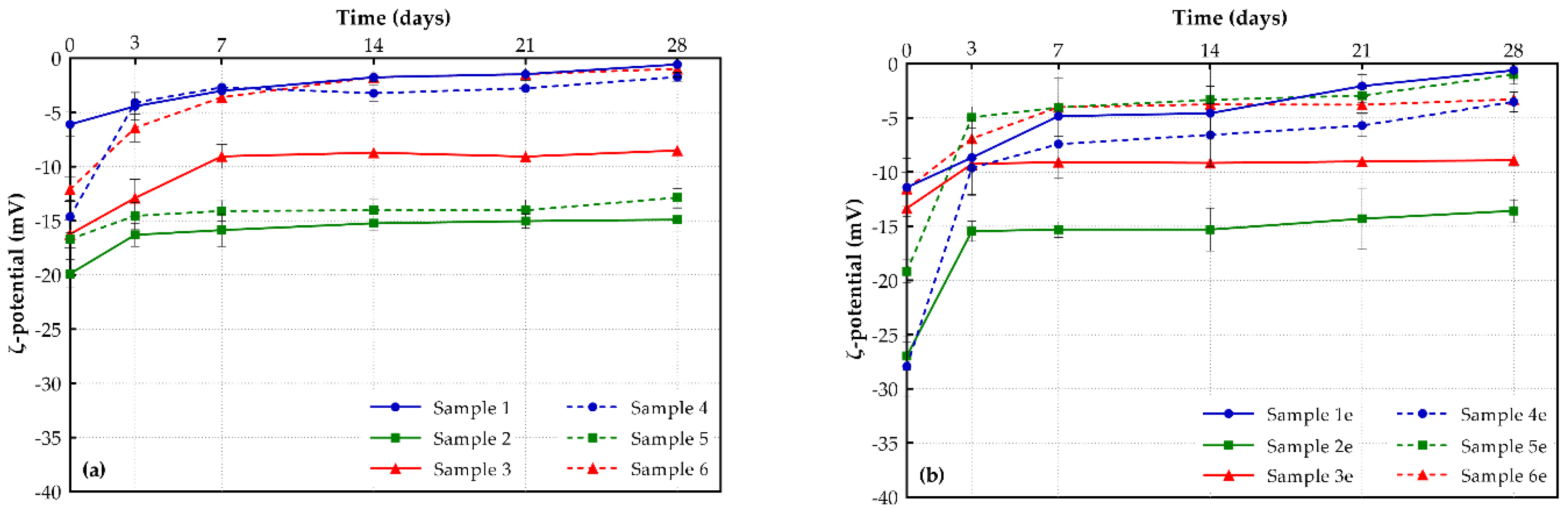
3.2.2. ζ-Potential
As depicted in Figure 5, the ζ-potential values of most samples remained stable (or reduced slightly) after their preservation at 4 °C. This indicates that the prepared samples maintained their initial charge, and as a result, their tendency for aggregation and flocculation was decreased. More precisely, DOPC conventional liposomes showed a lower rate of ζ-potential decrease (3.6%) in comparison to DSPC, EPC and DPPC (10.4%, 43.1%, and 30.5%, respectively) [29]. The addition of DSPG to DOPC and DSPC lipids led to similar rates of decrease (7.3% and 3.4%, respectively). Liposomes with DSPC/DSPG (sample 3) showed slight decreases in their ζ-potential values until day 7 and after that period, their ζ-potential values remained stable. On the other hand, DOPC/DSPG samples did not show any significant reduction during their residence at 4 °C for 28 days. Corresponding drug-free samples seemed to follow the same trend.
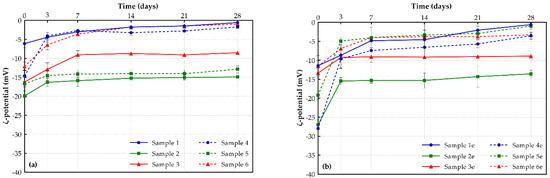
Figure 5.
ζ-potential values of (a) drug-loaded and (b) drug-free liposomal formulations (storage conditions: 4°C, 28 days).
PEGylated shikonin-loaded liposomes (samples 4, 5, and 6) showed similar ζ-potential reduction rates to the conventional ones (samples 1, 2 and 3). Briefly, formulations with DOPC lipids (sample 4) showed lower rates of ζ-potential decrease (15.4%), compared to DSPC, EPC and DPPC lipids (26.8%, 28.5%, and 29%, respectively [29]. Their ζ-potential values only decreased until day 3 and from then on, they remained stable. The same results arose when DSPG was added either to DOPC or DSPC lipids, with PEGylated liposomes showing similar ζ-potential reduction rates (5.6% and 9.5%, respectively).
The study of the corresponding “empty” formulations resulted in similar observations. However, the DOPC/DSPG lipid mixtures (samples 2e and 5e) showed different behavior. More precisely, their ζ-potential values increased after day 3, in contrast with the corresponding drug-loaded samples (2 and 5) where the ζ-potential values remained almost stable across the entire 28-day period. This may be attributed to the presence of shikonin that helps this specific lipid mixture to be more stable, maintaining low ζ-potential values and retaining the initial charge, and therefore, limiting the tendency for agglomeration and flocculation.
3.2.3. Drug Leakage
Since, drug leakage is a crucial parameter for drug delivery systems, all samples were assessed for it over the 28-day stability period at 4 °C.
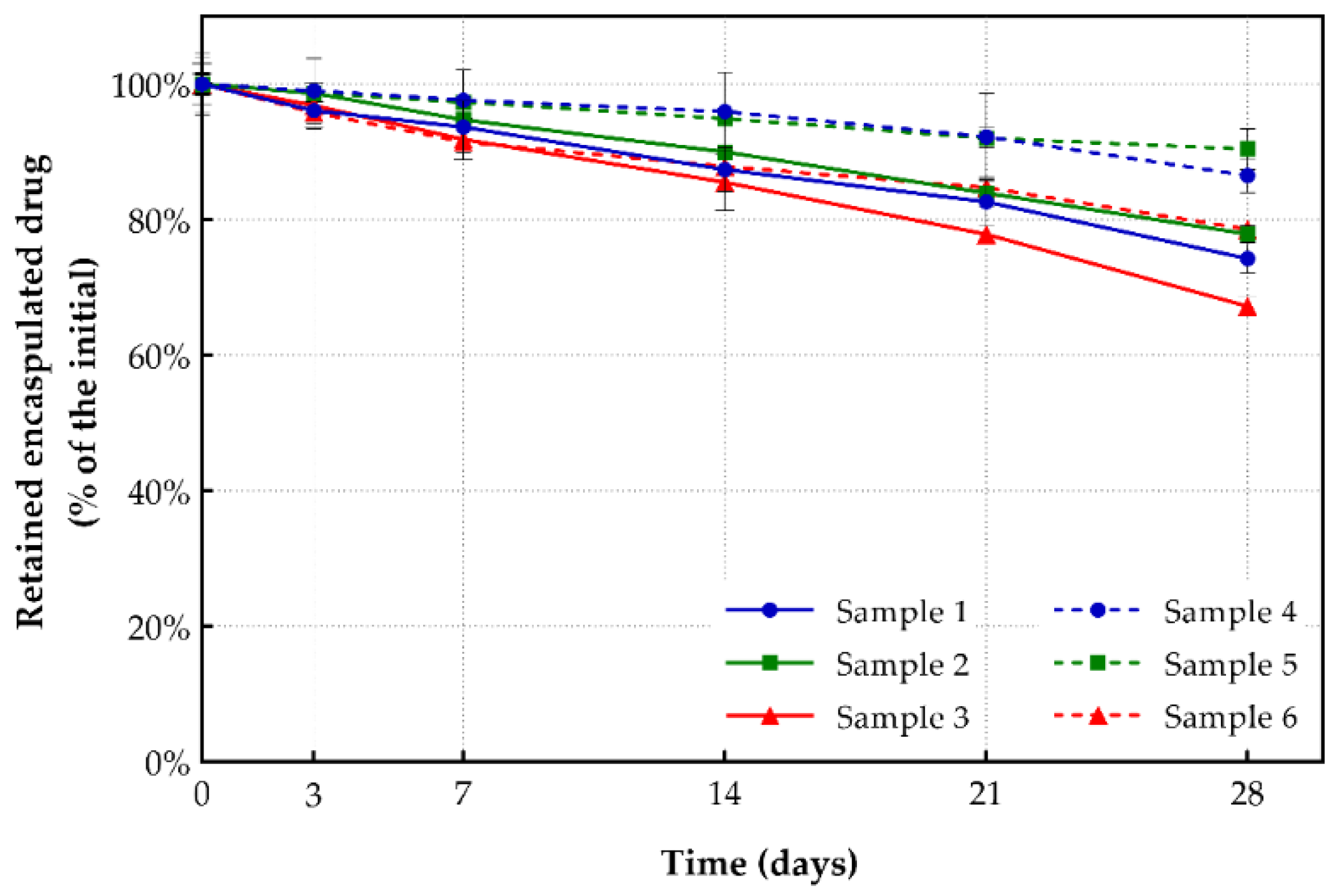
As depicted in Figure 6, all conventional shikonin-loaded liposomes (samples 1, 2 and 3) remained stable for 28 days, retaining more than 67.2% of their initially incorporated shikonin. More specifically, formulations prepared with DOPC lipid (sample 1) retained 74.3% of the initially incorporated drug, a significantly higher percentage compared to liposomes formed with DPPC lipid (62.3%) and a bit lower than those prepared with DSPC and EPC (81.4% and 83.1%, respectively) [29]. On the other hand, PEGylated formulations exhibited a higher amount of retention after 28 days, compared to all conventional samples, retaining, on average, ~85% of the initially incorporated drug. More precisely, the PEGylated liposomal formulation with DOPC lipids (sample 4) retained 86.5% of its initial drug content, almost the same as DSPC and EPC shikonin-loaded liposomes (85.2% and 86.4%) and a higher percentage of retention than DPPC (83.7%) [29]. In cases where DSPG was added to DOPC, there was an increment in drug stability for both conventional and PEGylated samples. On the contrary, if DSPG was mixed with DSPC (samples 3 and 6), the result was a higher drug leakage from the prepared liposomes.
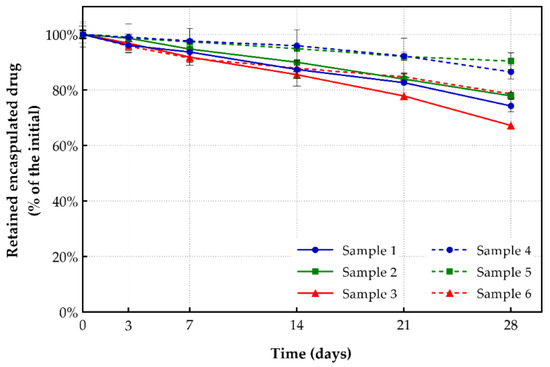
Figure 6.
Drug retention of drug-loaded liposomal formulations (storage conditions: 4 °C, 28 days).
4. Conclusions
Liposomes represent an advanced nanoscale drug delivery system that could deliver bioactive constituents to a specific site of action. Alkannin, shikonin and their derivatives have proved to be potent chemotherapeutic and chemo-preventive agents that should be further exploited as novel chemotherapeutics and for effective combination chemotherapy. Therefore, drug delivery systems for shikonin need to be further developed and optimized to provide effective and safe administration and this paper is a continuation of the research of our group in this direction.
Shikonin-loaded conventional and PEGylated liposomes were successfully prepared using three types of lipid (DOPC, DOPC/DSPG, and DSPC/DSPG). All samples were assessed for their physicochemical characteristics, entrapment efficiency, drug release and stability during residence at 4 °C for 28 days.
All shikonin-loaded liposomes showed desirable drug entrapment efficiencies, varying from 66.9 to 89.4% for PEGylated types, to 56.5 to 78.4% for conventional systems, regardless of the type of lipid used. The use of negatively charged lipid (DSPG) combined with DOPC, increased incorporation efficiency values and helped to form liposomes with reduced particle sizes. Furthermore, both conventional and PEGylated shikonin-loaded liposome with DOPC/DSPG lipids remained particularly stable in terms of their particle size distribution and ζ-potential. Even after 28 days of residence at 4 °C, PEGylated shikonin-loaded formulations preserved more than 85% (on average) of the initially incorporated drug.
The presence of DSPE-mPEG2000 in the outer surface of the lipid bilayer significantly modified the characteristics of formulations. More specifically, DSPE-mPEG2000 caused a decrease in the mean particle size, regardless of the type of lipid used (reduction varied between 17%–45% for drug-loaded liposomes and 17%–21% for drug-free liposomes). The minimum liposome size was obtained with DOPC/DSPG lipids. Furthermore, PEGylated samples showed higher entrapment efficiencies compared to conventional samples (increments varied from 13 to 18%) and higher values were obtained with DOPC/DSPG lipids. Concerning drug release profiles, PEGylated formulations released approximately 20%–30% more shikonin over a prolonged time period, compared to the corresponding conventional formulations.
In conclusion, PEGylated formulations appear to be more advantageous than conventional ones and should be further exploited to increase the therapeutic index of shikonin. The addition of the charged lipid, DSPG, improved, in most cases, the physicochemical and pharmaceutical characteristics of liposomes. Conventional shikonin-loaded liposomes prepared with DOPC/DSPG showed the most promising results and stability (in terms of almost all parameters) compared to all other conventional shikonin-loaded formulations studied so far, with characteristics close enough to PEGylated systems. Respectively, the shikonin-loaded PEGylated sample with DOPC/DSPG lipids, showed the most satisfactory characteristics among all studied samples so far from our group. However, such systems are much too complicated and should be further examined in terms of their physicochemical interactions.
Author Contributions
Vassilios P. Papageorgiou and Andreana N. Assimopoulou had the initial conception of this work; Konstantinos N. Kontogiannopoulos designed the experiments; Stella K. Tsermentseli performed the experiments and acquired the data; Konstantinos N. Kontogiannopoulos analyzed the data, performed the visualization and wrote the original draft; Andreana N. Assimopoulou and Vassilios P. Papageorgiou had the overall supervision and project administration as well as they revised the manuscript. This work was part of the Ph.D. thesis of S. K. Tsermentseli.
Acknowledgments
The authors would like to thank Lipoid Company (Lipoid GmbH, Ludwigshafen, Germany) for generously donating 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-distearoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt) (DSPG). The authors are grateful to Em. Professor Constantinos Kiparissides (School of Chemical Engineering, AUTh) and Associate Professor Dimitrios Fatouros (School of Pharmacy, AUTh) for giving open access to their laboratories.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ordoudi, S.A.; Tsermentseli, S.K.; Nenadis, N.; Assimopoulou, A.N.; Tsimidou, M.Z.; Papageorgiou, V.P. Structure-radical scavenging activity relationship of alkannin/shikonin derivatives. Food Chem. 2011, 124, 171–176. [Google Scholar] [CrossRef]
- Papageorgiou, V.P. Naturally occurring isohexenylnaphthazarin pigments: A new class of drugs. Planta Med. 1980, 38, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Ballis, A.C. Alkannins and shikonins: A new class of wound healing agents. Curr. Med. Chem. 2008, 15, 3248–3267. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 1999, 38, 270–300. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Samanidou, V.F.; Papadoyannis, I.N. Recent advances in chemistry, biology and biotechnology of alkannins and shikonins. Curr. Org. Chem. 2006, 10, 2123–2142. [Google Scholar] [CrossRef]
- Karapanagioti, E.G.; Assimopoulou, A.N. Naturally occurring wound healing agents: An evidence-based review. Curr. Med. Chem. 2016, 23, 3285–3321. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jia-Hua, C.; Qing-Qing, M.; Shao-Shun, L.; Wen, Z.; Sui, X. Advance in anti-tumor mechanisms of shikonin, alkannin and their derivatives. Mini-Rev. Med. Chem. 2018, 18, 164–172. [Google Scholar]
- Xie, Y.; Hou, X.L.; Wu, C.L. The research progress of cell apoptosis induced by shikonin and signal pathway of apoptosis. Chin. J. Pharm. Biotechnol. 2016, 23, 173–178. [Google Scholar]
- Zhao, Q.; Assimopoulou, A.N.; Klauck, S.M.; Damianakos, H.; Chinou, I.; Kretschmer, N.; Rios, J.L.; Papageorgiou, V.P.; Bauer, R.; Efferth, T. Inhibition of c-MYC with involvement of ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and its derivatives in killing leukemia cells. Oncotarget 2015, 6, 38934–38951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-Y.; Hu, Y.; Que, Z.-Y.; Wang, P.; Liu, Y.-H.; Wang, Z.-H.; Xue, Y.-X. Shikonin inhibits the migration and invasion of human glioblastoma cells by targeting phosphorylated β-catenin and phosphorylated PI3K/Akt: A potential mechanism for the anti-glioma efficacy of a traditional chinese herbal medicine. Int. J. Mol. Sci. 2015, 16, 23823–28848. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Zhang, B.; Yao, J.; Liu, Y.; Fang, J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic. Biol. Med. 2014, 70, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Wiench, B.; Eichhorn, T.; Paulsen, M.; Efferth, T. Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 726025. [Google Scholar] [CrossRef] [PubMed]
- Spyrelli, E.D.; Kyriazou, A.V.; Virgiliou, C.; Nakas, A.; Deda, O.; Papageorgiou, V.P.; Assimopoulou, A.N.; Gika, H.G. Metabolic profiling study of shikonin’s cytotoxic activity in the Huh7 human hepatoma cell line. Mol. BioSyst. 2017, 13, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q. Shikonin and NRF2 Chemoprevention; University of Maryland Baltimore; National Cancer Institute: Baltimore, MD, USA, 2014. Available online: http://projectreporter.nih.gov/project_info_description.cfm?aid=8685189 (accessed on 25 May 2018).
- Ni, F.; Huang, X.; Chen, Z.; Qian, W.; Tong, X. Shikonin exerts antitumor activity in Burkitt’s lymphoma by inhibiting C-MYC and PI3K/AKT/mTOR pathway and acts synergistically with doxorubicin. Sci. Rep. 2018, 8, 3317. [Google Scholar] [CrossRef] [PubMed]
- Kozako, T.; Arima, N.; Yoshimitsu, M.; Honda, S.I.; Soeda, S. Liposomes and nanotechnology in drug development: Focus on oncotargets. Int. J. Nanomed. 2012, 7, 4943–4951. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Rhee, J.W.; Richie, J.P.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. New frontiers in nanotechnology for cancer treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Wills, E.J.; Swain, C.P.; Tavill, A.S. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet 1974, 1, 1313–1316. [Google Scholar] [CrossRef]
- Gumulec, J.; Fojtu, M.; Raudenska, M.; Sztalmachova, M.; Skotakova, A.; Vlachova, J.; Skalickova, S.; Nejdl, L.; Kopel, P.; Knopfova, L.; et al. Modulation of induced cytotoxicity of doxorubicin by using apoferritin and liposomal cages. Int. J. Mol. Sci. 2014, 15, 22960–22977. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Polanska, H.; Merlos Rodrigo, M.A.; Guran, R.; Kulich, P.; Kopel, P.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Kizek, R.; et al. Prostate tumor attenuation in the nu/nu murine model due to anti-sarcosine antibodies in folate-targeted liposomes. Sci. Rep. 2016, 6, 33379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.C.; Lasic, D.D. Sterically stabilized liposomes. Biochim. Biophys. Acta 1992, 1113, 171–199. [Google Scholar] [CrossRef]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–744. [Google Scholar] [PubMed]
- Andresen, T.L.; Jensen, S.S.; Jørgensen, K. Advanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug release. Prog. Lipid Res. 2005, 44, 68–97. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.H.; Alzghari, S.K.; Chee, W.; Sankari, S.S.; La-Beck, N.M. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control. Release 2016, 232, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Tang, C.; Gui, H.; Wang, X.; Qi, J.; Wang, X.; Yang, Y. Preparation, cellular uptake and angiogenic suppression of shikonin-containing liposomes in vitro and in vivo. Biosci. Rep. 2013, 33, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Dimas, K.; Papageorgiou, V.P. Shikonin–loaded liposomes as a new drug delivery system: Physicochemical characterization and in vitro cytotoxicity. Eur. J. Lipid Sci. Technol. 2011, 113, 1113–1123. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Tsermentseli, S.K.; Assimopoulou, A.N.; Papageorgiou, V.P. Sterically stabilized liposomes as a potent carrier for shikonin. J. Liposome Res. 2014, 24, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; Shin, H.J.; Ha, D.H. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. Eur. J. Pharm. Sci. 2012, 46, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Crosasso, P.; Ceruti, M.; Brusa, P.; Arpicco, S.; Dosio, F.; Cattel, L. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J. Control. Release 2000, 63, 19–30. [Google Scholar] [CrossRef]
- Kelly, C.; Jefferies, C.; Cryan, S.A. Targeted liposomal drug delivery to monocytes andmacrophages. J. Drug Deliv. 2011, 2011, 727241. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Ganzera, M.; Stuppner, H.; Papageorgiou, V.P. Simultaneous determination of monomeric and oligomeric alkannins and shikonins by high-performance liquid chromatography–diode array detection–mass spectrometry. Biomed. Chromatogr. 2008, 22, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Krasnici, S.; Werner, A.; Eichhorn, M.E.; Schmitt-Sody, M.; Pahernik, S.A.; Sauer, B.; Schulze, B.; Teifel, M.; Michaelis, U.; Naujoks, K.; et al. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int. J. Cancer 2003, 105, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Lim, H.J.; Shim, J.; Kim, J.; Chang, I.S. Improved stability of liposome in oil/water emulsion by association of amphiphilic polymer with liposome and its effect on bioactive skin permeation. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 160–168. [Google Scholar] [CrossRef]
- Gardikis, K.; Hatziantoniou, S.; Bucos, M.A.; Fessas, D.; Signorelli, M.; Felekis, T.; Zervou, M.; Screttas, C.G.; Steele, B.R.; Ionov, M.; et al. New drug delivery nanosystem combining liposomal and dendrimeric technology (liposomal locked-in dendrimers) for cancer therapy. J. Pharm. Sci. 2010, 99, 3561–3571. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Colloidal soft matter as drug delivery system. J. Pharm. Sci. 2009, 98, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Guidance for Industry Liposome Drug Products. Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation; Center for Drug Evaluation and Research (CDER), U.S. Department of Health and Human Services, Food and Drug Administration: Washington, DC, USA, 2002.
- Tenzel, R.A.; Aitcheson, D.F. Preparation of uniform-size liposomes and other lipid structures. WO1989011335A1, 30 November 1989. [Google Scholar]
- Dadashzadeh, S.; Mirahmadi, N.; Babaei, M.H.; Vali, A.M. Peritoneal retention of liposomes: Effects of lipid composition, PEG coating and liposome charge. J. Control. Release 2010, 148, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sriwongsitanont, S.; Ueno, M. Effect of a PEG lipid (DSPE-PEG2000) and freeze-thawing process on phospholipid vesicle size and lamellarity. Colloid Polym. Sci. 2004, 282, 753–760. [Google Scholar] [CrossRef]
- Shenoy, V.S.; Gude, R.P.; Murthy, R.S.R. Investigations on paclitaxel loaded HSPC based conventional and PEGylated liposomes: In vitro release and cytotoxic studies. Asian J. Pharm. Sci. 2011, 6, 1–7. [Google Scholar]
- Muller, R.H. Colloidal Carriers for Controlled Drug Delivery and Targeting: Modification, Characterization, and in vivo Distribution; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Gentile, E.; Cilurzo, F.; Di Marzio, L.; Carafa, M.; Ventura, C.A.; Wolfram, J.; Paolino, D.; Celia, C. Liposomal chemotherapeutics. Future Oncol. 2013, 9, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.A.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Cui, F.D.; Choi, M.K.; Cho, J.W.; Chung, S.J.; Shim, C.K.; Kim, D.D. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. Int. J. Pharm. 2007, 338, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Vali, A.M.; Toliyat, T.; Shafaghi, B.; Dadashzadeh, S. Preparation, optimization, and characterization of topotecan loaded PEGylated liposomes using factorial design. Drug Dev. Ind. Pharm. 2008, 34, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Mourelatou, E.A.; Libster, D.; Nir, I.; Hatziantoniou, S.; Aserin, A.; Garti, N.; Demetzos, C. Type and location of interaction between hyperbranched polymers and liposomes. Relevance to design of a potentially advanced drug delivery nanosystem (aDDnS). J. Phys. Chem. B 2011, 115, 3400–3408. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, D.G.; Antimisiaris, S.G. Effect of amphiphilic drugs on the stability and zeta-potential of their liposome formulations: A study with prednisolone, diazepam, and griseofulvin. J. Colloid Interface Sci. 2002, 251, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, S.; Vali, A.M.; Rezaie, M. The effect of PEG coating on in vitro cytotoxicity and in vivo disposition of topotecan loaded liposomes in rats. Int. J. Pharm. 2008, 353, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Huang, Y.Y. Encapsulating protein into preformed liposomes by ethanol-destabilized method. Artif. Cells Nanomed. Biotechnol. 2003, 31, 303–312. [Google Scholar] [CrossRef]
- Abra, R.M.; Mihalko, P.J.; Schreier, H. The effect of lipid composition upon the encapsulation and in vitro leakage of metaproterenol sulfate from 0.2 μm diameter, extruded, multilamellar liposomes. J. Control. Release 1990, 14, 71–78. [Google Scholar] [CrossRef]
- Colletier, J.P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef]
- Haeri, A.; Alinaghian, B.; Daeihamed, M.; Dadashzadeh, S. Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iran. J. Pharm. Res. 2014, 13, 3–14. [Google Scholar] [PubMed]
- Ho, E.A.; Osooly, M.; Strutt, D.; Masin, D.; Yang, Y.; Yan, H.; Bally, M. Characterization of long-circulating cationic nanoparticle formulations consisting of a two-stage PEGylation step for the delivery of siRNA in a breast cancer tumor model. J. Pharm. Sci. 2013, 102, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, X.; Guo, Y.; Zhang, Z. Lipid-enveloped hybrid nanoparticles for drug delivery. Nanoscale 2013, 5, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.Y.; Abbulu, K.; Sudhakar, M. Preparation, characterization and in-vitro release study of flurbiprofen loaded stealth liposomes. Chem. Sci. Trans. 2012, 1, 201–209. [Google Scholar] [CrossRef][Green Version]
- Pippa, N.; Psarommati, F.; Pispas, S.; Demetzos, C. The shape/morphology balance: A study of stealth liposomes via fractal analysis and drug encapsulation. Pharm. Res. 2013, 30, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).