Changes in Physical Parameters of CO2 Containing Impurities in the Exhaust Gas of the Purification Plant and Selection of Equations of State
Abstract
1. Introduction
2. Characteristics of Components Before and After Carbon Capture in the Exhaust Gas of X Purification Plant
2.1. Components of Hydrodesulfurization Exhaust Gas
2.2. Components After Carbon Capture
3. Phase State and Physical Property Test of CO2 Gas Containing Impurities
3.1. Experimental Test Samples and Methods
3.1.1. Experimental Test Sample
3.1.2. Experimental Setup and Testing Methods
3.2. Experimental Results and Physical Property Trends of Compression Factor
3.2.1. Experimental Results
3.2.2. Compression Factor Changes in CO2 Containing Impurities
- (1)
- The compression factor diminishes with the rising pressure and subsequently increases after reaching the critical value.
- (2)
- The lower the temperature, the smaller the critical compression factor for a given CO2 content, and the lower the critical pressure when the critical compression factor is reached.
- (3)
- At a constant temperature, the higher the CO2 content, the smaller the compression factor when the critical pressure is reached. Typically, the higher the CO2 content at the same pressure, the smaller the compression factor. Comparatively, the compression factor of pure CO2 is higher than that of 90% CO2 at 20 °C and 0 °C under the same pressure (provided that the pressure exceeds 15 MPa and 5 MPa, respectively) due to the fact that 90% CO2 contains more C2 and heavier components.
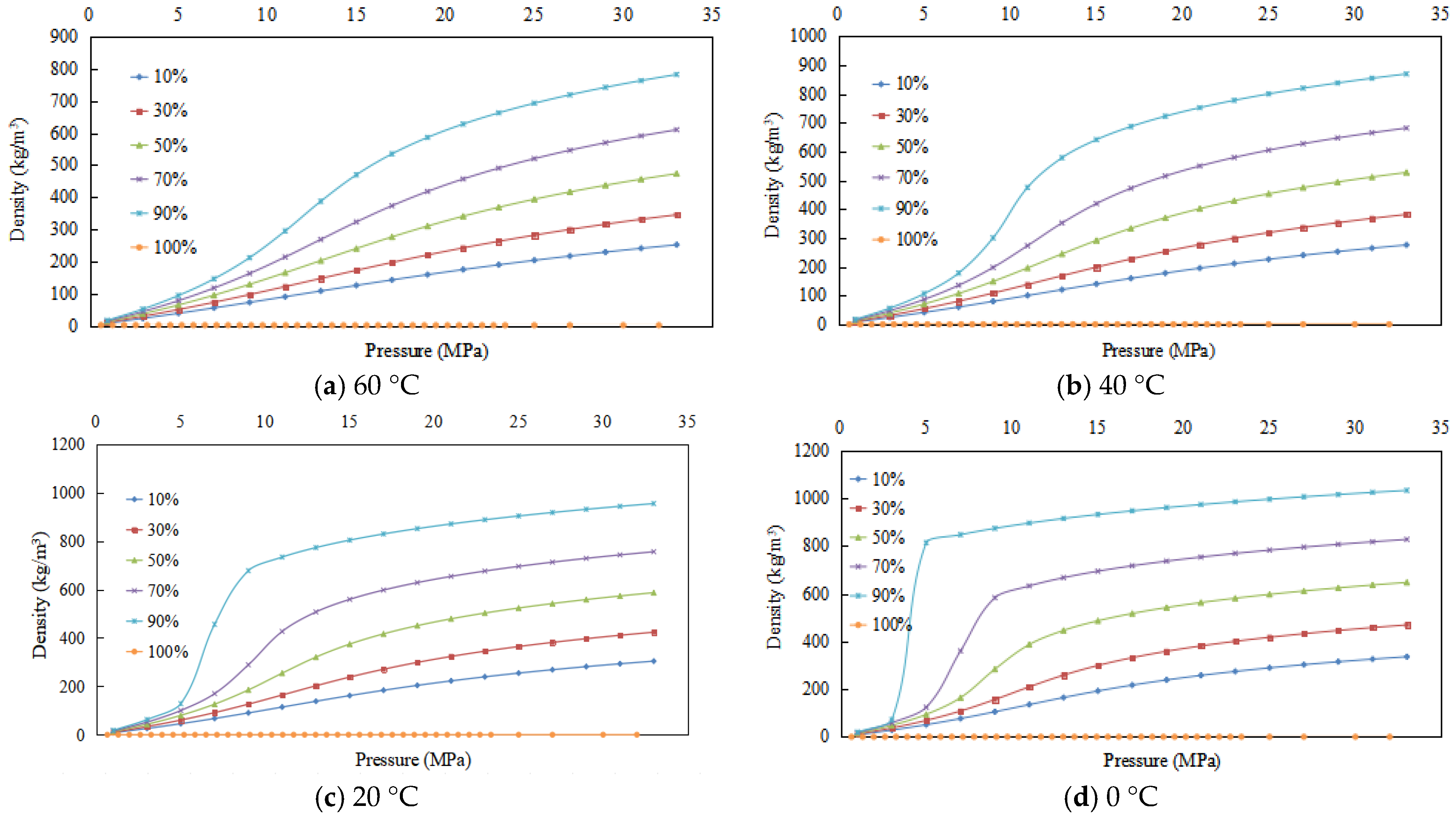
3.3. Experimental Results and Physical Property Trends of Density
3.3.1. Experimental Results
3.3.2. Density Changes of CO2 Containing Impurities
- (1)
- As pressure escalates, density rises at a faster rate, followed by a gradual deceleration.
- (2)
- The density of a given CO2 content is higher at lower temperatures.
- (3)
- At a constant temperature, for gas mixtures excluding pure CO2, higher CO2 content leads to a higher density under the same pressure. This phenomenon is linked to the presence of C2 and heavier hydrocarbons in the exhaust gas from purification plants.
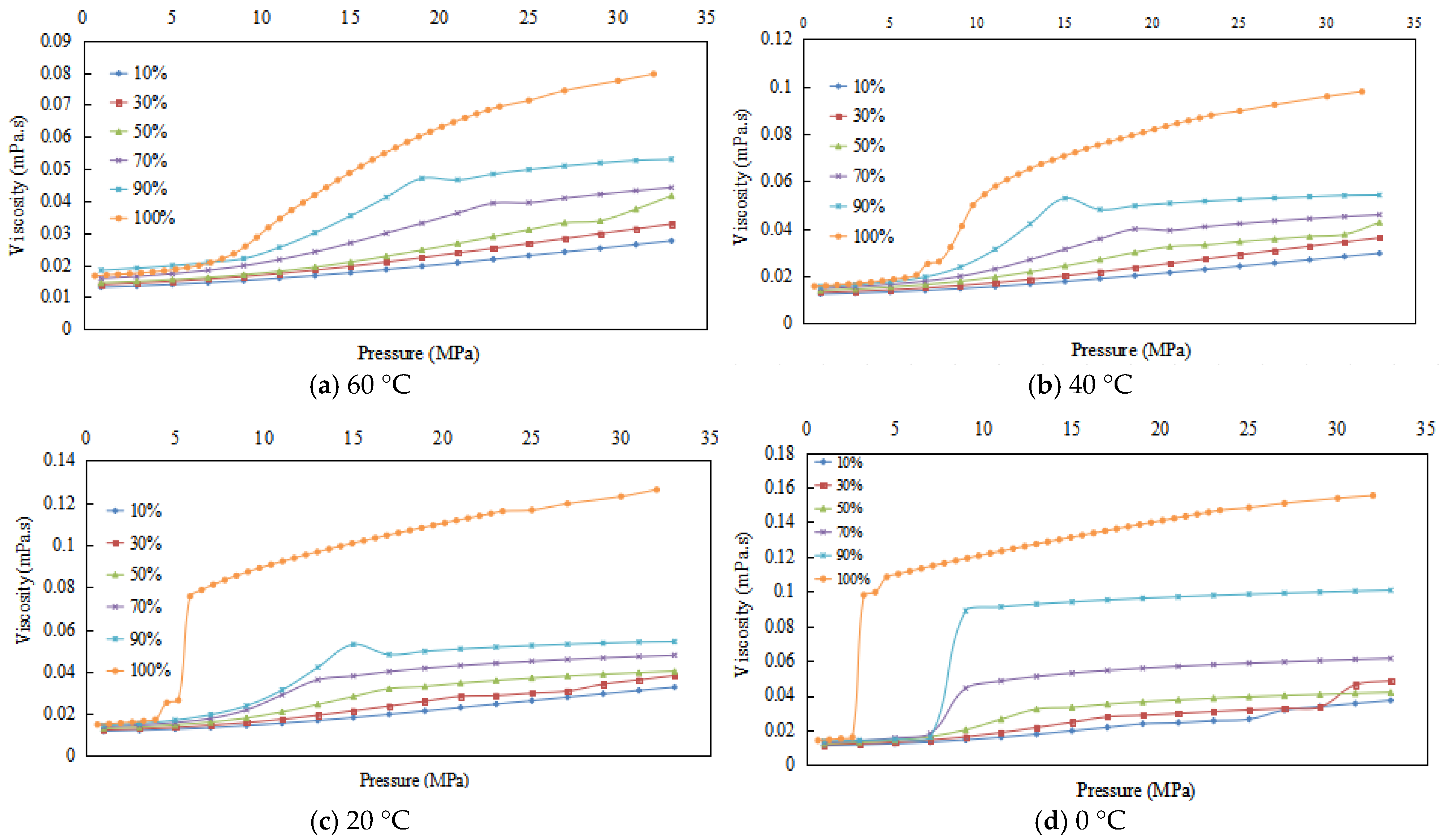
3.4. Experimental Results and Physical Property Trends in Viscosity
3.4.1. Experimental Results
3.4.2. Viscosity Changes of CO2 Containing Impurities
- (1)
- Viscosity increases with pressure, with the growth rate changing from slow to fast, then slowing again.
- (2)
- The lower the temperature, the higher the viscosity of the given CO2 content under the same pressure, and the lower the pressure at which viscosity growth slows.
- (3)
- At the same temperature, the higher the CO2 content, the higher the viscosity under the same pressure, and the lower the pressure at which viscosity growth slows.
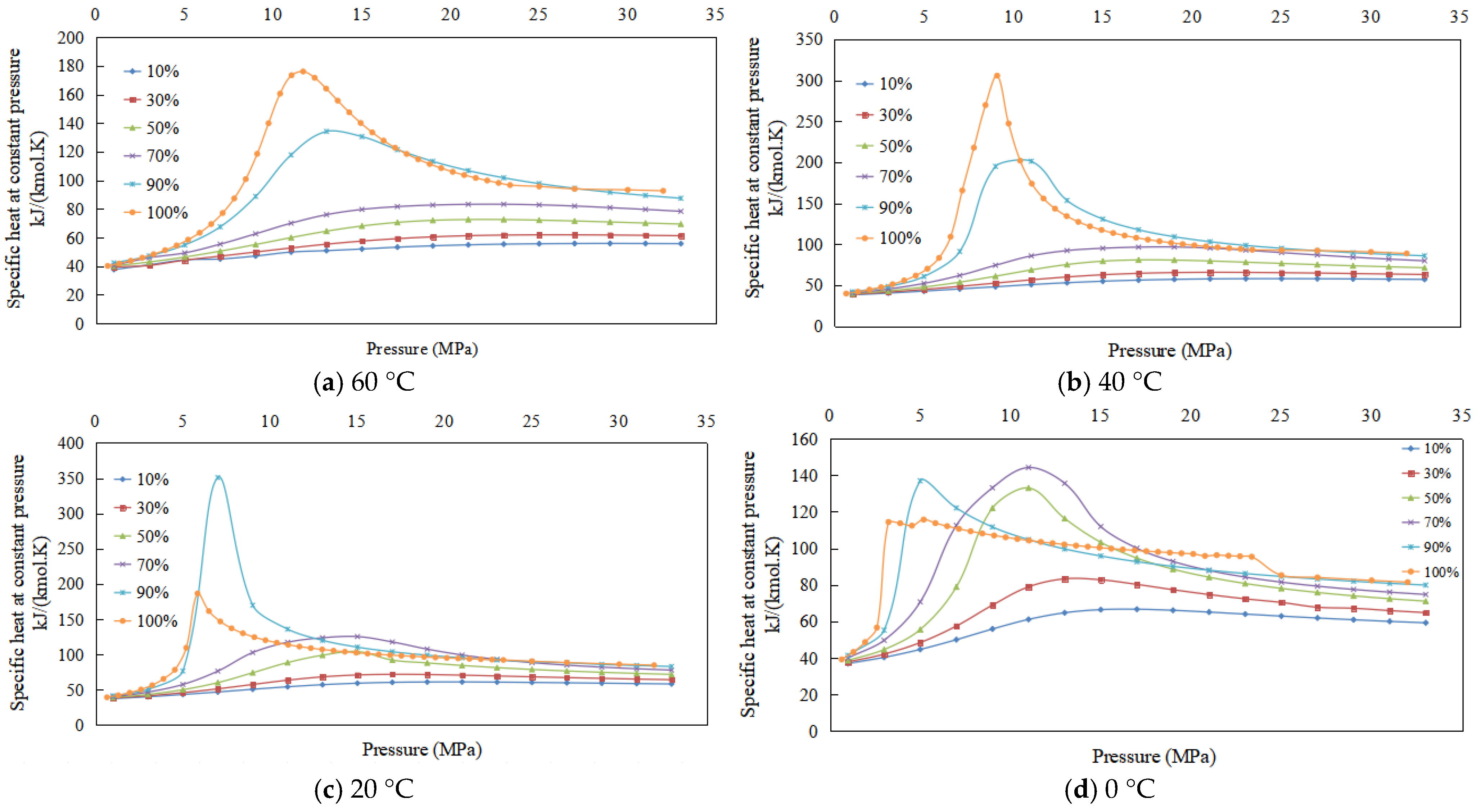
3.5. Experimental Results and Physical Property Trends of Specific Heat at Constant Pressure
3.5.1. Experimental Results
3.5.2. Changes in Specific Heat at Constant Pressure of CO2 Containing Impurities
- (1)
- Specific heat at constant pressure increases and then decreases with the increase in pressure.
- (2)
- The lower the temperature for a given CO2 content, the specific heat at constant pressure initially increases and subsequently decreases.
- (3)
- At the same temperature, the higher the CO2 content, the higher the specific heat at constant pressure under the same pressure; as the temperature decreases, the CO2 content of the highest specific heat at constant pressure decreases.
4. Calculation and Evaluation of Equations of State
4.1. Basic Parameters and Error Calculation Methods
4.2. Compression Factor Calculation
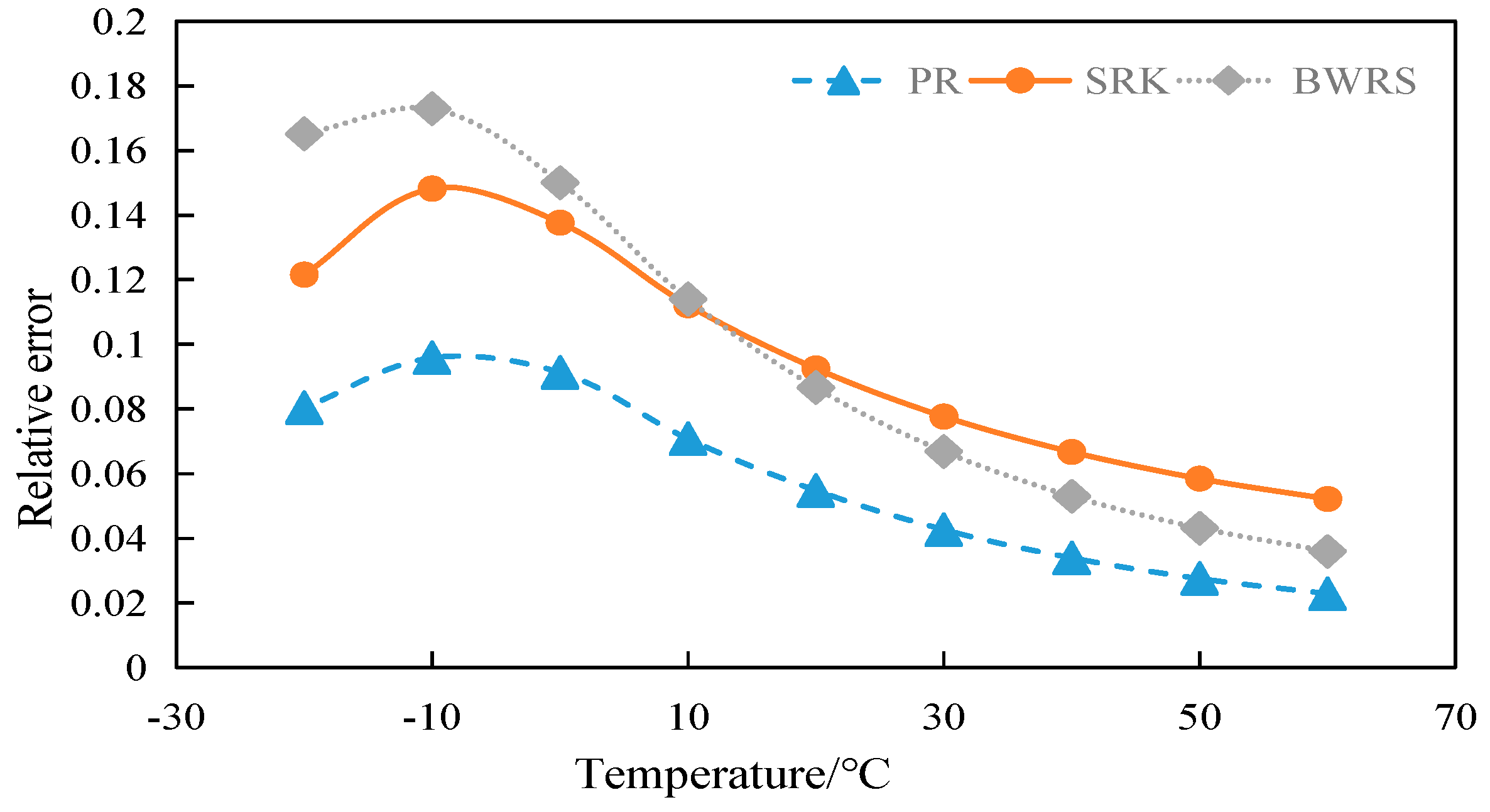
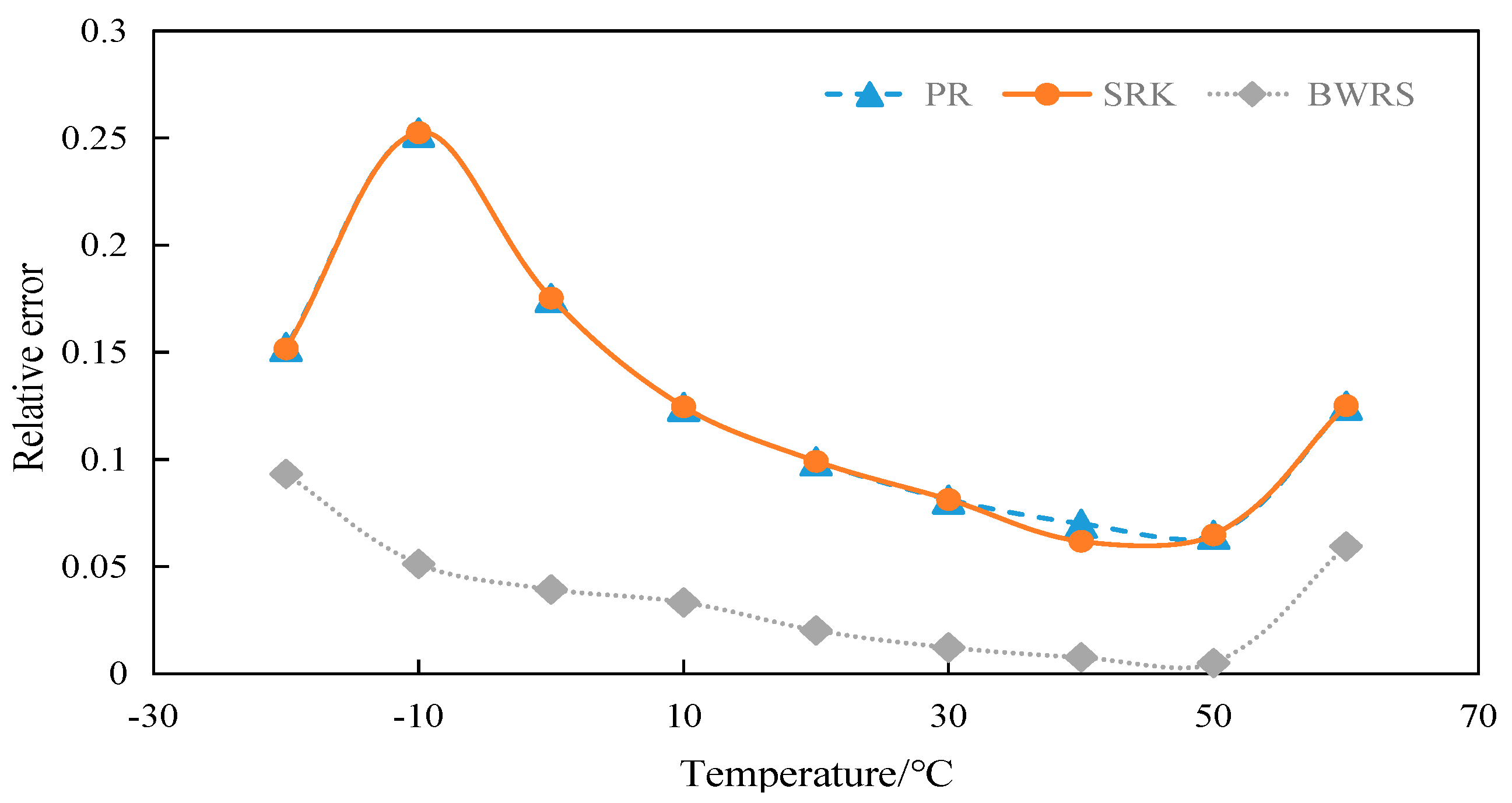
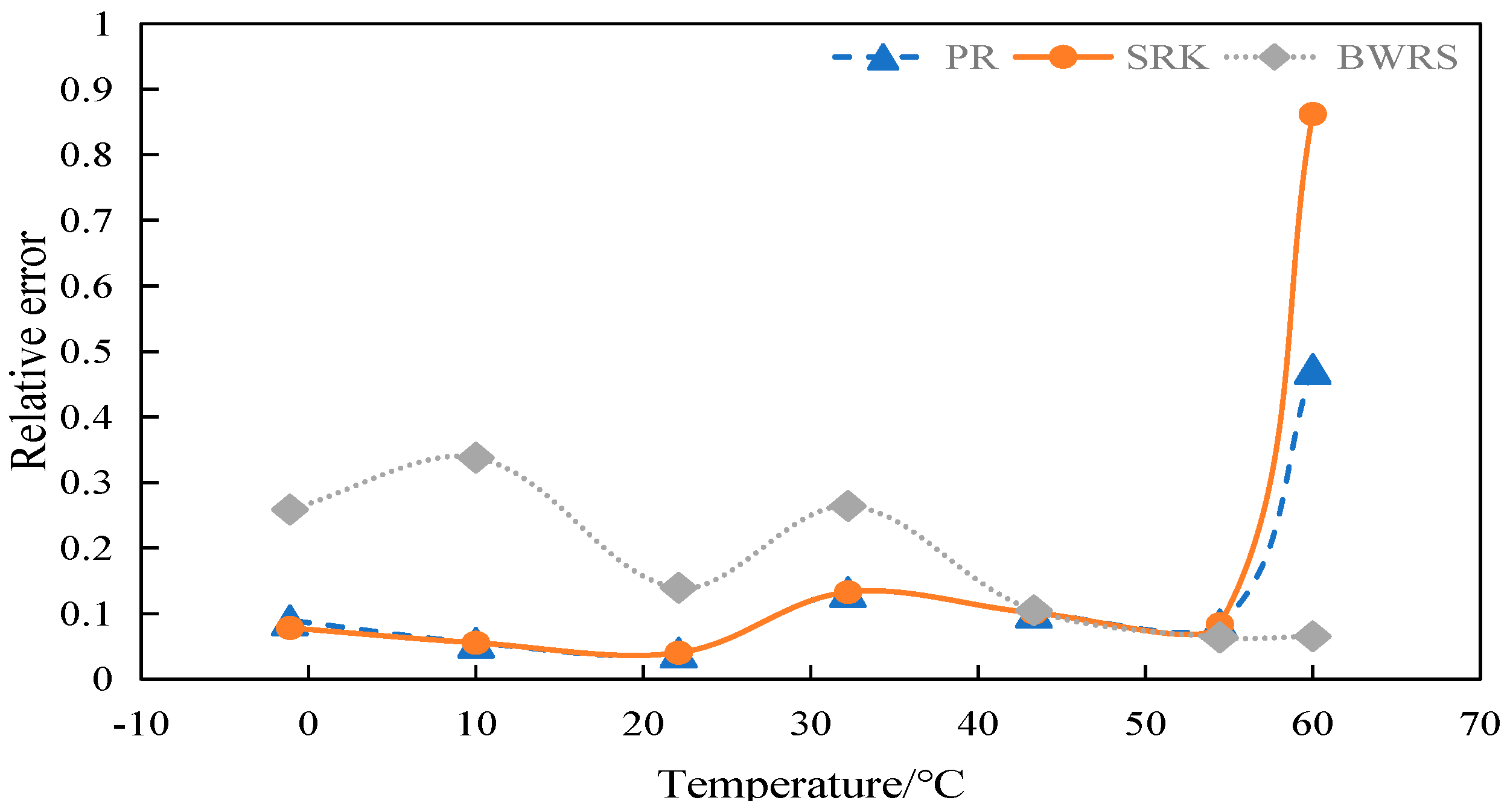
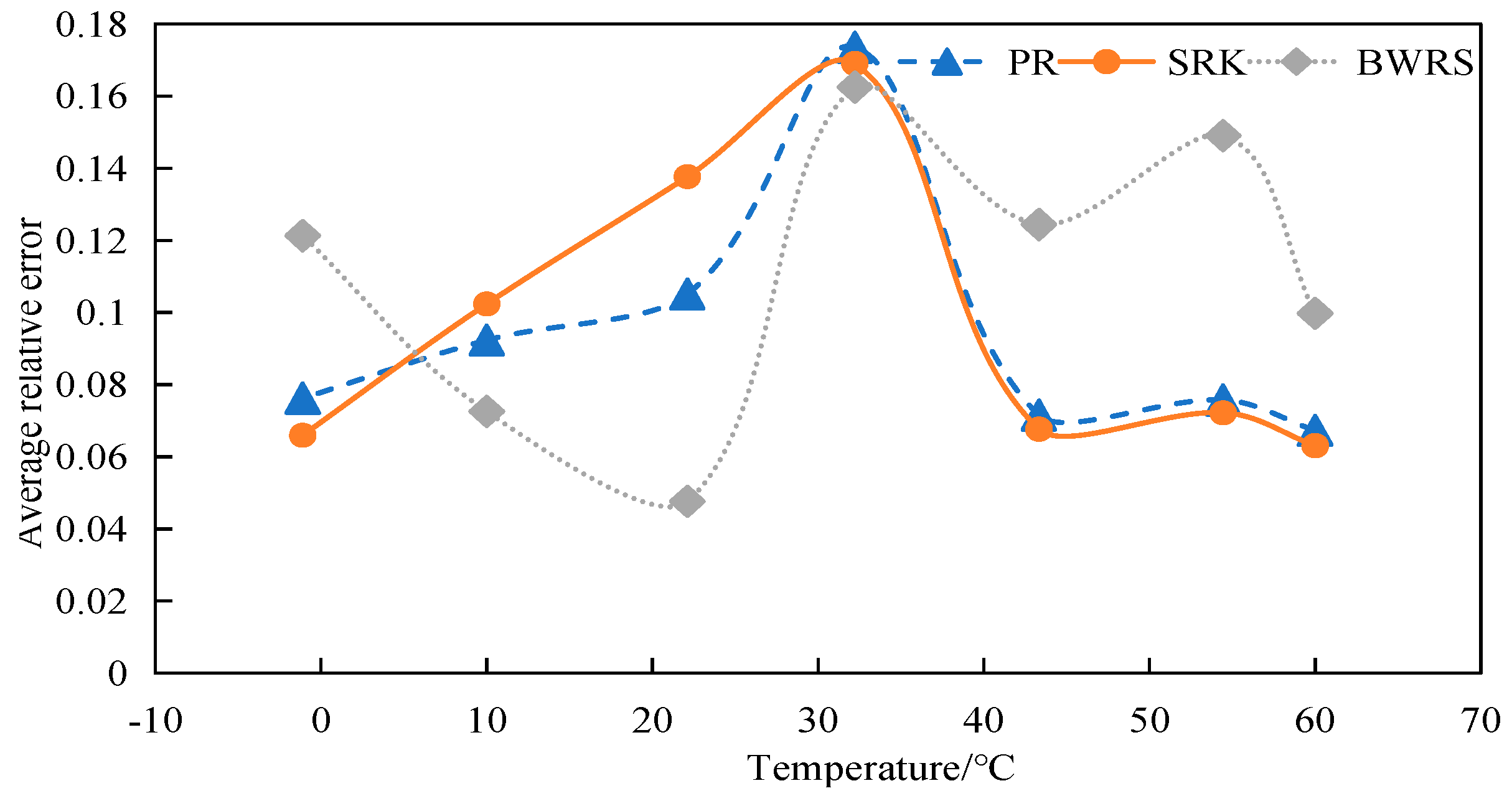
4.2.1. Calculation and Evaluation of Compression Factor of 50% CO2
4.2.2. Calculation and Evaluation of Compression Factor of 100% CO2
4.3. Density Calculation
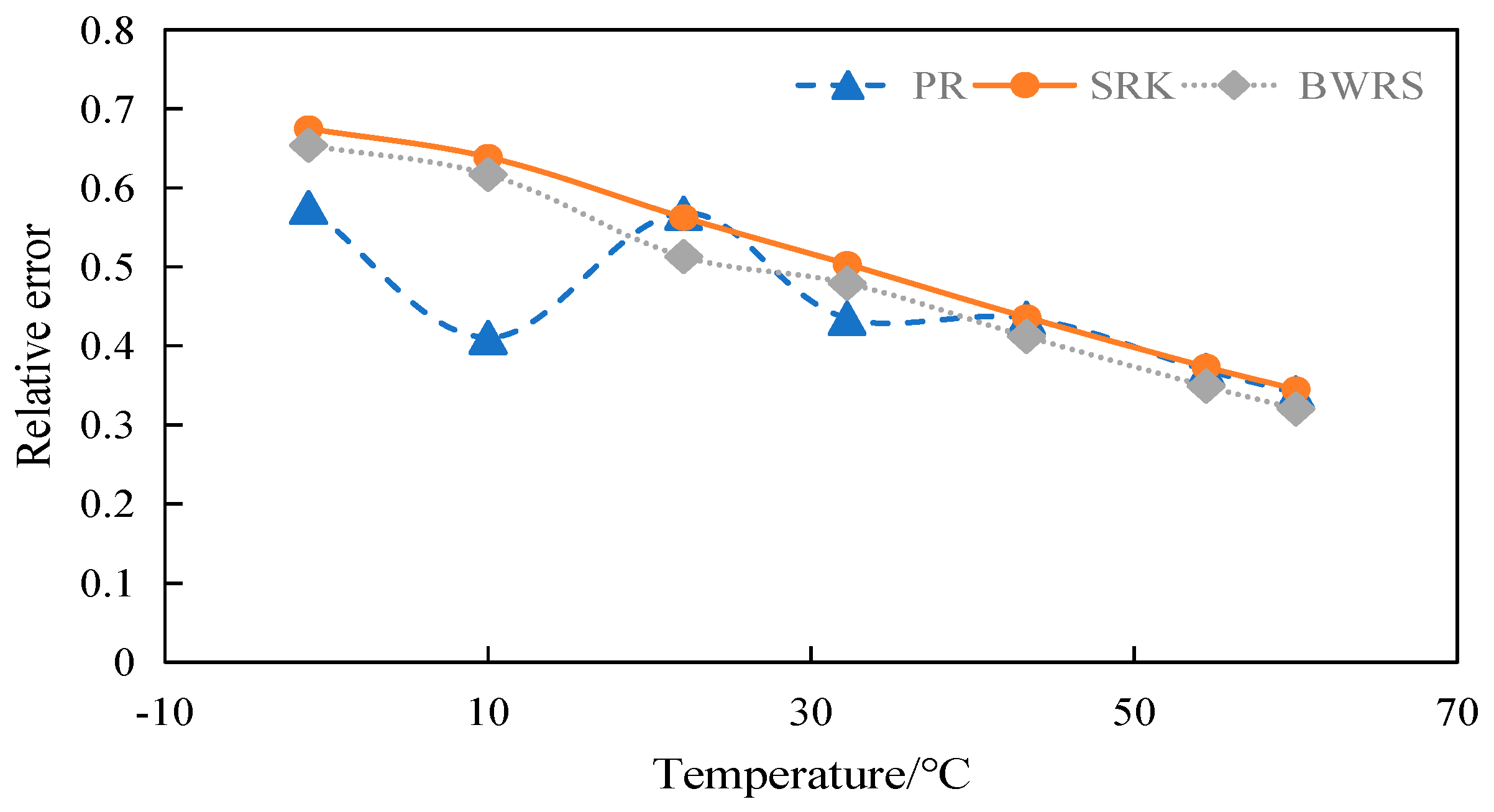
4.3.1. Calculation and Evaluation of Density of 50% CO2
4.3.2. Calculation and Evaluation of Density of 100% CO2
4.4. Viscosity Calculation
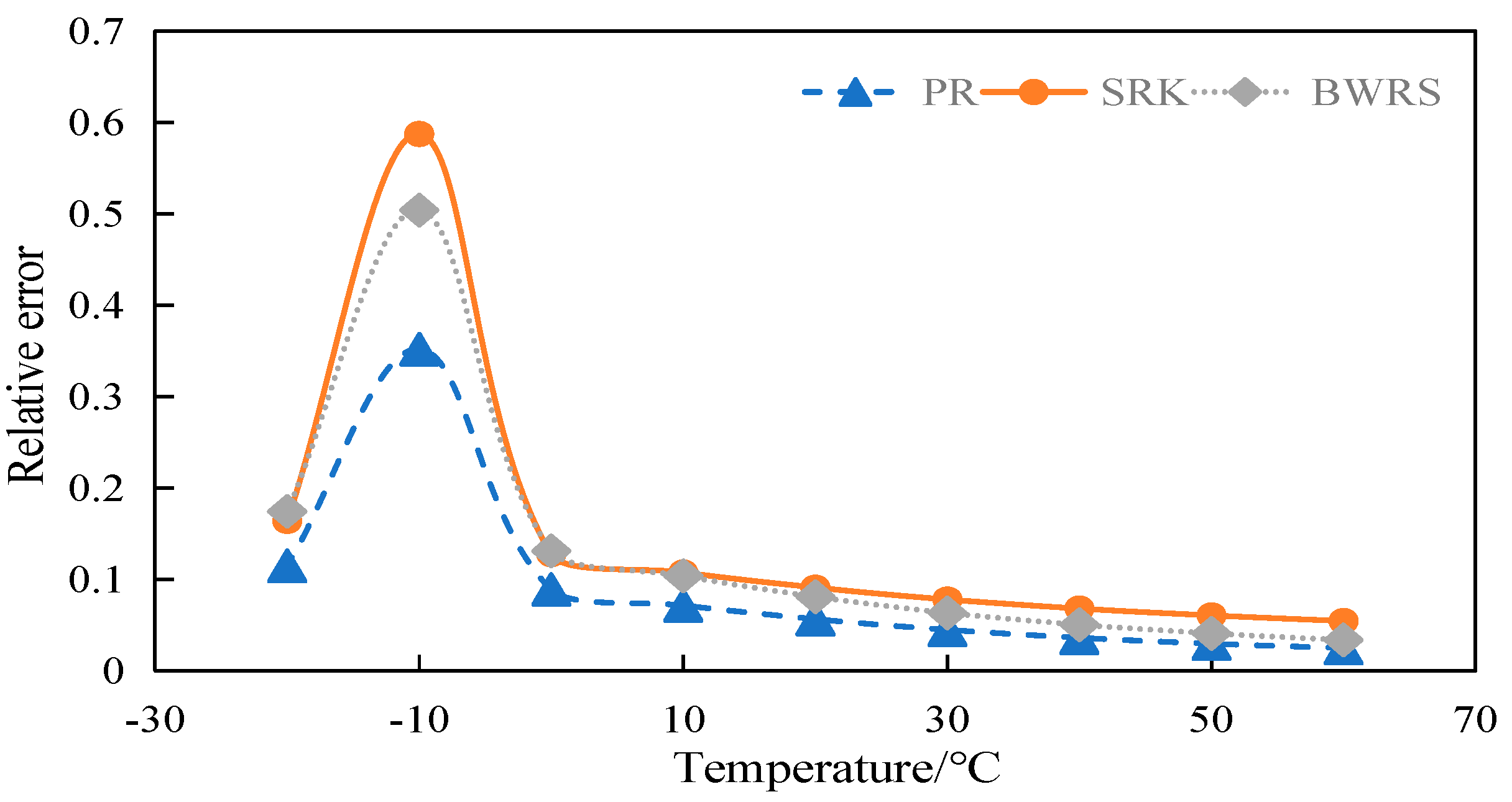
4.4.1. Calculation and Evaluation of Viscosity of 50% CO2
4.4.2. Calculation and Evaluation of Viscosity of 100% CO2
4.5. Calculation of Specific Heat at Constant Pressure
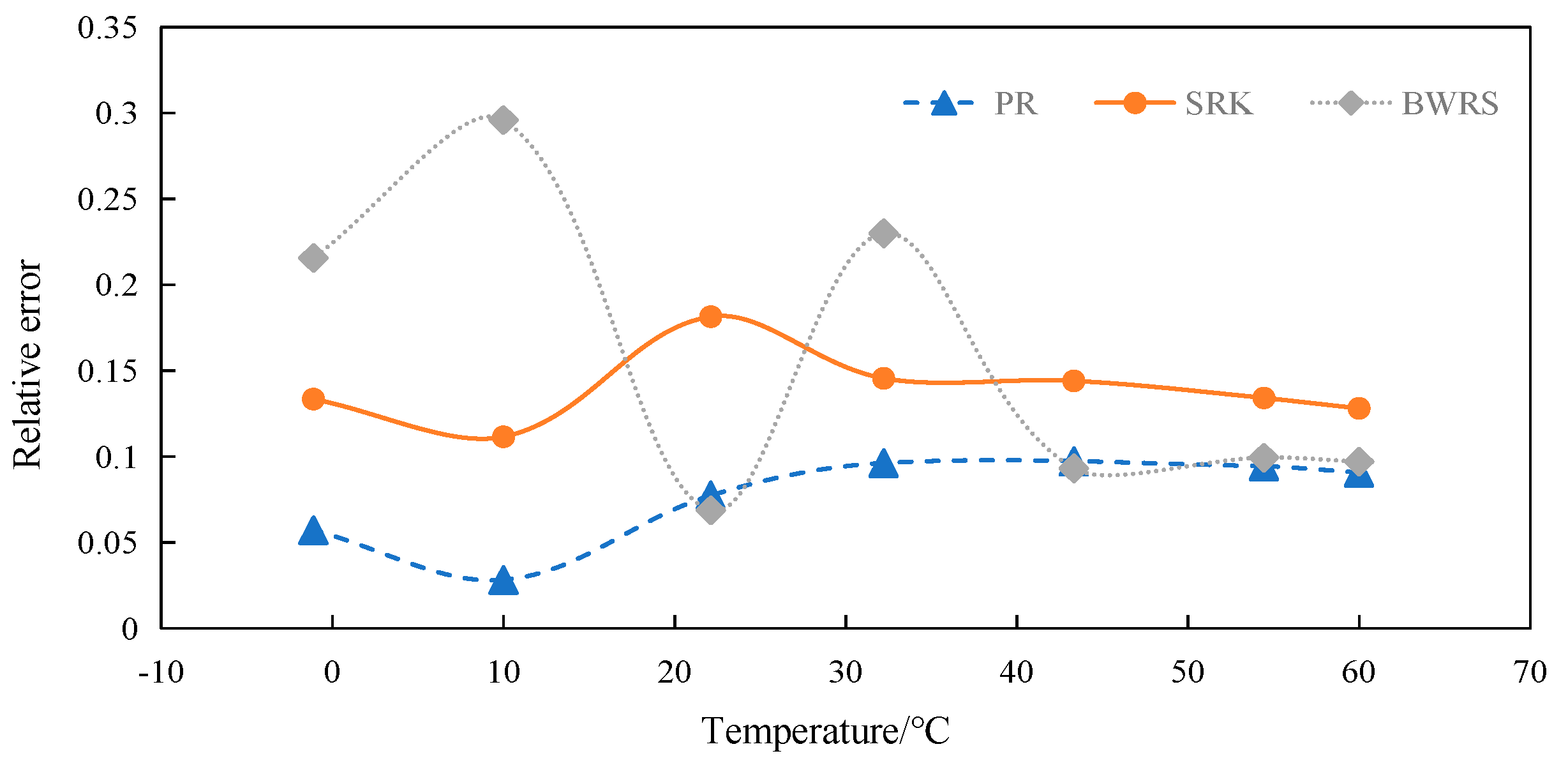
4.5.1. Calculation and Evaluation of Specific Heat at Constant Pressure of 50% CO2
4.5.2. Calculation and Evaluation of Specific Heat at Constant Pressure of 100% CO2
4.6. Summary
- (1)
- Error between compression factor and experimental data
- (2)
- Error between density and experimental data
- (3)
- Error between viscosity and experimental data
- (4)
- Error between specific heat at constant pressure and experimental data
- (5)
- Results summary
5. Conclusions
- (1)
- The compression factor first decreases and then increases with the increase in pressure, and the lower the temperature, the smaller the critical compression factor. At the same temperature, the higher the CO2 content, the smaller the compression factor under the same pressure. Density and viscosity increase with the rise in pressure, and the lower the temperature, the higher the corresponding density and viscosity. At the same temperature, the higher the CO2 content, the higher the density and viscosity under the same pressure. The specific heat at constant pressure increases first and then decreases with the increase in pressure, and the lower the temperature, the specific heat at constant pressure increases first and then decreases. At the same temperature, the higher the CO2 content, the higher the specific heat at the same pressure.
- (2)
- The BWRS equation of state shows great accuracy fluctuation in physical property parameter calculations. Under lower temperature conditions, a substantial error will arise between the calculated values and the experimental values. Conversely, the equation demonstrates higher-accuracy calculation within the elevated temperature range. Nevertheless, such an elevated temperature range is infrequently reached in real-world isothermal transportation. Furthermore, the BWRS equation is characterized by mass coefficients, which complicates the calculation procedure. Consequently, it is inadvisable to apply this equation while exploring the characteristics of multi-component CO2 in pipeline transport.
- (3)
- The SRK equation of state exhibits consistent calculation results. However, the predicted value is lower than the experimental value. There is a large deviation from the actual density value of CO2 in the conventional temperature range of pipeline transport.
- (4)
- The PR equation features accurate simulation calculations and straightforward calculation procedures, and its calculation results closely align with the experimental values. It is recommended to use the PR equation of state in the pipeline transport of CO2.
- (5)
- Based on the investigation of the influence mechanism of carbon dioxide containing impurities, it is concluded that when H2S/H2O impurities are present, the built-in polarity correction term of BWRS makes the calculation more stable. When the temperature is less than 40 °C and it is necessary to predict the freezing point of CO2 or the formation of hydrates, BWRS can describe the solid-phase equilibrium. When supercritical CO2 extraction is carried out, the accuracy of BWRS is significantly better than that of the PR and SRK equations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Carbon Dioxide Capture and Storage; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Czernichowski-Lauriol, I.; Berenblyum, R.; Bigi, S.; Car, M.; Gastine, M.; Persoglia, S.; Poulsen, N.; Schmidt-Hattenberger, C.; Stead, R.; Vincent, C.J.; et al. CO2 GeoNet actions in Europe for advancing CCUS through global cooperation. Energy Procedia 2018, 154, 73–79. [Google Scholar] [CrossRef]
- Bhave, A.; Taylor, R.H.; Fennell, P.; Livingston, W.R.; Shah, N.; Mac Dowell, N.; Dennis, J.; Kraft, M.; Pourkashanian, M.; Insa, M.; et al. Screening and techno-economic assessment of biomass-based power generation with CCS technologies to meet 2050 CO2 targets. Appl. Energy 2017, 190, 481–489. [Google Scholar] [CrossRef]
- Reiner, D.M. Learning through a portfolio of carbon capture and storage demonstration projects. Nat. Energy 2016, 1, 15011. [Google Scholar] [CrossRef]
- Lilliestam, J.; Bielicki, J.M.; Patt, A.G. Comparing carbon capture and storage (CCS) with concentrating solar power (CSP): Potentials, costs, risks, and barriers. Energy Policy 2012, 47, 447–455. [Google Scholar] [CrossRef]
- Gale, J.; Davison, J. Transmission of CO2—Safety and economic considerations. Energy 2004, 29, 1319–1328. [Google Scholar] [CrossRef]
- Bears, D.A.; Wied, R.F.; Martin, A.D.; Doyle, R.P. Paradis CO2 flood gathering, injection, and production systems. J. Pet. Technol. 1984, 36, 1312–1320. [Google Scholar] [CrossRef]
- Kleinstelber, S.W. The Wertz Tensleep CO2 flood: Design and initial performance. J. Pet. Technol. 1990, 42, 630–636. [Google Scholar] [CrossRef]
- Wilson, A. CO2 enhanced-oil-recovery project injects new life into Madison Reservoir in Wyoming. J. Pet. Technol. 2012, 64, 112–115. [Google Scholar] [CrossRef]
- Brown, S.; Martynov, S.; Mahgerefteh, H.; Fairweather, M.; Woolley, R.M.; Wareing, C.J.; Falle, S.A.; Rutters, H.; Niemi, A.; Zhang, Y.C.; et al. CO2QUEST: Techno-economic assessment of CO2 quality effect on its storage and transport. Energy Procedia 2014, 63, 2622–2629. [Google Scholar] [CrossRef]
- Mohitpour, M.; Seevam, P.; Botros, K.K.; Rothwell, B.; Ennis, C. Pipeline Transportation of Carbon Dioxide Containing Impurities; ASME Press: New York, NY, USA, 2012. [Google Scholar]
- Nezhad, S.; Williams, C. Bravo Dome Subsurface and Surface Network Modeling. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014. [Google Scholar]
- Jung, W.; Nicot, J.P. Impurities in CO2-rich mixtures impact CO2 pipeline design: Implications for calculating CO2 transport capacity. In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, New Orleans, LA, USA, 10–12 November 2010. SPE-139712-MS. [Google Scholar]
- McCollough, D.E.; Stiles, R.L. Operation of the Central Basin CO2 pipeline system. In Proceedings of the SPE Western Regional Meeting, Ventura, CA, USA, 8–10 April 1987. SPE-16329-MS. [Google Scholar]
- Towler, B.F.; Agarwal, D.; Mokhatab, S. Modeling Wyoming’s carbon dioxide pipeline network. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 30, 259–270. [Google Scholar] [CrossRef]
- De Nier, M.; Maas, W.; Gray, L.; Wiwchar, T. Maturing CCS Technology through Demonstration Quest: Learning from CCS Implementation in Canada. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 9–12 November 2015. [Google Scholar]
- IEA. Energy Technology Perspectives 2020; IEA: Paris, France, 2020. [Google Scholar]
- Edward, S.R.; John, E.D.; Howard, J.H. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control. 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Government of the United Kingdom. Re-Use of Oil and Gas Assets for Carbon Capture Usage and Storage Projects; Government of the United Kingdom: London, UK, 2019.
- Lone, S.; Cockerill, T.; Macchietto, S. The techno-economics of a phased approach to developing a UK carbon dioxide pipeline network. J. Pipeline Eng. 2010, 9, 223. [Google Scholar]
- Sandana, D.; Dale, M.; Charles, E.; Race, J. Transport of gaseous and dense carbon dioxide in pipelines: Is there an internal corrosion risk. J. Pipeline Eng. 2012, 11, 229–238. [Google Scholar]
- Choi, Y.S.; Nesic, S.; Young, D. Effect of impurities on the corrosion behavior of CO2 transmission pipeline steel in supercritical CO2-water environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Cole, I.S.; Bocher, F.; Corrigan, P.; Gamage, R.P.; Ukwattage, N.; Birbilis, N. Investigating the effect of salt and acid impurities in supercritical CO2 as relevant to the corrosion of carbon capture and storage pipelines. Int. J. Greenh. Gas Control 2013, 17, 534–541. [Google Scholar] [CrossRef]
- Froydis, E. Safe pipeline transmission of CO2. Pipeline Gas J. 2008, 235, 76–77. [Google Scholar]
- Zhang, Z.; Pan, S.Y.; Li, H. Recent Advances in Carbon Dioxide Utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Munkejord, S.T.; Austegard, A.; Deng, H.; Hammer, M.; Stang, H.J.; Løvseth, S.W. Depressurization of CO2 in a pipe: High-resolution pressure and temperature data and comparison with model predictions. Energy 2020, 211, 118560. [Google Scholar] [CrossRef]
- Vanchugov, I.M.; Rezanov, K.S.; Shestakov, R.A. On the issue of transportation of liquefied carbon dioxide. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2023, 334, 190–209. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Fenghour, A.; Wakeham, W.A.; Vesovic, V. The viscosity of carbon dioxide. J. Phys. Chem. Ref. Data 1996, 25, 1599–1610. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Folas, G.K. Thermodynamic Models for Industrial Applications: From Classical and Advanced Mixing Rules to Association Theories; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Li, H.; Jakobsen, J.P. PVTxy properties of CO2 mixtures relevant for CO2 capture, transport and storage: Review of available experimental data and theoretical models. Appl. Energy 2013, 111, 815–826. [Google Scholar] [CrossRef]
- Gernert, G.J.; Span, R. EOS-CG: A Helmholtz energy mixture model for humid gases and CCS mixtures. J. Chem. Thermodyn. 2016, 93, 274–293. [Google Scholar] [CrossRef]
- Ahmadi, P.; Chapoy, A. Experimental and modelling studies on the phase behaviour of (CO2+H2S) binary and (CO2+H2S+CH4) ternary systems. J. Chem. Thermodyn. 2017, 115, 127–138. [Google Scholar]
- ISO 20486:2017; Petroleum and Natural Gas Industries—Fluid Properties—Determination of Reservoir Fluid Properties by Laboratory Analysis. International Organization for Standardization: Geneva, Switzerland, 2017.
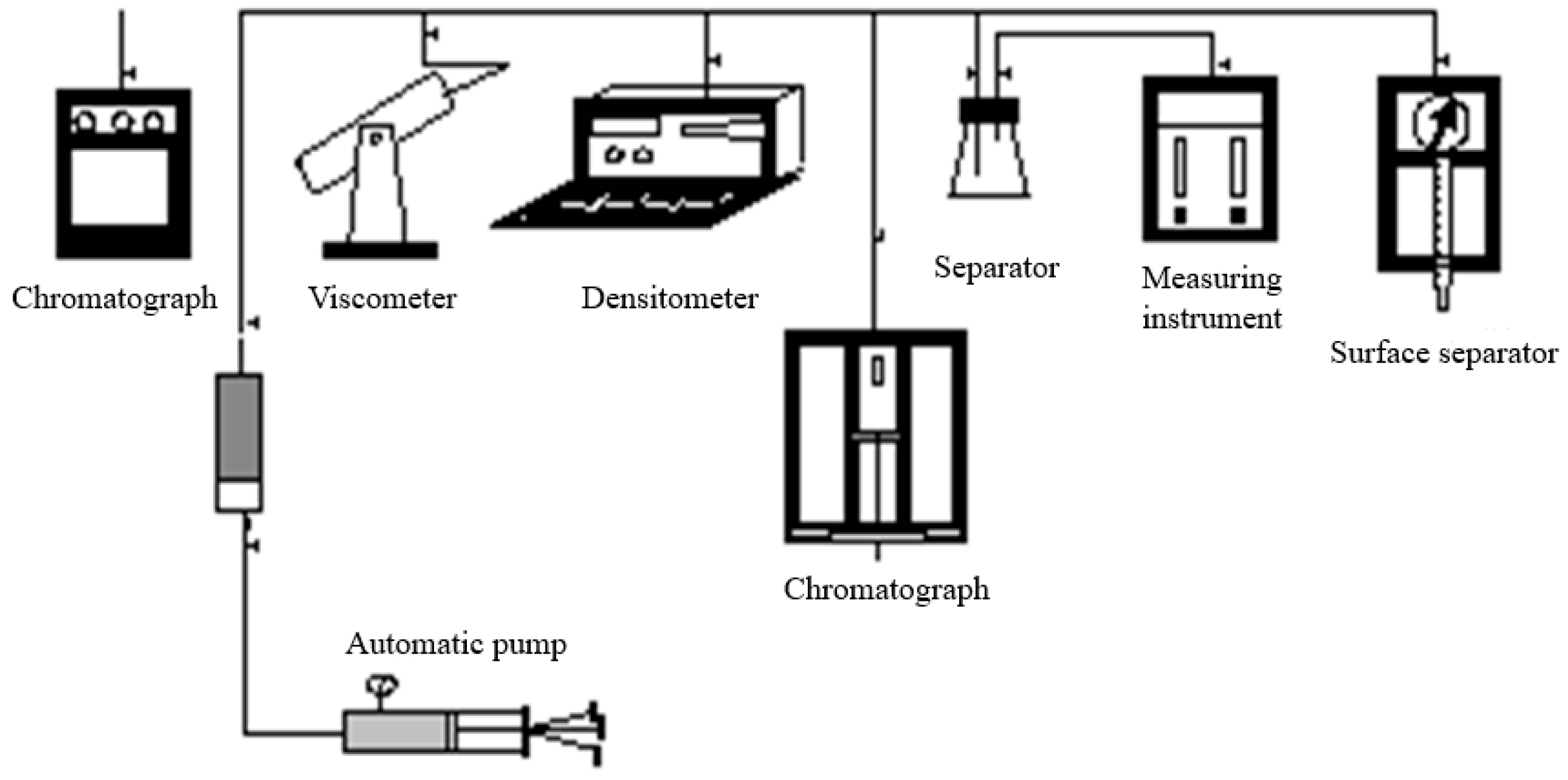



| Components | CO2 | H2O | N2 | H2 | CO | COS | H2S | Total |
|---|---|---|---|---|---|---|---|---|
| Content mol% | 42.2644 | 5.2810 | 49.3489 | 2.4568 | 0.1013 | 0.0032 | 0.0140 | 100 |
| Components | CO2 | H2O | N2 | H2 | CO | COS | H2S | Total |
|---|---|---|---|---|---|---|---|---|
| Content mol% | 99.9883 | 0.0017 | 0.0089 | 0.0006 | 0.0001 | 0.0001 | 0.0003 | 100 |
| CO2 Content (%) | 100 | 90 | 70 | 50 | 30 | 10 |
|---|---|---|---|---|---|---|
| CO2 | 100 | 91.77 | 70.98 | 50.99 | 28.86 | 9.84 |
| N2 | - | 0.58 | 1.14 | 0.83 | 1.24 | 2.05 |
| C1 | - | - | 27.32 | 46.54 | 67.73 | 86.02 |
| C2 | - | 7.60 | 0.46 | 1.16 | 1.59 | 1.67 |
| C3 | - | - | 0.08 | 0.33 | 0.42 | 0.31 |
| iC4 | - | 0.02 | 0.01 | 0.05 | 0.06 | 0.04 |
| nC4 | - | - | 0.01 | 0.06 | 0.07 | 0.05 |
| iC5 | - | - | 0.01 | 0.02 | 0.03 | 0.01 |
| nC5 | - | - | - | 0.01 | 0.01 | - |
| C6+ | - | 0.02 | - | 0.01 | - | - |
| Equation | Characteristic | Applicability | Formula |
|---|---|---|---|
| BWRS | It is improved from the BWR (Benedict–Webb–Rubin) equation and can accurately describe the PVT characteristics under high pressure and low temperature, which is suitable for calculating CO2 and the density near the critical pressure. | It is applicable to high-purity/high-pressure CO2 systems, acidic gases containing H2S/H2O, and scenarios where the generation of solid CO2 needs to be predicted. | |
| SRK | The calculation of parameters such as compression factor and heat is relatively accurate, and the calculation accuracy of the physical properties of gaseous CO2 is relatively high. | It is suitable for medium and low-pressure hydrocarbon mixtures, scenarios with relatively low CO2 concentration (<30%), and no strong polar impurities. | |
| PR | It is improved from the Clapeyron equation and has high accuracy in the calculation of gas-liquid phase equilibrium and physical properties of polar systems such as CO2. | It is suitable for CO2-hydrocarbon mixed systems, medium-and high-pressure processes (<15 MPa), and scenarios where a balance between precision and speed is required. |
| Composition | Calculation Parameters | PR/% | SRK/% | BWRS/% | Recommended Equations of State |
|---|---|---|---|---|---|
| 50% CO2 | Compression factor | 2.28~9.58 | 34.45~67.51 | 5.21~14.82 | PR |
| 50% CO2 | Density | 2.47~35.19 | 5.44~58.71 | 3.37~50.40 | PR |
| 50% CO2 | Viscosity | 6.48~25.25 | 6.48~25.25 | 0.50~9.32 | BWRS |
| 50% CO2 | Specific heat at constant pressure | 1.26~4.43 | 0.53~3.15 | 1.41~11.37 | PR |
| 100% CO2 | Compression factor | 34.04~57.54 | 34.45~67.51 | 32.05~65.37 | PR |
| 100% CO2 | Density | 2.82~9.72 | 11.15~18.15 | 6.88~29.59 | PR |
| 100% CO2 | Viscosity | 4.06~13.28 | 4.06~13.28 | 6.37~33.77 | PR |
| 100% CO2 | Specific heat at constant pressure | 6.74~17.36 | 6.31~16.91 | 4.77~25.86 | SRK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Dai, Z.; Wang, F.; Bie, Q.; Fu, W.; Shan, C.; Zheng, S.; Sun, J. Changes in Physical Parameters of CO2 Containing Impurities in the Exhaust Gas of the Purification Plant and Selection of Equations of State. Fluids 2025, 10, 189. https://doi.org/10.3390/fluids10080189
Wang X, Dai Z, Wang F, Bie Q, Fu W, Shan C, Zheng S, Sun J. Changes in Physical Parameters of CO2 Containing Impurities in the Exhaust Gas of the Purification Plant and Selection of Equations of State. Fluids. 2025; 10(8):189. https://doi.org/10.3390/fluids10080189
Chicago/Turabian StyleWang, Xinyi, Zhixiang Dai, Feng Wang, Qin Bie, Wendi Fu, Congxin Shan, Sijia Zheng, and Jie Sun. 2025. "Changes in Physical Parameters of CO2 Containing Impurities in the Exhaust Gas of the Purification Plant and Selection of Equations of State" Fluids 10, no. 8: 189. https://doi.org/10.3390/fluids10080189
APA StyleWang, X., Dai, Z., Wang, F., Bie, Q., Fu, W., Shan, C., Zheng, S., & Sun, J. (2025). Changes in Physical Parameters of CO2 Containing Impurities in the Exhaust Gas of the Purification Plant and Selection of Equations of State. Fluids, 10(8), 189. https://doi.org/10.3390/fluids10080189







