Physics-Informed Neural Networks Simulation and Validation of Airflows in Three-Dimensional Upper Respiratory Tracts
Abstract
1. Introduction
- (1)
- Test the PINN model in three geometries with increasing complexity.
- (2)
- Assess PINN training via the Hessian matrix vs. flow speeds and shape complexity.
- (3)
- Nondimensionalize the Navier–Stokes equations to improve training robustness.
- (4)
- Develop an inference module to visualize the PINN results in ANSYS Fluent 21.
- (5)
- Compare PINN and CFD results of airflows qualitatively and quantitatively.
- (6)
- Compare fidelity between the PINN laminar and SDF–mixing-length models.
2. Materials and Methods
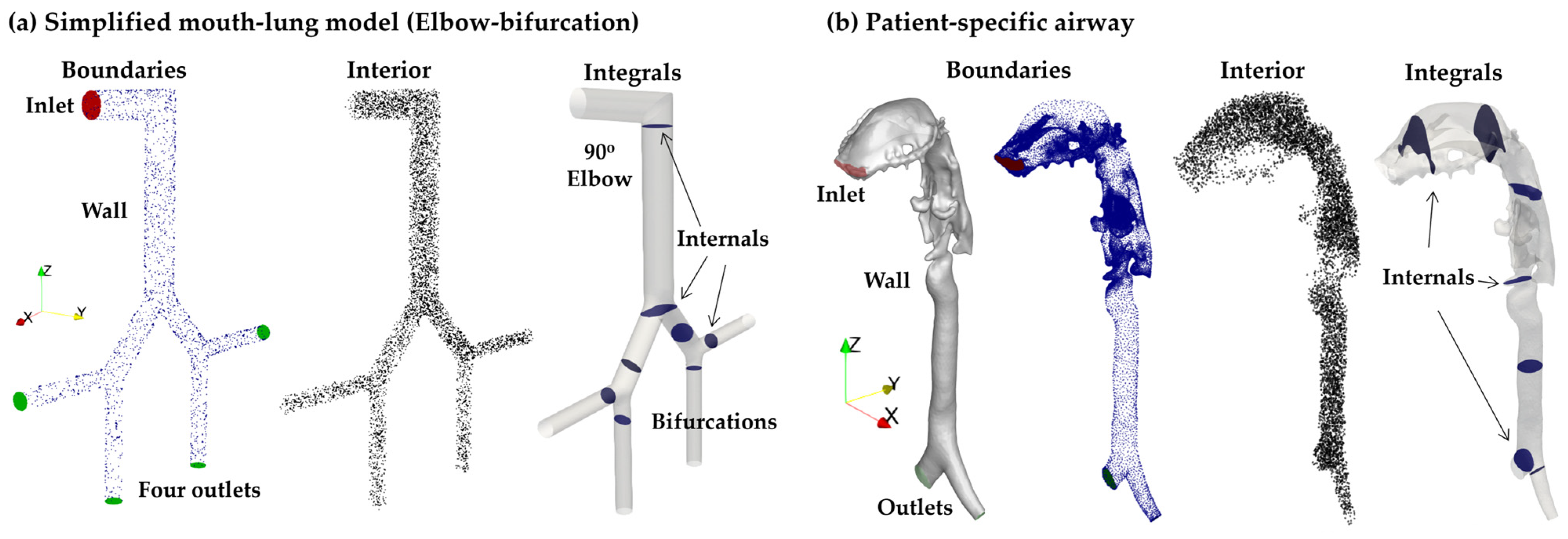
2.1. Testing Geometries
2.2. CFD Numerical Methods
2.3. PINN
2.3.1. Loss Function
2.3.2. Network Architecture and Hyperparameters
2.3.3. Nondimensionalization
2.3.4. Hessian Matrix and Control Number
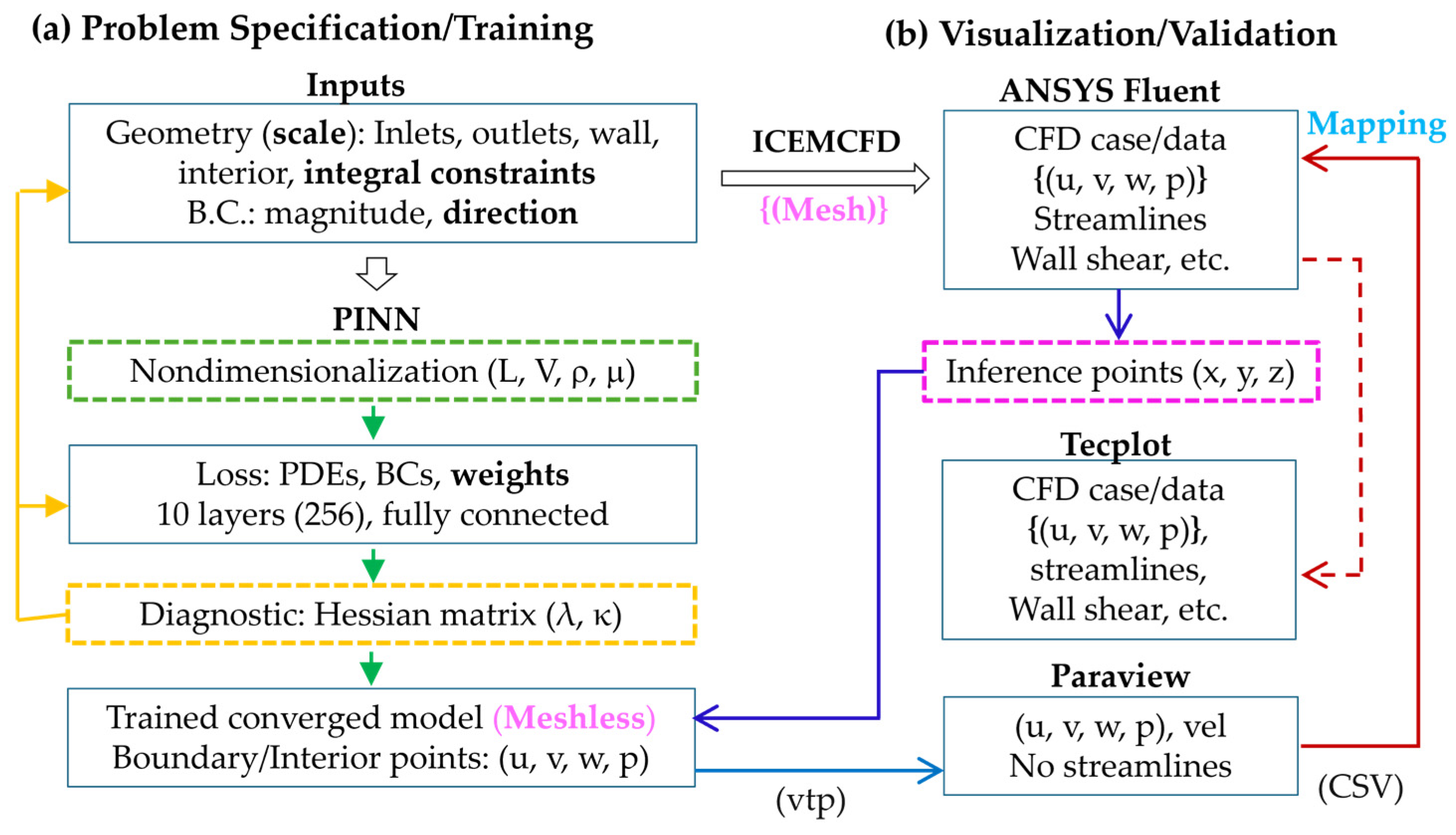
2.4. PINN Training and Visualization
2.4.1. Problem Specification and Training
2.4.2. Fluent-Based Visualization and Validation
2.4.3. PINN Inference Methodology
3. Results
3.1. Duct Flow
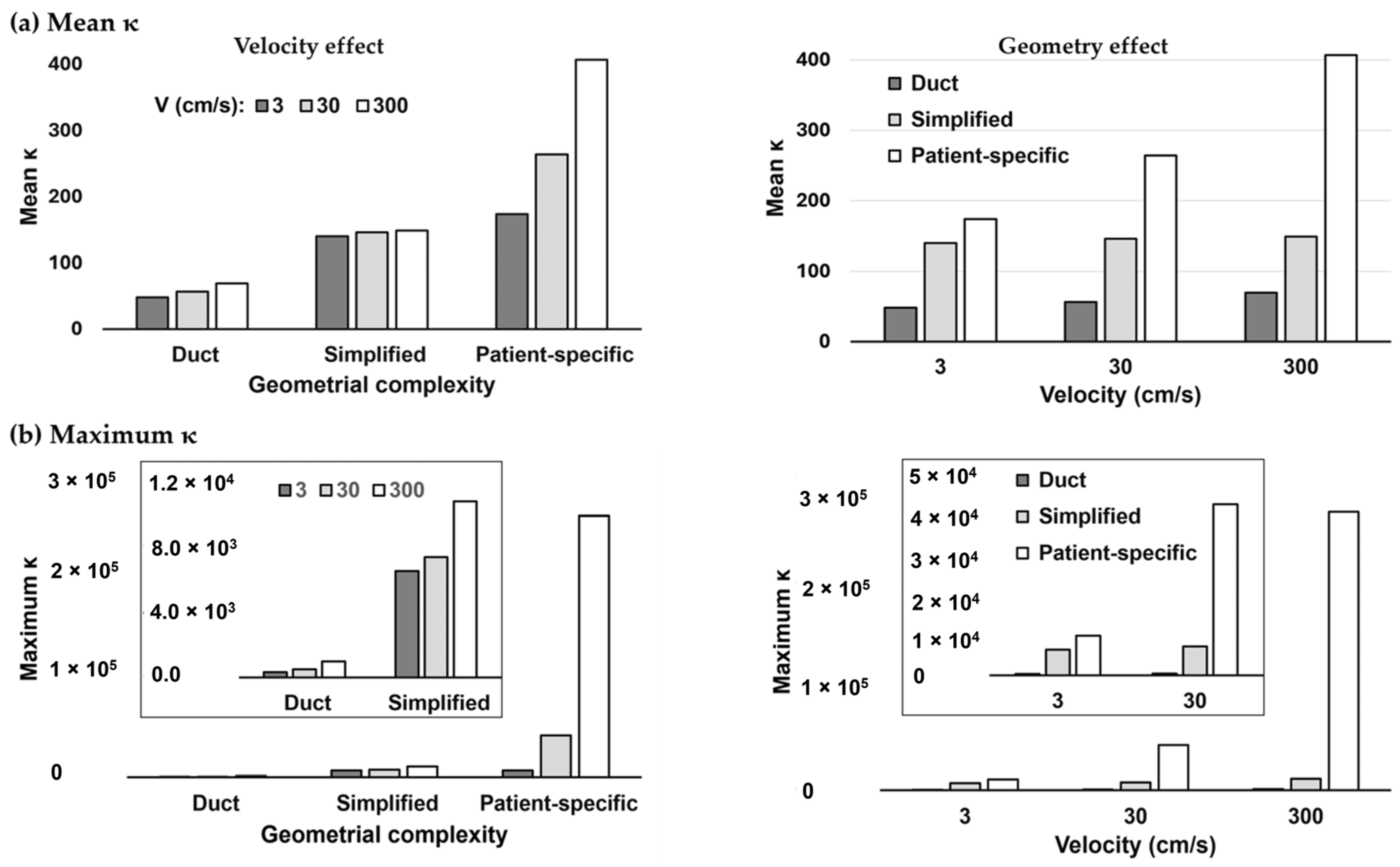
3.2. Hessian-Matrix-Based Condition Number κ
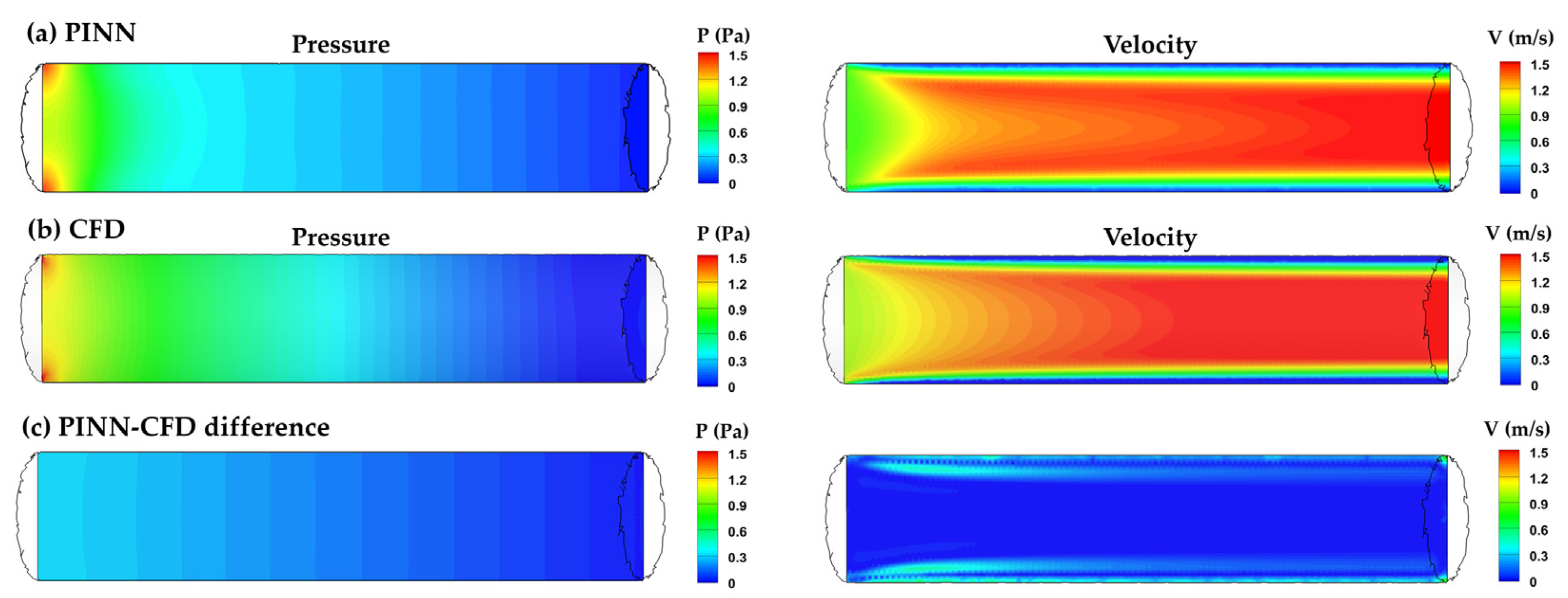
3.3. Simplified Mouth–Lung Geometry
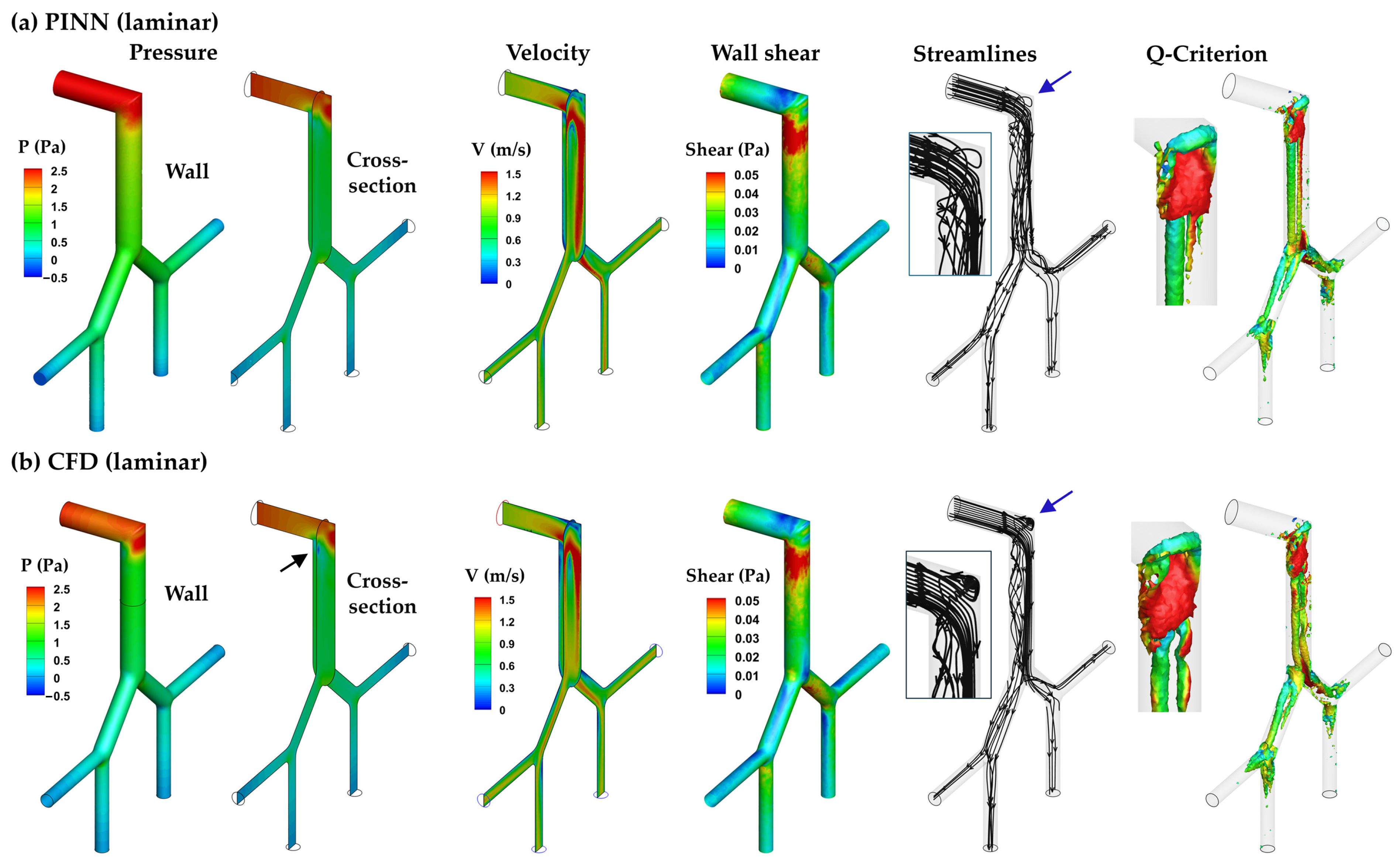
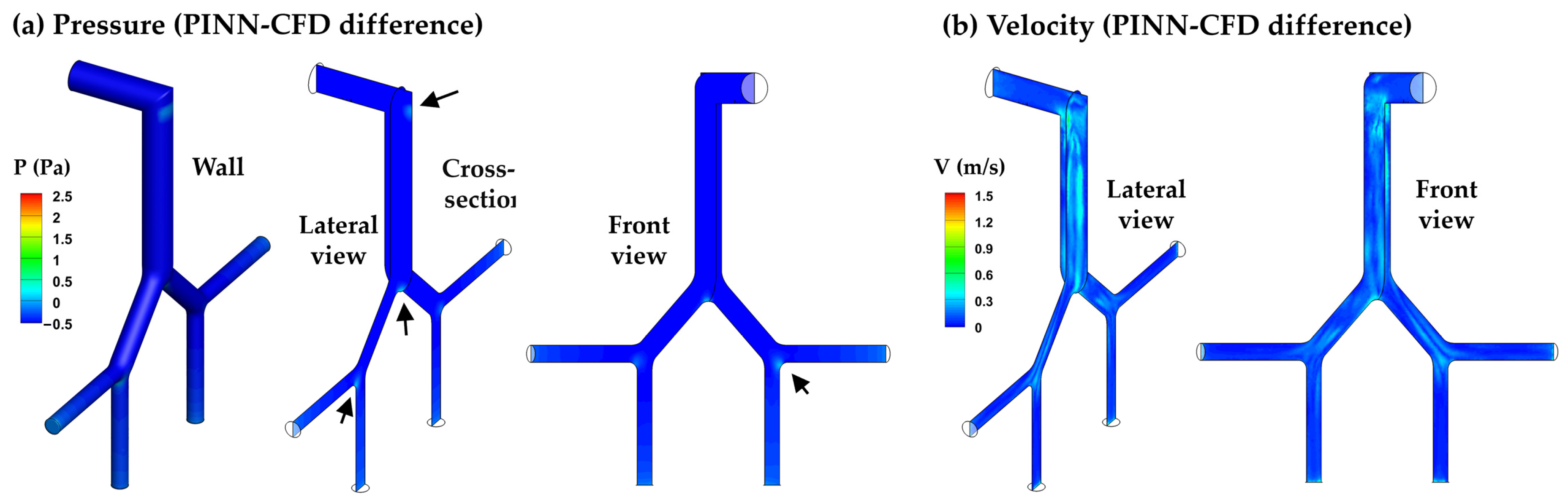
3.3.1. PINN vs. CFD: Laminar Model
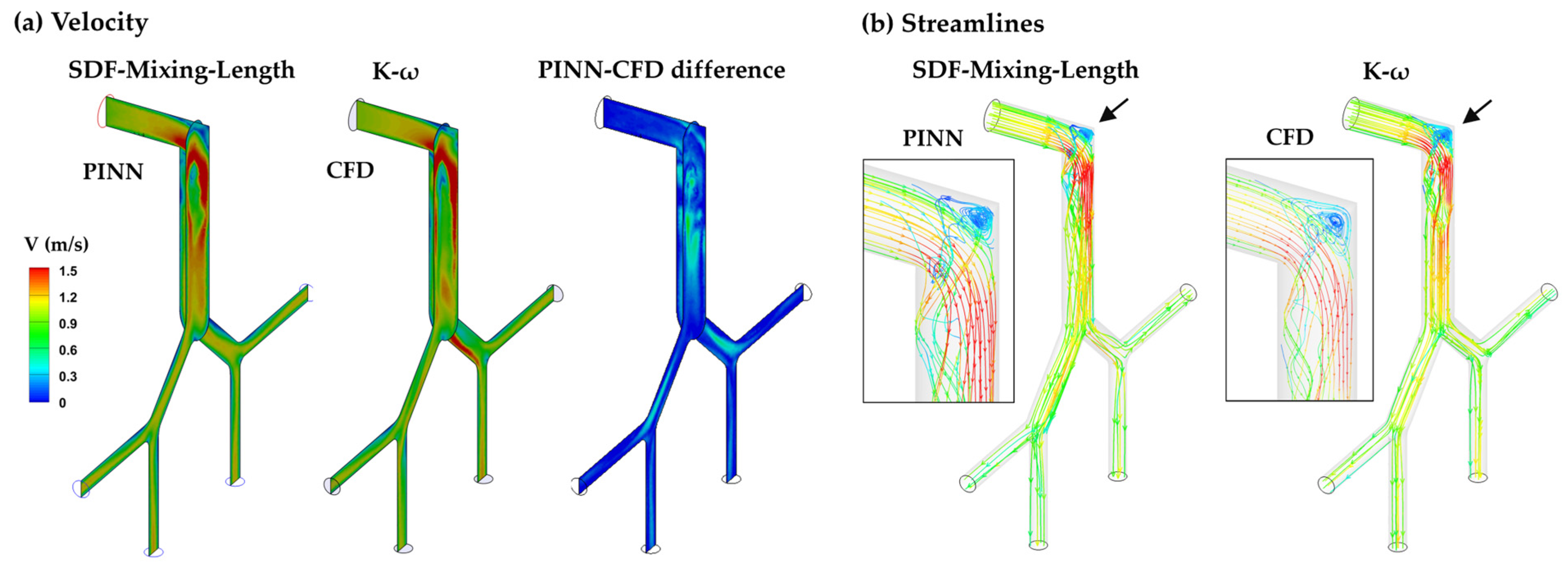
3.3.2. SDF–Mixing-Length PINN vs. CFD Turbulence Model
3.4. Patient-Specific Airway Model
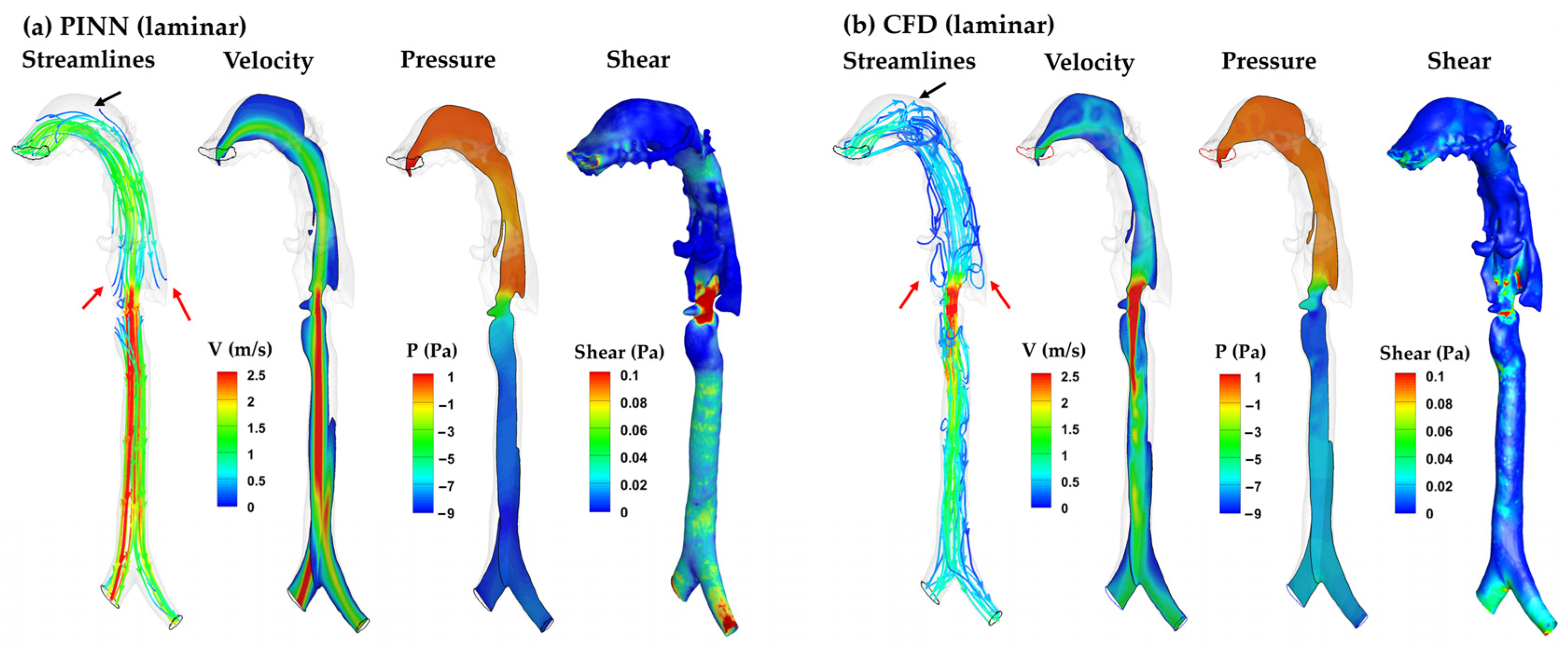
3.4.1. PINN vs. CFD: Laminar Model
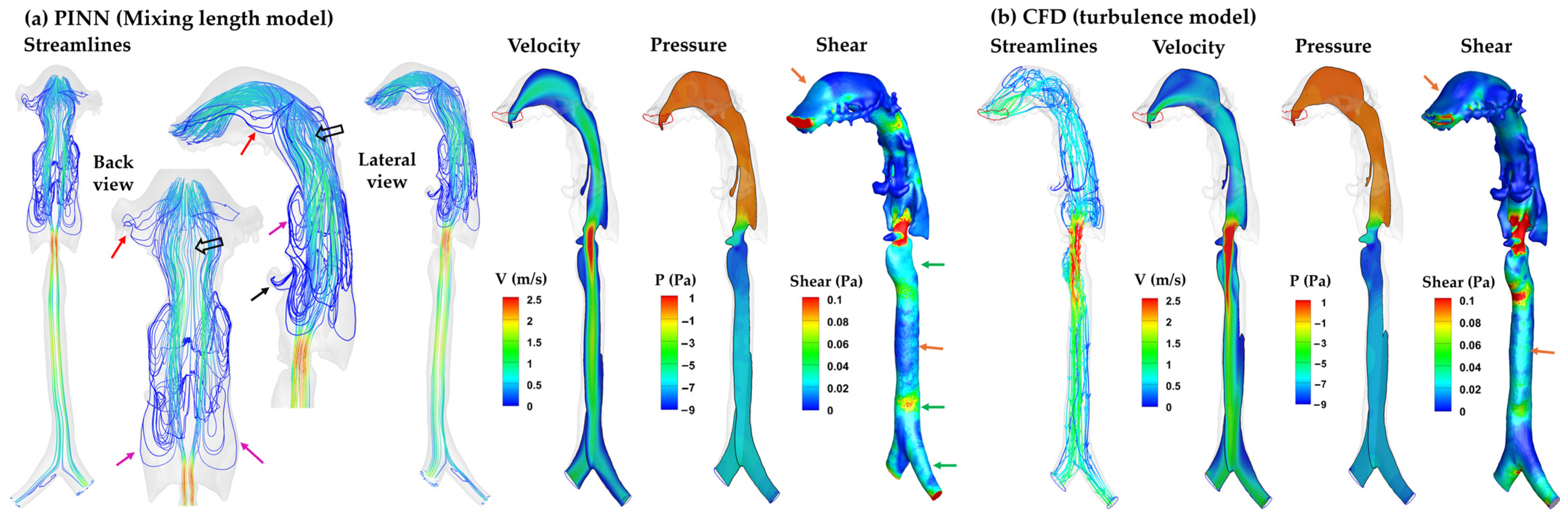
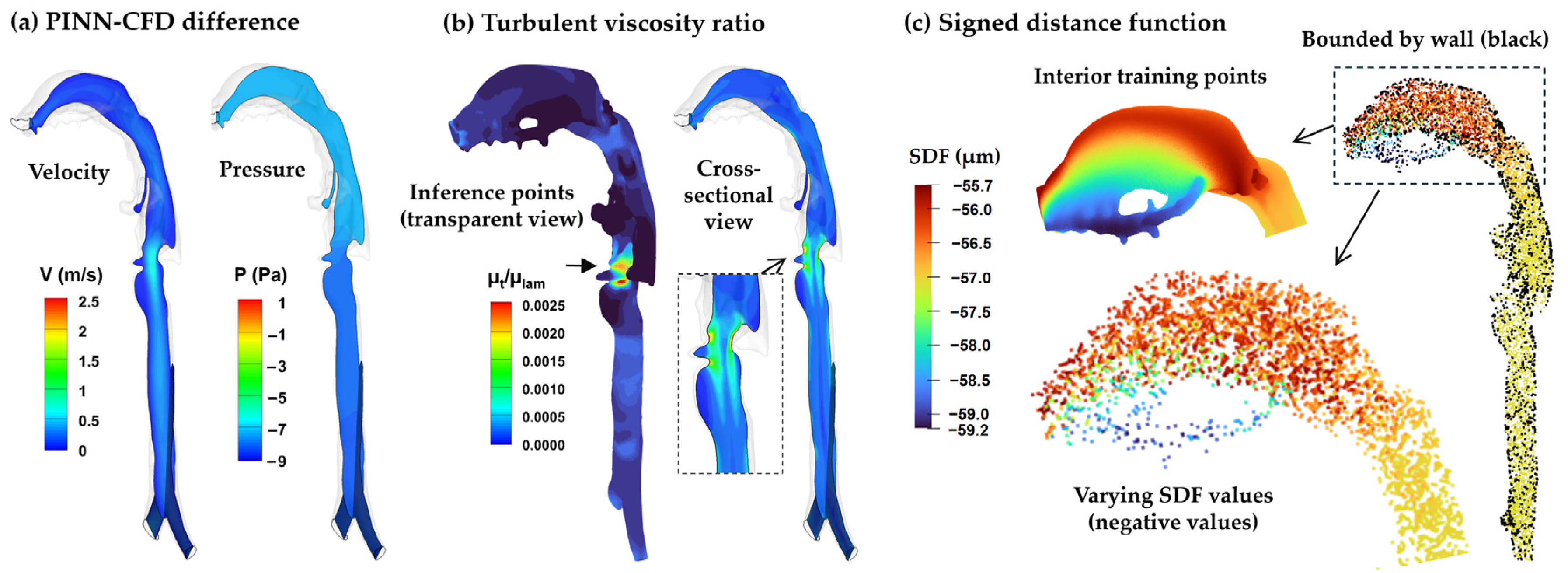
3.4.2. SDF–Mixing-Length PINN vs. CFD Turbulence Model
4. Discussion
4.1. Novelties Compared to Previous Studies
4.2. Challenges and Best Practices in Implementing and Training PINN
4.2.1. Nondimensional Governing Equations
4.2.2. Flow Constraints and GPU Memory Limitation
4.2.3. Near-Wall Treatment via Signed Distance Function (SDF)
4.3. Implications
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawman, L.; Mukherjee, A.; Sethi, T.; Agrawal, A.; Kabra, S.K.; Lodha, R. Role of impulse oscillometry in assessing asthma control in children. Indian Pediatr. 2020, 57, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wang, Z.; Talaat, K.; Glide-Hurst, C.; Dong, H. Numerical study of dynamic glottis and tidal breathing on respiratory sounds in a human upper airway model. Sleep Breath. 2018, 22, 463–479. [Google Scholar] [CrossRef]
- Si, X.; Xi, J.; Kim, J. Effect of laryngopharyngeal anatomy on expiratory airflow and submicrometer particle deposition in human extrathoracic airways. Open J. Fluid Dyn. 2013, 3, 286–301. [Google Scholar] [CrossRef]
- Lancmanová, A.; Bodnár, T. Numerical simulations of human respiratory flows: A review. Discov. Appl. Sci. 2025, 7, 242. [Google Scholar] [CrossRef]
- Faizal, W.M.; Ghazali, N.N.N.; Khor, C.Y.; Badruddin, I.A.; Zainon, M.Z.; Yazid, A.A.; Ibrahim, N.B.; Razi, R.M. Computational fluid dynamics modelling of human upper airway: A review. Comput. Methods Programs Biomed. 2020, 196, 105627. [Google Scholar] [CrossRef]
- Lauria, M.; Singhrao, K.; Stiehl, B.; Low, D.; Goldin, J.; Barjaktarevic, I.; Santhanam, A. Automatic triangulated mesh generation of pulmonary airways from segmented lung 3DCTs for computational fluid dynamics. Int. J. Comput. Assit. Radiol. Surg. 2022, 17, 185–197. [Google Scholar] [CrossRef]
- Jing, H.; Ge, H.; Wang, L.; Choi, S.; Farnoud, A.; An, Z.; Lai, W.; Cui, X. Investigating unsteady airflow characteristics in the human upper airway based on the clinical inspiration data. Phys. Fluids 2023, 35, 101911. [Google Scholar] [CrossRef]
- Jing, H.; Ge, H.; Wang, L.; Zhou, Q.; Chen, L.; Choi, S.; Cui, X. Large eddy simulation study of the airflow characteristics in a human whole-lung airway model. Phys. Fluids 2023, 35, 071903. [Google Scholar] [CrossRef]
- Vara Almirall, B.; Calmet, H.; Ang, H.Q.; Inthavong, K. Flow behavior in idealized & realistic upper airway geometries. Comput. Biol. Med. 2025, 194, 110449. [Google Scholar] [CrossRef]
- Jiang, F.; Hirano, T.; Liang, C.; Zhang, G.; Matsunaga, K.; Chen, X. Multi-scale simulations of pulmonary airflow based on a coupled 3D-1D-0D model. Comput. Biol. Med. 2024, 171, 108150. [Google Scholar] [CrossRef]
- Dey, R.; Patni, H.K.; Anand, S. Improved aerosol deposition predictions in human upper respiratory tract using coupled mesh phantom-based computational model. Sci. Rep. 2025, 15, 14260. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, C.C.; Schuh, A.; Higano, N.S.; Woods, J.C.; Bates, A.J. The effect of airway motion and breathing phase during imaging on CFD simulations of respiratory airflow. Comput. Biol. Med. 2020, 127, 104099. [Google Scholar] [CrossRef]
- Kim, M.; Chau, N.-K.; Park, S.; Nguyen, P.C.H.; Baek, S.S.; Choi, S. Physics-aware machine learning for computational fluid dynamics surrogate model to estimate ventilation performance. Phys. Fluids 2025, 37, 027130. [Google Scholar] [CrossRef]
- Gao, H.; Qian, W.; Dong, J.; Liu, J. Rapid prediction of indoor airflow field using operator neural network with small dataset. Build. Environ. 2024, 251, 111175. [Google Scholar] [CrossRef]
- Hao, Y.; Song, F. Fourier neural operator networks for solving reaction–diffusion equations. Fluids 2024, 9, 258. [Google Scholar] [CrossRef]
- Vinuesa, R.; Brunton, S.L. Enhancing computational fluid dynamics with machine learning. Nat. Computat. Sci. 2022, 2, 358–366. [Google Scholar] [CrossRef]
- Raissi, M.; Perdikaris, P.; Karniadakis, G.E. Physics-informed neural networks: A deep learning framework for solving forward and inverse problems involving nonlinear partial differential equations. J. Comput. Phys. 2019, 378, 686–707. [Google Scholar] [CrossRef]
- Raissi, M.; Yazdani, A.; Karniadakis, G.E. Hidden fluid mechanics: Learning velocity and pressure fields from flow visualizations. Science 2020, 367, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Mao, Z.; Wang, Z.; Yin, M.; Karniadakis, G.E. Physics-informed neural networks (PINNs) for fluid mechanics: A review. Acta Mech. Sin. 2022, 37, 1727–1738. [Google Scholar] [CrossRef]
- Arzani, A.; Wang, J.-X.; D’Souza, R.M. Uncovering near-wall blood flow from sparse data with physics-informed neural networks. Phys. Fluids 2021, 33, 071905. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, B.; Che, Y.; Kang, J.; Luo, M.; Qiao, A.; Liu, Y.; Anzai, H.; Ohta, M.; Guo, Y.; et al. Physics-informed neural networks (PINNs) for 4D hemodynamics prediction: An investigation of optimal framework based on vascular morphology. Comput. Biol. Med. 2023, 164, 107287. [Google Scholar] [CrossRef] [PubMed]
- Alzhanov, N.; Ng, E.Y.K.; Zhao, Y. Three-dimensional physics-informed neural network simulation in coronary artery trees. Fluids 2024, 9, 153. [Google Scholar] [CrossRef]
- Kumar, A.K.; Jain, S.; Jain, S.; Ritam, M.; Xia, Y.; Chandra, R. Physics-informed neural entangled-ladder network for inhalation impedance of the respiratory system. Comput. Methods Programs Biomed. 2023, 231, 107421. [Google Scholar] [CrossRef]
- Jagtap, A.D.; Karniadakis, G.E. Extended physics-informed neural networks (XPINNs): A generalized space-time domain decomposition based deep learning framework for nonlinear partial differential equations. Commun. Comput. Phys. 2020, 28, 2002–2041. [Google Scholar] [CrossRef]
- McClenny, L.D.; Braga-Neto, U.M. Self-adaptive physics-informed neural networks. J. Comput. Phys. 2023, 474, 111722. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Perdikaris, P. When and why PINNs fail to train: A neural tangent kernel perspective. J. Comput. Phys. 2022, 449, 110768. [Google Scholar] [CrossRef]
- Gu, L.; Qin, S.; Xu, L.; Chen, R. Physics-informed neural networks with domain decomposition for the incompressible Navier–Stokes equations. Phys. Fluids 2024, 36, 021914. [Google Scholar] [CrossRef]
- Pioch, F.; Harmening, J.H.; Müller, A.M.; Peitzmann, F.-J.; Schramm, D.; el Moctar, O. Turbulence modeling for physics-informed neural networks: Comparison of different RANS models for the backward-facing step Flow. Fluids 2023, 8, 43. [Google Scholar] [CrossRef]
- Jin, X.; Cai, S.; Li, H.; Karniadakis, G.E. NSFnets (Navier-Stokes flow nets): Physics-informed neural networks for the incompressible Navier-Stokes equations. J. Comput. Phys. 2021, 426, 109951. [Google Scholar] [CrossRef]
- Yu, T.; Qi, Y.; Oseledets, I.; Chen, S. Spectral informed neural network: An efficient and low-memory PINN. arXiv 2024, arXiv:2408.16414. [Google Scholar]
- Huang, Y.; Zhang, Z.; Zhang, X. A direct-forcing immersed boundary method for incompressible flows based on physics-informed neural network. Fluids 2022, 7, 56. [Google Scholar] [CrossRef]
- Karniadakis, G.E.; Kevrekidis, I.G.; Lu, L.; Perdikaris, P.; Wang, S.; Liu, Y. Physics-informed machine learning. Nat. Rev. Phys. 2021, 3, 422–440. [Google Scholar] [CrossRef]
- Kundu, P.K.; Cohen, I.M.; Dowling, D.R. Fluid Mechanics, 6th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Anderson, J.D. Fundamentals of Aeodynamics; McGraw-Hill Education: Columbus, OH, USA, 2017. [Google Scholar]
- Krishnapriyan, A.; Gholami, A.; Zhe, S.; Kirby, R.; Mahoney, M.W. Characterizing possible failure modes in physics-informed neural networks. Adv. Neural Inf. Process. Syst. 2021, 34, 26548–26560. [Google Scholar]
- Talaat, M.; Si, X.A.; Xi, J. Lower inspiratory breathing depth enhances pulmonary delivery efficiency of ProAir sprays. Pharmaceuticals 2022, 15, 706. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, J.; Cheng, Y.S. Comparison of deposition in the USP and physical mouth-throat models with solid and liquid particles. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 277–284. [Google Scholar] [CrossRef]
- Longest, P.W.; Bass, K.; Dutta, R.; Rani, V.; Thomas, M.L.; El-Achwah, A.; Hindle, M. Use of computational fluid dynamics deposition modeling in respiratory drug delivery. Expert Opin Drug Deliv 2019, 16, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Corley, R.A.; Kabilan, S.; Kuprat, A.P.; Carson, J.P.; Minard, K.R.; Jacob, R.E.; Timchalk, C.; Glenny, R.; Pipavath, S.; Cox, T.; et al. Comparative computational modeling of airflows and vapor dosimetry in the respiratory tracts of rat, monkey, and human. Toxicol. Sci. 2012, 128, 500–516. [Google Scholar] [CrossRef]
- Xi, J.; Longest, P.W.; Martonen, T.B. Effects of the laryngeal jet on nano- and microparticle transport and deposition in an approximate model of the upper tracheobronchial airways. J. Appl. Physiol. 2008, 104, 1761–1777. [Google Scholar] [CrossRef] [PubMed]
- Longest, P.W.; Spence, B.; Hindle, M. Devices for improved delivery of nebulized pharmaceutical aerosols to the lungs. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 317–339. [Google Scholar] [CrossRef]
- He, Q.; Tartakovsky, A.M. Physics-informed neural network method for forward and backward advection-dispersion equations. Water Resour. Res. 2021, 57, e2020WR029479. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, J.; Liu, Y.-S.; Han, Z. Towards better gradient consistency for neural signed distance functions via level set alignment. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Vancouver, BC, Canada, 17–24 June 2023; pp. 17724–17734. [Google Scholar]
- NVIDIA Docs Hub Homepage. NVIDIA PhysicsNeMo Sym. Available online: https://docs.nvidia.com/deeplearning/physicsnemo/physicsnemo-sym/user_guide/basics/lid_driven_cavity_flow.html?utm_source (accessed on 30 July 2025).
- Hennigh, O.; Narasimhan, S.; Nabian, M.A.; Subramaniam, A.; Tangsali, K.; Fang, Z.; Rietmann, M.; Byeon, W.; Choudhry, S. NVIDIA SimNet™: An AI-accelerated multi-physics simulation framework. In Proceedings of the Computational Science—ICCS 2021, Krakow, Poland, 16–18 June 2021; pp. 447–461. [Google Scholar]
- Buckingham, E. On physically similar systems; illustrations of the use of dimensional equations. Phys. Rev. 1914, 135, 345–376. [Google Scholar] [CrossRef]
- Barenblatt, G.I. Scaling, Self-Similarity, and Intermediate Asymptotics; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Le-Duc, T.; Lee, S.; Nguyen-Xuan, H.; Lee, J. A hierarchically normalized physics-informed neural network for solving differential equations: Application for solid mechanics problems. Eng. Appl. Artif. Intell. 2024, 133, 108400. [Google Scholar] [CrossRef]
- Rasht-Behesht, M.; Huber, C.; Shukla, K.; Karniadakis, G.E. Physics-informed neural networks (PINNs) for wave propagation and full waveform inversions. J. Geophys. Res. Solid Earth 2022, 127, e2021JB023120. [Google Scholar] [CrossRef]
- Wang, R.; Kashinath, K.; Mustafa, M.; Albert, A.; Yu, R. Towards physics-informed deep learning for turbulent flow prediction. In Proceedings of the 26th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Virtual Event, 6–10 July 2020; pp. 1457–1466. [Google Scholar]
- Xiang, Z.; Peng, W.; Liu, X.; Yao, W. Self-adaptive loss balanced physics-informed neural networks. Neurocomputing 2021, 496, 11–34. [Google Scholar] [CrossRef]
- Urbán, J.F.; Stefanou, P.; Pons, J.A. Unveiling the optimization process of physics informed neural networks: How accurate and competitive can PINNs be? J. Comput. Phys. 2025, 523, 113656. [Google Scholar] [CrossRef]
- Li, S.; Feng, X. Dynamic weight strategy of physics-informed neural networks for the 2D Navier-Stokes equations. Entropy 2022, 24, 1254. [Google Scholar] [CrossRef]
- Dastoorian, F.; Pakzad, L.; Kozinski, J.; Behzadfar, E. A CFD investigation on the aerosol drug delivery in the mouth–throat airway using a pressurized metered-dose inhaler device. Processes 2022, 10, 1230. [Google Scholar] [CrossRef]
- Xi, J.; Talaat, M.; Si, X.; Dong, H. Flow dynamics and acoustics from glottal vibrations at different frequencies. Acoustics 2022, 4, 915–933. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, J.; Tian, L.; Inthavong, K.; Tu, J. Numerical and experimental analysis of inhalation airflow dynamics in a human pharyngeal airway. Int. J. Environ. Res. Public Health 2020, 17, 1556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Shi, X.-Q.; Cai, W.; Li, B.; Zhao, Y. Computational fluid–structure interaction analysis of flapping uvula on aerodynamics and pharyngeal vibration in a pediatric airway. Sci. Rep. 2023, 13, 2013. [Google Scholar] [CrossRef]
- Kou, G.; Li, X.; Wang, Y.; Lin, M.; Zeng, Y.; Yang, X.; Yang, Y.; Gan, Z. CFD simulation of airflow dynamics during cough based on CT-scanned respiratory airway geometries. Symmetry 2018, 10, 595. [Google Scholar] [CrossRef]
- Bottou, L.; Curtis, F.E.; Nocedal, J. Optimization methods for large-scale machine learning. SIAM Rev. 2018, 60, 223–311. [Google Scholar] [CrossRef]
- Duchi, J.; Hazan, E.; Singer, Y. Adaptive subgradient methods for online learning and stochastic optimization. J. Mach. Learn. Res. 2011, 12, 2121–2159. [Google Scholar]
- Higham, N.J. Accuracy and Stability of Numerical Algorithms; SIAM: Philadelphia, PA, USA, 2002. [Google Scholar]
- Jacot, A.; Gabriel, F.; Ged, F.; Hongler, C. Order and chaos: NTK views on DNN normalization, checkerboard and boundary artifacts. arXiv 2019, arXiv:1907.05715. [Google Scholar]
- Jacot, A.; Gabriel, F.; Hongler, C. Neural tangent kernel: Convergence and generalization in neural networks. Adv. Neural Inf. Process. Syst. 2018, 31, 8571–8580. [Google Scholar]
- Wang, S.; Sankaran, S.; Perdikaris, P. Respecting causality is all you need for training physics-informed neural networks. arXiv 2022, arXiv:2203.07404. [Google Scholar] [CrossRef]
- Yu, T.; Kumar, S.; Gupta, A.; Levine, S.; Hausman, K.; Finn, C. Gradient surgery for multi-task learning. Adv. Neural Inf. Process. Syst. 2020, 33, 5824–5836. [Google Scholar]
- Gupta, V.; Koren, T.; Singer, Y. Shampoo: Preconditioned stochastic tensor optimization. arXiv 2018, arXiv:1802.09568. [Google Scholar] [CrossRef]
- Ren, Z.; Zhou, S.; Liu, D.; Liu, Q. Physics-informed neural networks: A review of methodological evolution, theoretical foundations, and interdisciplinary frontiers toward next-generation scientific computing. Appl. Sci. 2025, 15, 8092. [Google Scholar] [CrossRef]
- Jagtap, A.D.; Kharazmi, E.; Karniadakis, G.E. Conservative physics-informed neural networks on discrete domains for conservation laws: Applications to forward and inverse problems. Comput. Methods Appl. Mech. Eng. 2020, 365, 113028. [Google Scholar] [CrossRef]
- Shukla, K.; Jagtap, A.D.; Karniadakis, G.E. Parallel physics-informed neural networks via domain decomposition. J. Comput. Phys. 2021, 447, 110683. [Google Scholar] [CrossRef]
- Sukumar, N.; Srivastava, A. Exact imposition of boundary conditions with distance functions in physics-informed deep neural networks. Comput. Methods Appl. Mech. Eng. 2022, 389, 114333. [Google Scholar] [CrossRef]
- Xiang, Z.; Peng, W.; Zhou, W.; Yao, W. Hybrid finite difference with the physics-informed neural network for solving PDE in complex geometries. arXiv 2022, arXiv:2202.07926. [Google Scholar] [CrossRef]
- Barschkis, S. Exact and soft boundary conditions in Physics-Informed Neural Networks for the Variable Coefficient Poisson equation. arXiv 2023, arXiv:2310.02548. [Google Scholar] [CrossRef]
- Plankovskyy, S.; Tsegelnyk, Y.; Shyshko, N.; Litvinchev, I.; Romanova, T.; Velarde Cantú, J.M. Review of physics-informed neural networks: Challenges in loss function design and geometric integration. Mathematics 2025, 13, 3289. [Google Scholar] [CrossRef]
- El Hassan, M.; Mjalled, A.; Miron, P.; Mönnigmann, M.; Bukharin, N. Machine learning in fluid dynamics—Physics-informed neural networks (PINNs) using sparse data: A review. Fluids 2025, 10, 226. [Google Scholar] [CrossRef]
- Martínez-Esteban, A.; Calvo-Barlés, P.; Martín-Moreno, L.; Rodrigom, S.G. Physics-informed neural networks with dynamical boundary constraints. arXiv 2025, arXiv:2507.21800. [Google Scholar] [CrossRef]
- Kalajahi, A.P.; Csala, H.; Naderi, F.; Mamun, Z.; Yadav, S.; Amili, O.; Arzani, A.; D’Souza, R. Input parameterized physics informed neural network for advanced 4d flow MRI processing. Eng. Appl. Artif. Intell. 2025, 150, 110600. [Google Scholar]
- Kang, J.; Jung, E.C.; Koo, H.J.; Yang, D.H.; Ha, H. Flow-rate constrained physics-informed neural networks for flow field error correction in four-dimensional flow magnetic resonance imaging. IEEE Trans. Med. Imaging, 2025; early access. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, A.; Brikis, G.O.; Dey, B. RANS-PINN based simulation surrogates for predicting turbulent flows. arXiv 2023, arXiv:2306.06034. [Google Scholar] [CrossRef]
- Barari, K.; Si, X.; Xi, J. Impacts of mask wearing and leakages on cyclic respiratory flows and facial thermoregulation. Fluids 2024, 9, 9. [Google Scholar] [CrossRef]
- Khaled, T.; Xi, J.; Baldez, P.; Hecht, A. Radiation dosimetry of inhaled radioactive aerosols: CFPD and MCNP transport simulation of Radionuclides in the lungs. Sci. Rep. 2019, 9, 17450. [Google Scholar] [CrossRef] [PubMed]
- Talaat, M.; Si, X.; Tanbour, H.; Xi, J. Numerical studies of nanoparticle transport and deposition in terminal alveolar models with varying complexities. Med One 2019, 4, e190018. [Google Scholar] [CrossRef]
- Si, X.A.; Talaat, M.; Xi, J. Effects of guiding vanes and orifice jet flow of a metered-dose inhaler on drug dosimetry in human respiratory tract. Exp. Comput. Multiph. Flow. 2023, 5, 247–261. [Google Scholar] [CrossRef]
- Talaat, M.; Si, X.; Xi, J. Evaporation dynamics and dosimetry methods in numerically assessing MDI performance in pulmonary drug delivery. Fluids 2024, 9, 286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talaat, M.; Si, X.; Dong, H.; Xi, J. Physics-Informed Neural Networks Simulation and Validation of Airflows in Three-Dimensional Upper Respiratory Tracts. Fluids 2025, 10, 306. https://doi.org/10.3390/fluids10120306
Talaat M, Si X, Dong H, Xi J. Physics-Informed Neural Networks Simulation and Validation of Airflows in Three-Dimensional Upper Respiratory Tracts. Fluids. 2025; 10(12):306. https://doi.org/10.3390/fluids10120306
Chicago/Turabian StyleTalaat, Mohamed, Xiuhua Si, Haibo Dong, and Jinxiang Xi. 2025. "Physics-Informed Neural Networks Simulation and Validation of Airflows in Three-Dimensional Upper Respiratory Tracts" Fluids 10, no. 12: 306. https://doi.org/10.3390/fluids10120306
APA StyleTalaat, M., Si, X., Dong, H., & Xi, J. (2025). Physics-Informed Neural Networks Simulation and Validation of Airflows in Three-Dimensional Upper Respiratory Tracts. Fluids, 10(12), 306. https://doi.org/10.3390/fluids10120306







