Physicochemical Properties and Cookie-Making Performance as Fat Replacer of Wax-Based Rice Bran Oil Oleogels
Abstract
1. Introduction
2. Results and Discussion
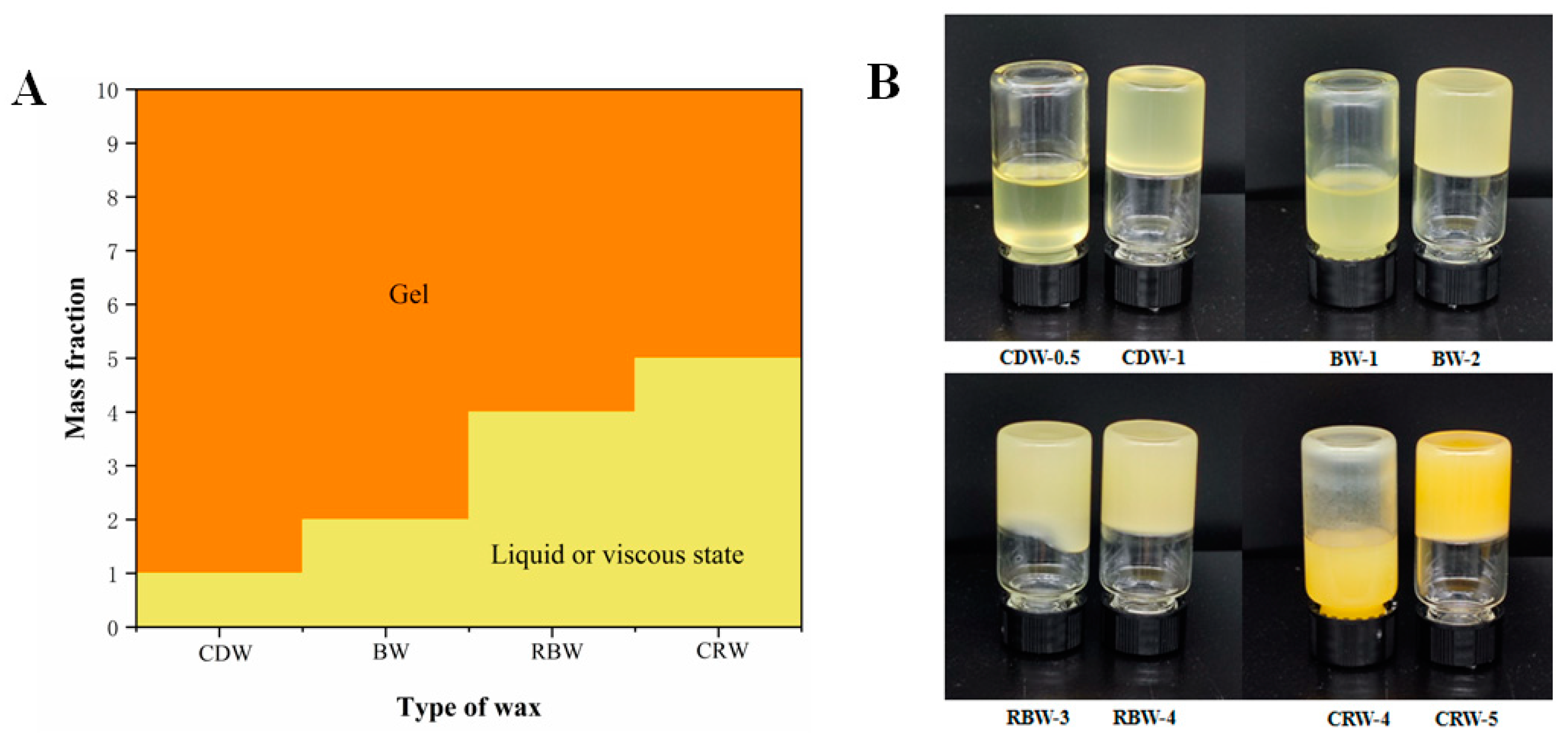
2.1. Determination of the Critical Concentration of Oleogels (25 °C)
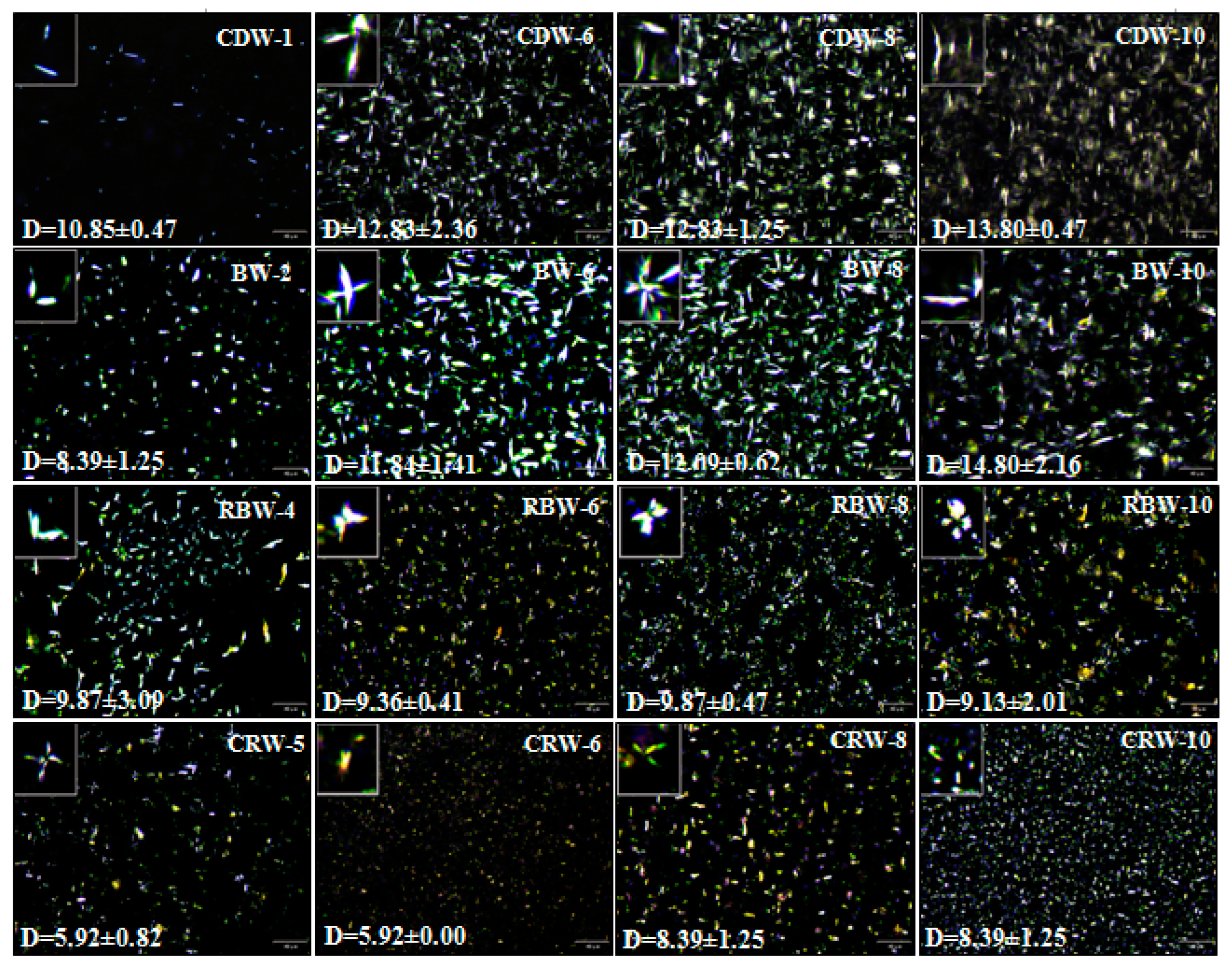
2.2. Microstructure Analysis of Oleogels (PLM)
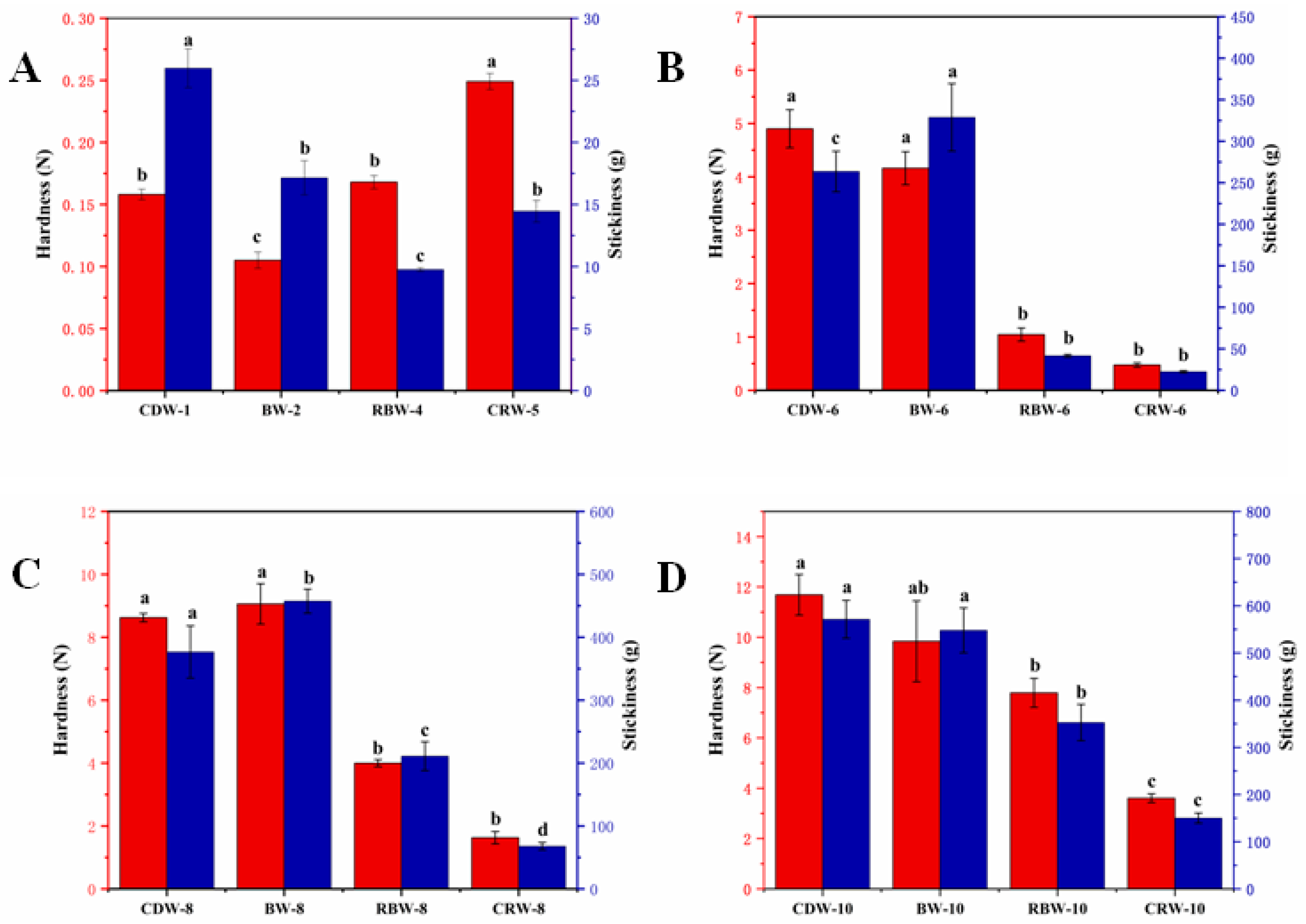
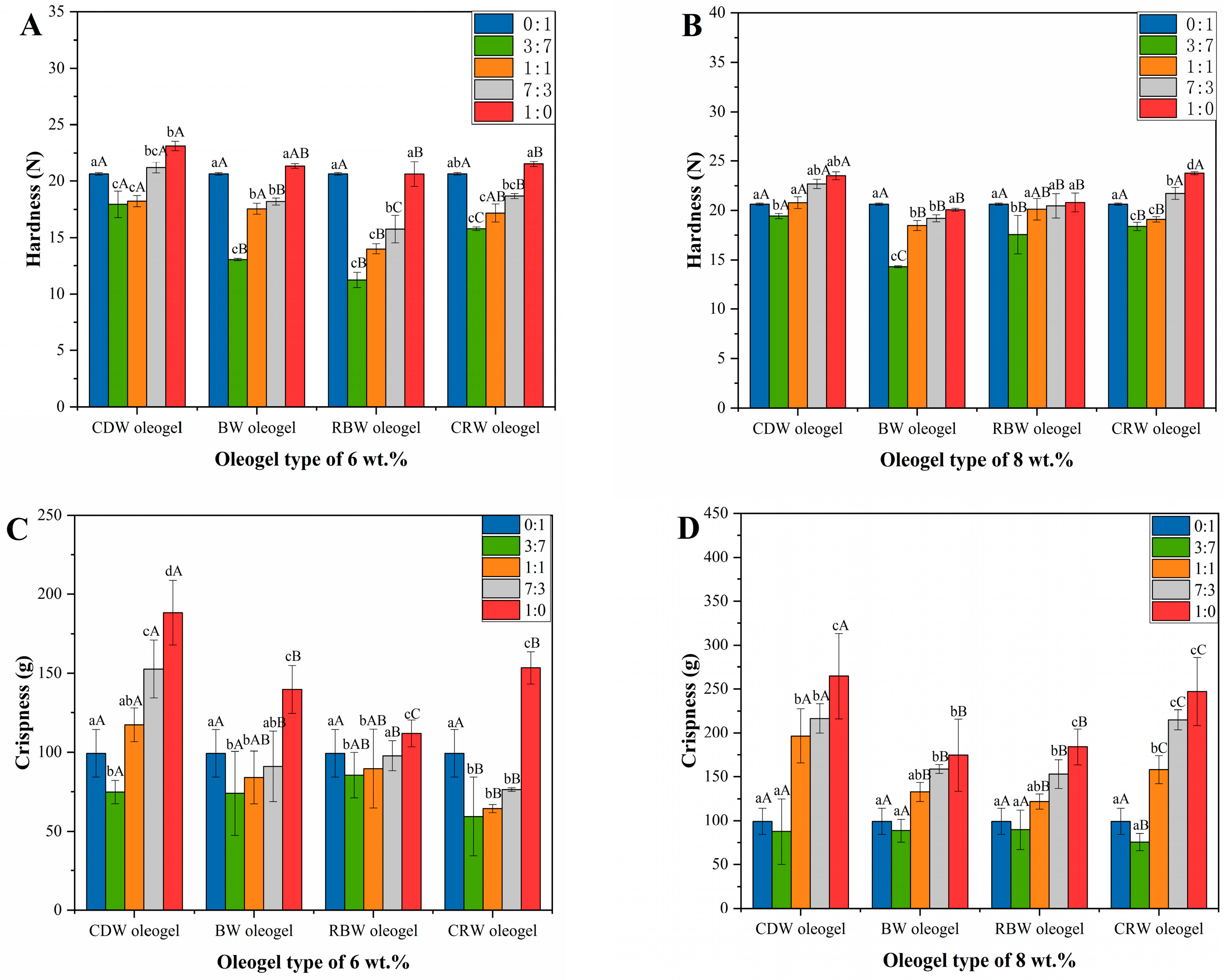
2.3. Texture Analysis of Oleogels
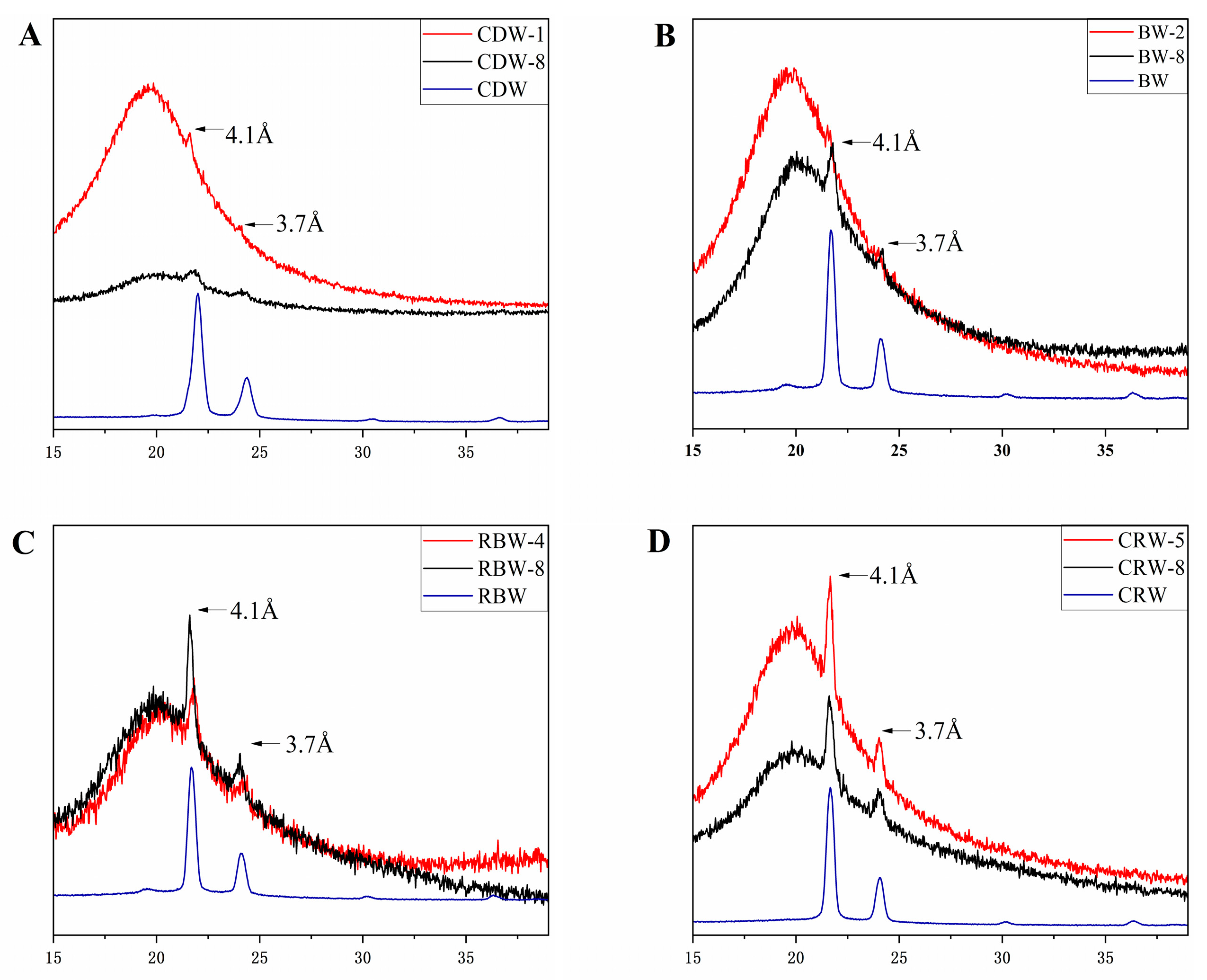
2.4. X-ray Diffraction Analysis (XRD) of Oleogels
2.5. Texture Analysis of Optimized Formulation of Oleogels in Cookies
2.6. Color Analysis of Optimized Formulation of Oleogels in Cookies
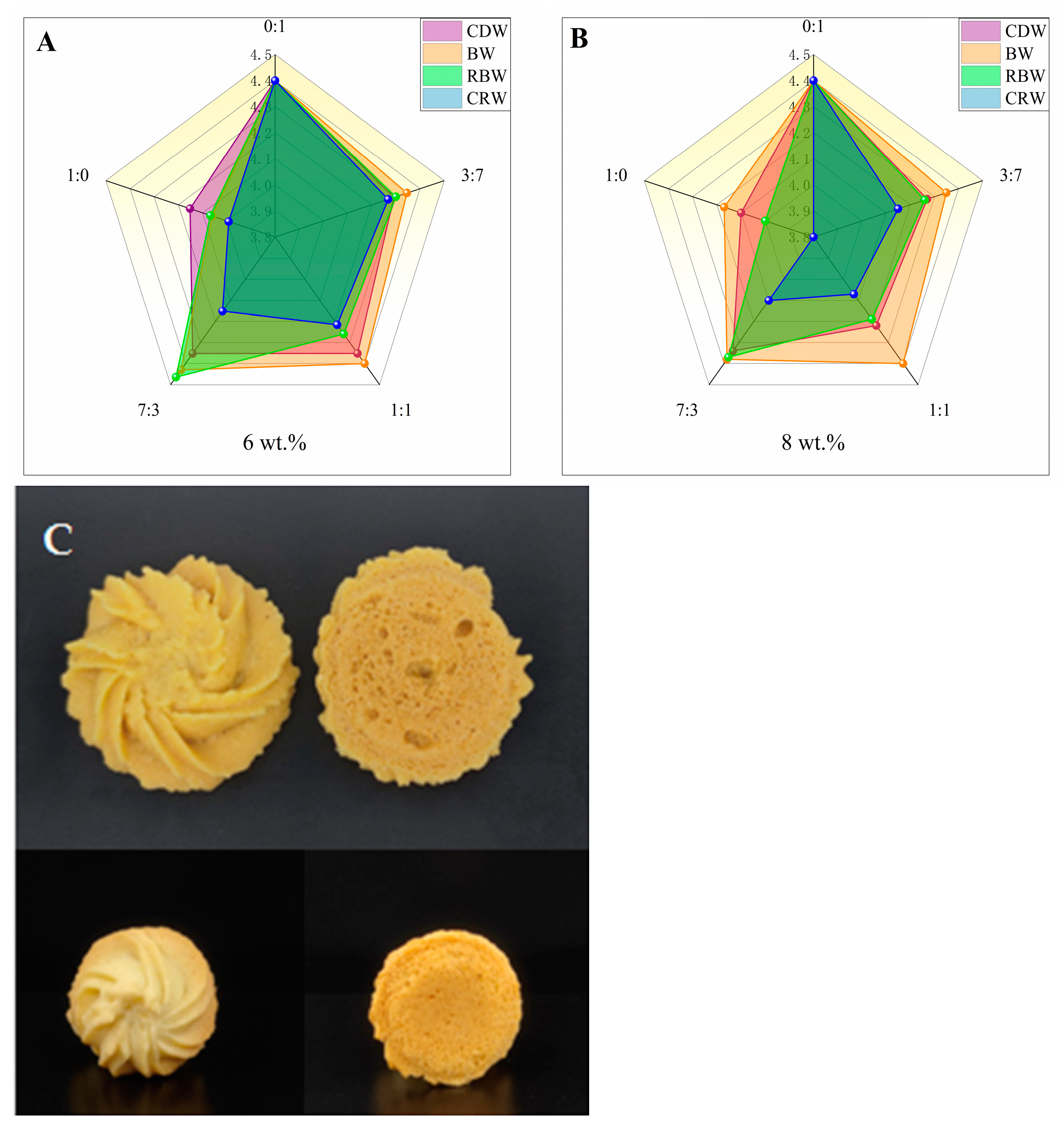
2.7. Sensory Analysis of Optimized Formulation of Oleogels in Cookies
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Oleogels
4.3. Preparation of Oleogel Application in Cookies
4.4. Determination of the Critical Concentration of Oleogels
4.5. Polarized Light Microscopy (PLM) of Oleogels
4.6. Texture Analysis of Oleogels
4.7. X-ray Diffraction Analysis (XRD) of Oleogels
4.8. Texture Analysis of the Optimized Formulation of Oleogels in Cookies
4.9. Color Analysis of the Optimized Formulation of Oleogels in Cookies
4.10. Sensory Analysis of the Optimized Formulation of Oleogels in Cookies
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viriato, R.L.S.; Queiros, M.D.S.; da Gama, M.A.S.; Ribeiro, A.P.B.; Gigante, M.L. Milk fat as a structuring agent of plastic lipid bases. Food Res. Int. 2018, 111, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.; Kamarulzaman, N.A.; Omar, Z. Influence of enzymatic and chemical interesterification on crystallisation properties of refined, bleached and deodourised (RBD) palm oil and RBD palm kernel oil blends. Food Res. Int. 2018, 106, 982–991. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Madhujith, T. Current trends in applications of enzymatic interesterification of fats and oils: A review. Lwt-Food Sci. Technol. 2020, 132, 109880. [Google Scholar] [CrossRef]
- Kadhum, A.A.H.; Shamma, M.N. Edible lipids modification processes: A review. Crit. Rev. Food Sci. 2017, 57, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Margarines: Historical approach, technological aspects, nutritional profile, and global trends. Food Res. Int. 2021, 147, 110486. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.A.; Wanders, A.J.; Katan, M.B. Trans fatty acids and cardiovascular health: Research completed? Eur. J. Clin. Nutr. 2013, 67, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.B.; Cunha, R.L. Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef]
- Dawczynski, C.; Lorkowski, S. Trans-fatty acids and cardiovascular risk: Does origin matter? Expert Rev. Cardiovasc. Ther. 2016, 14, 1001–1005. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.Y.; Guo, Y.; Wang, Y.; Liu, Y.F. Effects of thickening agents on the formation and properties of edible oleogels based on hydroxypropyl methyl cellulose. Food Chem. 2018, 246, 137–149. [Google Scholar] [CrossRef]
- Khiabani, A.A.; Tabibiazar, M.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Preparation and characterization of carnauba wax/adipic acid oleogel: A new reinforced oleogel for application in cake and beef burger. Food Chem. 2020, 333, 127446. [Google Scholar] [CrossRef]
- Li, S.Y.; Wu, G.C.; Li, X.J.; Jin, Q.Z.; Wang, X.G.; Zhang, H. Roles of gelator type and gelation technology on texture and sensory properties of cookies prepared with oleogels. Food Chem. 2021, 356, 129667. [Google Scholar] [CrossRef] [PubMed]
- Moriano, M.E.; Alamprese, C. Organogels as novel ingredients for low saturated fat ice creams. Lwt-Food Sci. Technol. 2017, 86, 371–376. [Google Scholar] [CrossRef]
- Sun, P.; Xia, B.; Ni, Z.J.; Wang, Y.; Elam, E.; Thakur, K.; Ma, Y.L.; Wei, Z.J. Characterization of functional chocolate formulated using oleogels derived from beta-sitosterol with gamma-oryzanol/lecithin/stearic acid. Food Chem. 2021, 360, 130017. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, G.Q.; Lin, Y.W. Physical and bloom stability of low-saturation chocolates with oleogels based on different gelation mechanisms. Lwt-Food Sci. Technol. 2021, 140, 110807. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Zhang, Q.Y.; Ju, X.R.; Wang, Z.G.; He, R. Study of monoglycerides enriched with unsaturated fatty acids at sn-2 position as oleogelators for oleogel preparation. Food Chem. 2021, 354, 129534. [Google Scholar] [CrossRef] [PubMed]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H.; Sharifimehr, S. Physicochemical properties of beef burger after partial incorporation of ethylcellulose oleogel instead of animal fat. J. Food Sci. Technol. 2021, 58, 4775–4784. [Google Scholar] [CrossRef]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative Study on the Ability of Different Oleogelators to Structure Sunflower Oil. Food Biophys. 2020, 15, 42–49. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, A.K.; Perez-Martinez, J.D.; Gallegos-Infante, J.A.; Toro-Vazquez, J.F.; Ornelas-Paz, J.J. Rheological properties of ethyl cellulose-monoglyceride-candelilla wax oleogel vis-a-vis edible shortenings. Carbohyd. Polym. 2021, 252, 117171. [Google Scholar] [CrossRef]
- Pang, M.; Shi, Z.; Lei, Z.; Ge, Y.; Jiang, S.; Cao, L. Structure and thermal properties of beeswax-based oleogels with different types of vegetable oil. Grasas Aceites 2020, 71, e380. [Google Scholar] [CrossRef]
- Hwang, H.S.; Winkler-Moser, J.K. Properties of margarines prepared from soybean oil oleogels with mixtuRes. of candelilla wax and beeswax. J. Food Sci. 2020, 85, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Scharfe, M.; Floter, E. Oleogelation: From Scientific Feasibility to Applicability in Food Products. Eur. J. Lipid Sci. Technol. 2020, 122, 2000213. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, T.; Tao, G.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Characterization and determination of free phytosterols and phytosterol conjugates: The potential phytochemicals to classify different rice bran oil and rice bran. Food Chem. 2021, 344, 128624. [Google Scholar] [CrossRef] [PubMed]
- Tangpromphan, P.; Duangsrisai, S.; Jaree, A. Development of separation method for Alpha-Tocopherol and Gamma-Oryzanol extracted from rice bran oil using Three-Zone simulated moving bed process. Sep. Purif. Technol. 2021, 272, 118930. [Google Scholar] [CrossRef]
- Imsanguan, P.; Roaysubtawee, A.; Borirak, R.; Pongamphai, S.; Douglas, S.; Douglas, P.L. Extraction of α-tocopherol and γ-oryzanol from rice bran. LWT-Food Sci. Technol. 2008, 41, 1417–1424. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Chang, M.; Tang, L.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Antioxidant interaction of alpha-tocopherol, gamma-oryzanol and phytosterol in rice bran oil. Food Chem. 2021, 343, 128431. [Google Scholar] [CrossRef]
- Pestana-Bauer, V.R.; Zambiazi, R.C.; Mendonca, C.R.; Beneito-Cambra, M.; Ramis-Ramos, G. gamma-Oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chem. 2012, 134, 1479–1483. [Google Scholar] [CrossRef]
- Sawada, K.; Nakagami, T.; Rahmania, H.; Matsuki, M.; Ito, J.; Mohri, T.; Ogura, Y.; Kuwahara, S.; Hashimoto, H.; Nakagawa, K. Isolation and structural elucidation of unique gamma-oryzanol species in rice bran oil. Food Chem. 2021, 337, 127956. [Google Scholar] [CrossRef] [PubMed]
- Wijarnprecha, K.; Aryusuk, K.; Santiwattana, P.; Sonwai, S.; Rousseau, D. Structure and rheology of oleogels made from rice bran wax and rice bran oil. Food Res. Int. 2018, 112, 199–208. [Google Scholar] [CrossRef]
- Wang, N.; Chen, J.; Zhou, Q.; Jiang, L.; Wang, L.; Dai, Y.; Yu, D.; Elfalleh, W. Crude Wax Extracted from Rice Bran Oil Improves Oleogel Properties and Oxidative Stability. Eur. J. Lipid Sci. Technol. 2021, 123, 2000091. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil-Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Issara, U. Improvement of Thai Sweet Sausage (Goon Chiang) Properties by Oleogel Made of Rice Bran Wax and Rice Bran Oil: A Textural, Sensorial, and Nutritional Aspect. IOP Conf. Ser. Earth Environ. Sci. 2022, 995, 012045. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Sintang, M.D.B.; Danthine, S.; Van de Walle, D.; Rimaux, T.; Dewettinck, K. Crystallization and Gelation Behavior of Low- and High Melting Waxes in Rice Bran Oil: A Case-Study on Berry Wax and Sunflower Wax. Food Biophys. 2016, 12, 97–108. [Google Scholar] [CrossRef]
- Dhal, S.; Qureshi, D.; Mohanty, B.; Maji, S.; Anis, A.; Kim, D.; Sarkar, P.; Pal, K. Kokum butter and rice bran oil-based oleogels as novel ocular drug delivery systems. In Advances and Challenges in Pharmaceutical Technology; Academic Press: Cambridge, MA, USA, 2021; pp. 147–179. [Google Scholar]
- Marangoni, A.; Garti, N. Edible Oleogels: Structure and Health Implications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–342. [Google Scholar]
- Wettlaufer, T.; Flöter, E. Wax based oleogels and their application in sponge cakes. Food Funct. 2022, 13, 9419–9433. [Google Scholar] [CrossRef] [PubMed]
- Mert, B.; Demirkesen, I. Reducing saturated fat with oleogel/shortening blends in a baked product. Food Chem. 2016, 199, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Bae, W.; Hwang, H.S.; Lee, H.G.; Lee, S. Evaluation of canola oil oleogels with candelilla wax as an alternative to shortening in baked goods. Food Chem. 2015, 187, 525–529. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L.; Wu, G.; Jin, Q.; Wang, X.; Zhang, H. Relationship between the microstructure and physical properties of emulsifier based oleogels and cookies quality. Food Chem. 2022, 377, 131966. [Google Scholar] [CrossRef]
- Kanya, T.; Rao, L.; Sastry, M. Characterization of wax esters, free fatty alcohols and free fatty acids of crude wax from sunflower seed oil refineries. Food Chem. 2007, 101, 1552–1557. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Sobolev, R.; Frolova, Y.; Sarkisyan, V.; Makarenko, M.; Kochetkova, A. Effect of beeswax and combinations of its fractions on the oxidative stability of oleogels. Food BioSci. 2022, 48, 101744. [Google Scholar] [CrossRef]
- Huang, Z.H.; Guo, B.Z.; Deng, C.; Tang, C.; Liu, C.M.; Hu, X.T. Fabrication and characterization of the W/O/W multiple emulsion through oleogelation of oil. Food Chem. 2021, 358, 129856. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Cao, L.; Kang, S.; Jiang, S.; Pang, M. Influence of wax type on characteristics of oleogels from camellia oil and medium chain triglycerides. Int. J. Food Sci. Technol. 2021, 57, 2003–2014. [Google Scholar] [CrossRef]
- Alvarez-Ramirez, J.; Vernon-Carter, E.J.; Carrera-Tarela, Y.; Garcia, A.; Roldan-Cruz, C. Effects of candelilla wax/canola oil oleogel on the rheology, texture, thermal properties and in vitro starch digestibility of wheat sponge cake bread. Lwt-Food Sci. Technol. 2020, 130, 109701. [Google Scholar] [CrossRef]
- Li, J.X.; Guo, R.H.; Bi, Y.L.; Zhang, H.; Xu, X.B. Comprehensive evaluation of saturated monoglycerides for the forming of oleogels. Lwt-Food Sci. Technol. 2021, 151, 112061. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. The gelation of oil using ethyl cellulose. Carbohyd. Polym. 2015, 117, 869–878. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chandrapala, J.; Truong, T.; Farahnaky, A. Oleogels prepared with low molecular weight gelators: Texture, rheology and sensory properties, a review. Crit. Rev. Food Sci. 2022, 1–45. [Google Scholar] [CrossRef]
- Yilmaz, E.; Ogutcu, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct. 2015, 6, 1194–1204. [Google Scholar] [CrossRef]
- Ogutcu, M.; Yilmaz, E.; Guneser, O. Influence of Storage on Physicochemical and Volatile Features of Enriched and Aromatized Wax Organogels. J. Am. Oil Chem. Soc. 2015, 92, 1429–1443. [Google Scholar] [CrossRef]
- Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The role of hydrogen bonds in TAG derivative-based oleogel structure and properties. Food Chem. 2021, 334, 127585. [Google Scholar] [CrossRef]
- Gao, Y.X.; Lei, Y.J.; Wu, Y.H.; Liang, H.S.; Li, J.; Pei, Y.; Li, Y.; Li, B.; Luo, X.G.; Liu, S.L. Beeswax: A potential self-emulsifying agent for the construction of thermal-sensitive food W/O emulsion. Food Chem. 2021, 349, 129203. [Google Scholar] [CrossRef]
| Sample | L* | a* | b* | ΔE* | Sample | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|---|---|---|---|
| Control | 66.70 ± 2.86 ab | 3.44 ± 1.16 b | 35.40 ± 1.74 b | 41.51 ± 1.89 b | |||||
| CDW | 72.59 ± 2.13 c | −2.90 ± 0.15 c | 34.37 ± 0.99 b | 37.96 ± 0.21 a | BW | 60.14 ± 1.23 b | −2.70 ± 0.18 c | 47.77 ± 1.12 a | 44.77 ± 1.30 b |
| CDW-6 | BW-6 | ||||||||
| 3:7 | 67.13 ± 1.01 a | 0.24 ± 0.38 a | 32.45 ± 0.75 ab | 36.65 ± 0.30 a | 3:7 | 66.56 ± 0.92 ab | 2.90 ± 0.95 b | 32.34 ± 0.47 b | 38.73 ± 0.69 a |
| 1:1 | 66.94 ± 1.39 a | 0.24 ± 0.44 a | 30.40 ± 1.21 a | 36.49 ± 1.33 a | 1:1 | 66.20 ± 1.15 ab | 2.74 ± 1.10 b | 31.57 ± 1.52 b | 38.11 ± 1.24 a |
| 7:3 | 64.09 ± 1.38 b | 1.99 ± 0.36 ab | 30.20 ± 1.33 a | 40.36 ± 1.31 ab | 7:3 | 65.77 ± 1.03 a | 3.49 ± 1.29 a | 30.25 ± 2.57 b | 37.75 ± 1.46 a |
| 1:0 | 63.37 ± 1.66 b | 2.23 ± 0.73 ab | 28.93 ± 1.01 c | 40.11 ± 1.02 ab | 1:0 | 64.50 ± 1.70 a | 4.52 ± 0.17 a | 29.13 ± 2.62 c | 40.79 ± 0.98 ab |
| CDW-8 | BW-8 | ||||||||
| 3:7 | 65.42 ± 1.18 ab | 1.54 ± 0.92 a | 30.47 ± 0.52 a | 37.63 ± 1.02 a | 3:7 | 65.20 ± 0.45 a | 3.04 ± 0.51 b | 33.42 ± 0.35 a | 40.96 ± 0.61 ab |
| 1:1 | 64.03 ± 0.32 b | 1.88 ± 1.33 ab | 29.33 ± 0.82 a | 36.85 ± 0.43 c | 1:1 | 65.13 ± 0.86 a | 2.56 ± 0.77 a | 32.39 ± 0.64 a | 38.85 ± 0.44 a |
| 7:3 | 63.12 ± 1.06 b | 2.46 ± 0.75 b | 28.46 ± 1.36 c | 39.65 ± 0.46 a | 7:3 | 64.70 ± 1.48 a | 2.35 ± 0.65 a | 30.15 ± 1.05 b | 39.07 ± 0.72 a |
| 1:0 | 62.57 ± 0.48 b | 2.56 ± 0.84 b | 27.85 ± 1.03 d | 40.06 ± 0.33 ab | 1:0 | 64.15 ± 1.38 b | 1.11 ± 0.33 c | 29.67 ± 0.93 c | 35.53 ± 0.49 c |
| RBW | 76.47 ± 2.49 c | −2.49 ± 0.49 c | 36.80 ± 2.34 b | 36.91 ± 0.96 a | CRW | 62.49 ± 0.54 b | 1.30 ± 0.37 c | 42.92 ± 0.74 a | 54.02 ± 0.68 d |
| RBW-6 | CRW-6 | ||||||||
| 3:7 | 70.40 ± 0.32 a | 3.40 ± 0.47 a | 33.85 ± 1.23 ab | 37.40 ± 0.88 a | 3:7 | 64.81 ± 1.89 a | 2.46 ± 1.00 a | 31.35 ± 2.38 b | 40.55 ± 0.73 ab |
| 1:1 | 67.44 ± 1.52 ab | 2.69 ± 0.61 a | 29.16 ± 0.28 c | 36.16 ± 1.13 c | 1:1 | 64.21 ± 0.69 a | 1.01 ± 0.61 c | 30.41 ± 1.20 a | 37.30 ± 1.33 a |
| 7:3 | 66.66 ± 2.81 ab | 2.08 ± 0.55 ab | 28.39 ± 2.35 c | 37.78 ± 1.10 a | 7:3 | 64.11 ± 1.16 a | 1.34 ± 0.17 c | 28.56 ± 0.69 c | 39.71 ± 0.51 a |
| 1:0 | 64.65 ± 0.99 b | 1.40 ± 0.07 b | 27.15 ± 0.53 d | 38.40 ± 0.57 a | 1:0 | 62.95 ± 0.80 b | 2.24 ± 1.04 b | 27.28 ± 1.73 c | 39.87 ± 0.54 a |
| RBW-8 | CRW-8 | ||||||||
| 3:7 | 69.90 ± 2.49 a | 3.08 ± 0.17 a | 31.41 ± 0.40 a | 36.14 ± 1.38 a | 3:7 | 64.28 ± 0.82 a | 1.43 ± 0.27 c | 28.98 ± 1.56 c | 39.65 ± 0.43 a |
| 1:1 | 67.18 ± 2.15 ab | 3.22 ± 0.24 a | 29.60 ± 0.85 a | 34.89 ± 1.36 c | 1:1 | 63.84 ± 0.76 b | 2.35 ± 0.76 b | 28.23 ± 1.21 c | 37.63 ± 0.82 a |
| 7:3 | 65.00 ± 3.40 b | 2.43 ± 0.19 ab | 28.56 ± 0.88 c | 38.15 ± 1.56 a | 7:3 | 62.45 ± 1.12 b | 2.44 ± 0.63 b | 27.65 ± 0.43 c | 40.10 ± 0.12 ab |
| 1:0 | 64.57 ± 0.18 b | 1.16 ± 0.22 b | 26.23 ± 1.82 d | 37.26 ± 0.44 a | 1:0 | 62.02 ± 0.74 c | 2.65 ± 1.48 ab | 26.59 ± 0.78 d | 41.82 ± 0.38 c |
| Sample | Shape (5′) | Color (5′) | Odor (5′) | Texture (5′) | Tatse (5′) | Total (5′) |
|---|---|---|---|---|---|---|
| 0:1 | 4.51 ± 0.18 a | 4.34 ± 0.12 a | 4.32 ± 0.32 a | 4.40 ± 0.29 a | 4.45 ± 0.33 a | 22.02 ± 0.07 a |
| CDW-6 | ||||||
| 3:7 | 4.40 ± 0.13 a | 4.28 ± 0.12 a | 4.32 ± 0.16 a | 4.20 ± 0.17 a | 4.26 ± 0.16 a | 21.46 ± 0.07 a |
| 1:1 | 4.44 ± 0.17 a | 4.32 ± 0.16 a | 4.30 ± 0.14 a | 4.34 ± 0.24 a | 4.36 ± 0.17 a | 21.76 ± 0.05 a |
| 7:3 | 4.46 ± 0.08 ab | 4.26 ± 0.21 a | 4.30 ± 0.19 a | 4.32 ± 0.16 a | 4.42 ± 0.17 ab | 21.76 ± 0.08 a |
| 1:0 | 4.36 ± 0.12 a | 4.30 ± 0.19 a | 4.12 ± 0.25 a | 3.96 ± 0.19 a | 4.02 ± 0.16 a | 20.76 ± 0.16 a |
| CDW-8 | ||||||
| 3:7 | 4.36 ± 0.17 a | 4.32 ± 0.21 a | 4.24 ± 0.16 a | 4.20 ± 0.19 ab | 4.24 ± 0.16 a | 21.36 ± 0.06 ab |
| 1:1 | 4.24 ± 0.16 ab | 4.30 ± 0.14 a | 4.14 ± 0.12 a | 4.24 ± 0.16 ab | 4.20 ± 0.19 ab | 21.12 ± 0.05 b |
| 7:3 | 4.48 ± 0.16 a | 4.36 ± 0.19 a | 4.26 ± 0.14 a | 4.30 ± 0.19 a | 4.30 ± 0.19 a | 21.7 ± 0.08 a |
| 1:0 | 4.04 ± 0.21 ab | 4.32 ± 0.16 a | 4.10 ± 0.13 ab | 4.04 ± 0.15 a | 3.98 ± 0.13 a | 20.48 ± 0.12 ab |
| BW-6 | ||||||
| 3:7 | 4.28 ± 0.19 a | 4.40 ± 0.12 a | 4.30 ± 0.10 a | 4.40 ± 0.12 a | 4.35 ± 0.12 a | 21.73 ± 0.05 a |
| 1:1 | 4.40 ± 0.12 a | 4.44 ± 0.21 a | 4.32 ± 0.25 a | 4.42 ± 0.29 a | 4.42 ± 0.22 a | 22.00 ± 0.04 a |
| 7:3 | 4.58 ± 0.10 a | 4.30 ± 0.19 a | 4.32 ± 0.10 a | 4.40 ± 0.25 a | 4.55 ± 0.19 a | 22.15 ± 0.12 a |
| 1:0 | 4.08 ± 0.28 ab | 4.16 ± 0.21 a | 4.00 ± 0.00 a | 4.04 ± 0.30 a | 4.02 ± 0.26 a | 20.30 ± 0.06 a |
| BW-8 | ||||||
| 3:7 | 4.36 ± 0.12 a | 4.26 ± 0.16 a | 4.32 ± 0.18 a | 4.42 ± 0.12 a | 4.40 ± 0.24 a | 21.76 ± 0.06 a |
| 1:1 | 4.50 ± 0.19 a | 4.36 ± 0.12 a | 4.32 ± 0.12 a | 4.40 ± 0.09 a | 4.48 ± 0.35 a | 22.06 ± 0.07 a |
| 7:3 | 4.46 ± 0.21 a | 4.34 ± 0.24 a | 4.26 ± 0.22 a | 4.36 ± 0.20 a | 4.46 ± 0.15 a | 21.88 ± 0.08 a |
| 1:0 | 4.30 ± 0.19 a | 4.30 ± 0.19 a | 4.16 ± 0.14 a | 4.10 ± 0.19 a | 4.00 ± 0.06 a | 20.86 ± 0.12 a |
| RBW-6 | ||||||
| 3:7 | 4.40 ± 0.13 a | 4.26 ± 0.16 a | 4.28 ± 0.20 a | 4.30 ± 0.11 a | 4.26 ± 0.16 a | 21.50 ± 0.05 a |
| 1:1 | 4.36 ± 0.10 a | 4.28 ± 0.17 a | 4.26 ± 0.16 a | 4.06 ± 0.22 a | 4.34 ± 0.10 a | 21.30 ± 0.11 a |
| 7:3 | 4.60 ± 0.13 a | 4.40 ± 0.13 a | 4.36 ± 0.12 a | 4.44 ± 0.17 a | 4.52 ± 0.13 a | 22.32 ± 0.09 a |
| 1:0 | 4.08 ± 0.19 ab | 4.18 ± 0.12 a | 4.02 ± 0.12 a | 4.02 ± 0.12 a | 4.04 ± 0.10 a | 20.34 ± 0.06 a |
| RBW-8 | ||||||
| 3:7 | 4.34 ± 0.10 a | 4.14 ± 0.22 a | 4.26 ± 0.14 a | 4.26 ± 0.16 ab | 4.32 ± 0.16 a | 21.32 ± 0.07 ab |
| 1:1 | 4.10 ± 0.13 b | 4.32 ± 0.16 a | 4.26 ± 0.16 a | 4.06 ± 0.12 b | 4.22 ± 0.20 ab | 20.96 ± 0.10 ab |
| 7:3 | 4.54 ± 0.21 a | 4.36 ± 0.12 a | 4.26 ± 0.16 a | 4.40 ± 0.18 a | 4.30 ± 0.19 a | 21.86 ± 0.10 a |
| 1:0 | 4.06 ± 0.08 ab | 4.16 ± 0.14 a | 4.02 ± 0.17 ab | 4.04 ± 0.10 a | 3.88 ± 0.12 a | 20.16 ± 0.09 b |
| CRW-6 | ||||||
| 3:7 | 4.32 ± 0.19 a | 4.28 ± 0.23 a | 4.28 ± 0.15 a | 4.22 ± 0.23 a | 4.24 ± 0.19 a | 21.34 ± 0.03 a |
| 1:1 | 4.26 ± 0.21 a | 4.26 ± 0.21 a | 4.22 ± 0.23 a | 4.12 ± 0.16 a | 4.22 ± 0.17 a | 21.08 ± 0.05 a |
| 7:3 | 4.22 ± 0.17 b | 4.24 ± 0.19 a | 4.06 ± 0.22 a | 4.14 ± 0.08 a | 4.10 ± 0.20 b | 20.76 ± 0.07 bc |
| 1:0 | 3.96 ± 0.16 b | 4.14 ± 0.15 a | 3.94 ± 0.17 a | 3.86 ± 0.14 a | 4.06 ± 0.08 a | 19.96 ± 0.10 a |
| CRW-8 | ||||||
| 3:7 | 4.20 ± 0.17 a | 4.18 ± 0.13 a | 4.16 ± 0.10 a | 4.06 ± 0.08 b | 4.16 ± 0.19 a | 20.76 ± 0.05 b |
| 1:1 | 4.14 ± 0.15 b | 4.18 ± 0.13 a | 4.10 ± 0.13 a | 3.98 ± 0.16 b | 3.94 ± 0.14 b | 20.34 ± 0.09 c |
| 7:3 | 4.16 ± 0.19 a | 4.14 ± 0.15 a | 4.18 ± 0.13 a | 3.90 ± 0.13 b | 4.14 ± 0.15 a | 20.52 ± 0.10 b |
| 1:0 | 3.82 ± 0.24 b | 4.18 ± 0.13 a | 3.78 ± 0.25 b | 3.76 ± 0.30 a | 3.80 ± 0.28 a | 19.34 ± 0.16 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, M.; Kang, S.; Liu, L.; Ma, T.; Zheng, Z.; Cao, L. Physicochemical Properties and Cookie-Making Performance as Fat Replacer of Wax-Based Rice Bran Oil Oleogels. Gels 2023, 9, 13. https://doi.org/10.3390/gels9010013
Pang M, Kang S, Liu L, Ma T, Zheng Z, Cao L. Physicochemical Properties and Cookie-Making Performance as Fat Replacer of Wax-Based Rice Bran Oil Oleogels. Gels. 2023; 9(1):13. https://doi.org/10.3390/gels9010013
Chicago/Turabian StylePang, Min, Shengmei Kang, Lin Liu, Tengfei Ma, Zhi Zheng, and Lili Cao. 2023. "Physicochemical Properties and Cookie-Making Performance as Fat Replacer of Wax-Based Rice Bran Oil Oleogels" Gels 9, no. 1: 13. https://doi.org/10.3390/gels9010013
APA StylePang, M., Kang, S., Liu, L., Ma, T., Zheng, Z., & Cao, L. (2023). Physicochemical Properties and Cookie-Making Performance as Fat Replacer of Wax-Based Rice Bran Oil Oleogels. Gels, 9(1), 13. https://doi.org/10.3390/gels9010013








