The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels
Abstract
:1. Introduction
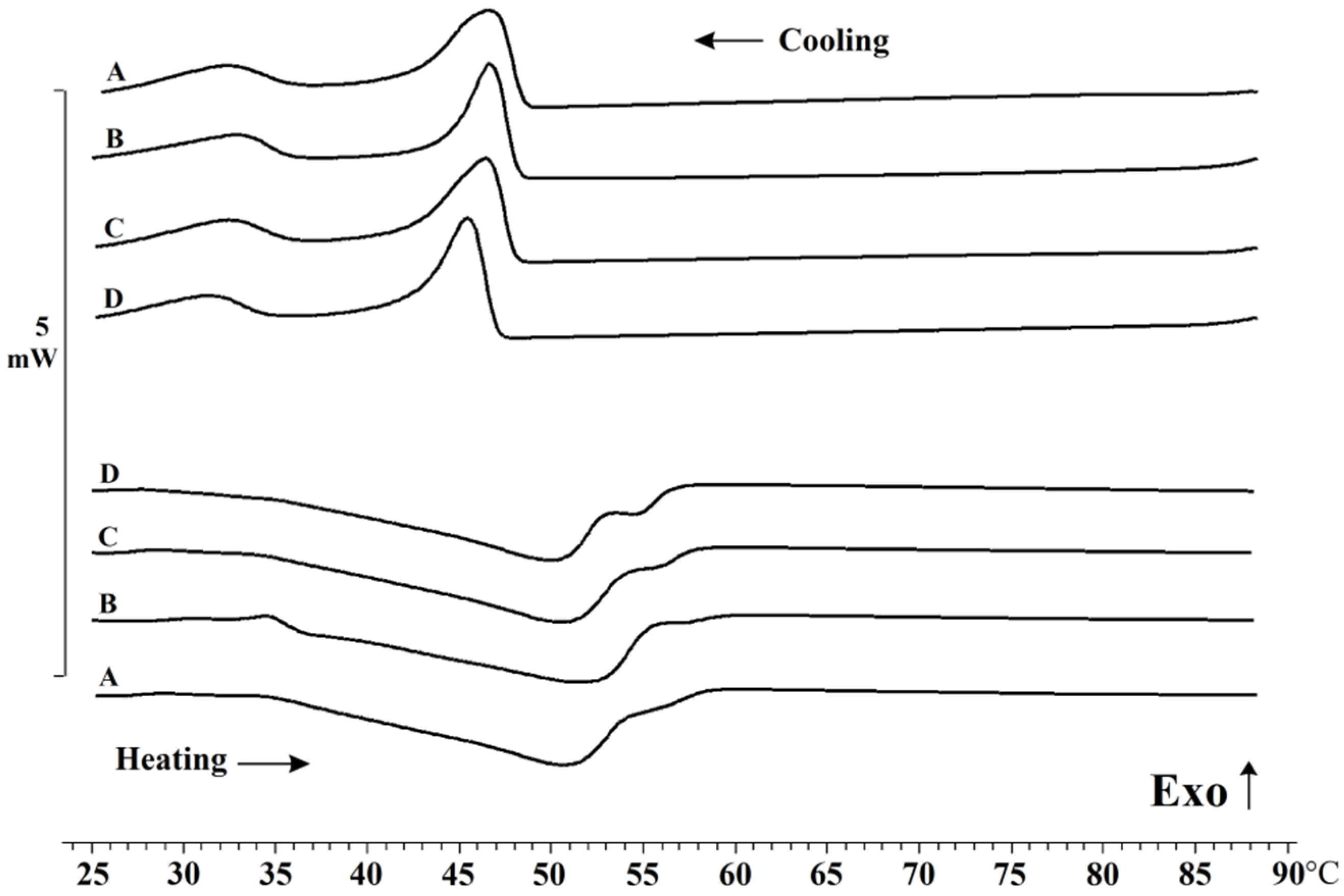
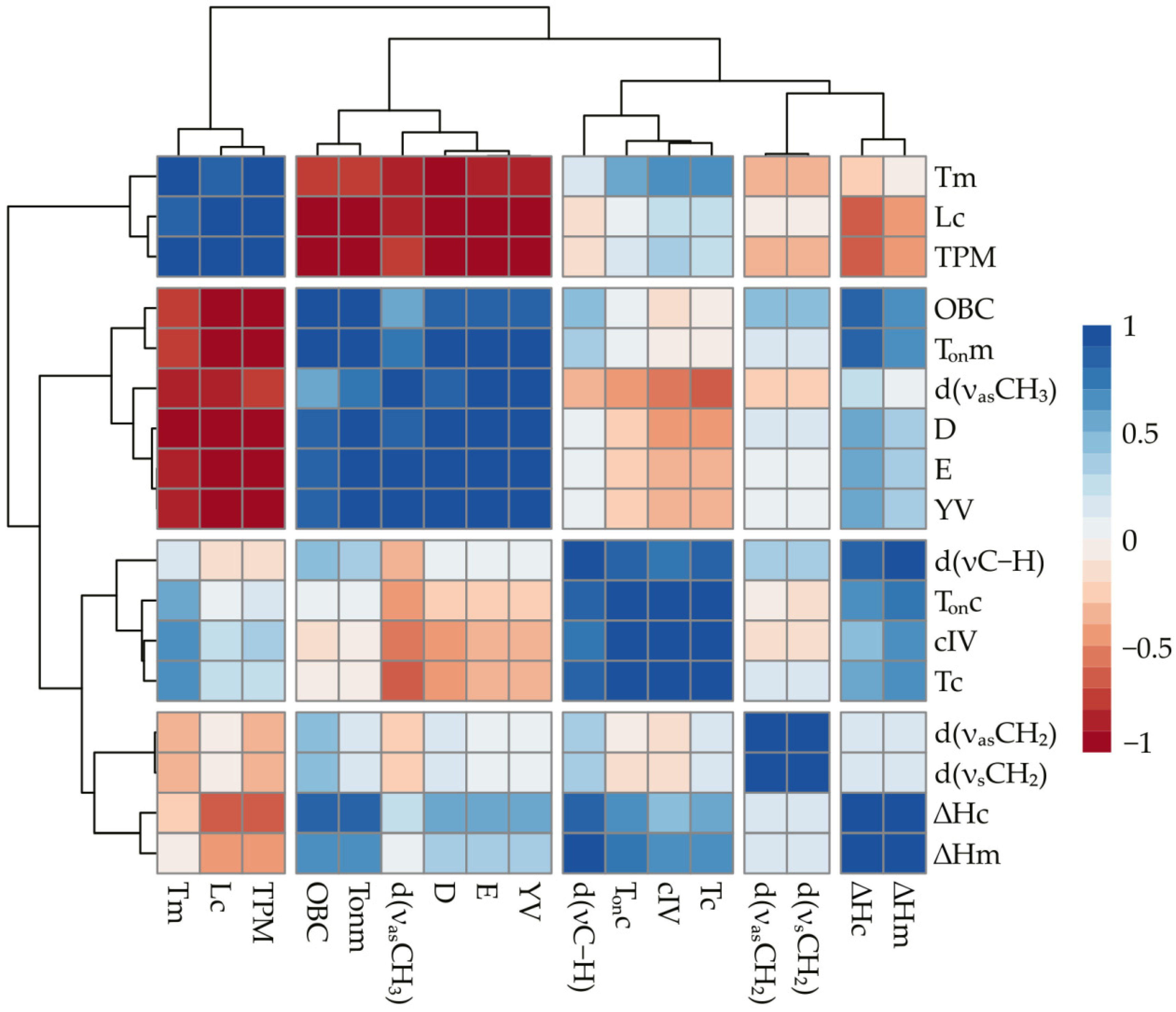
2. Results and Discussion
2.1. Edible Oils Analysis
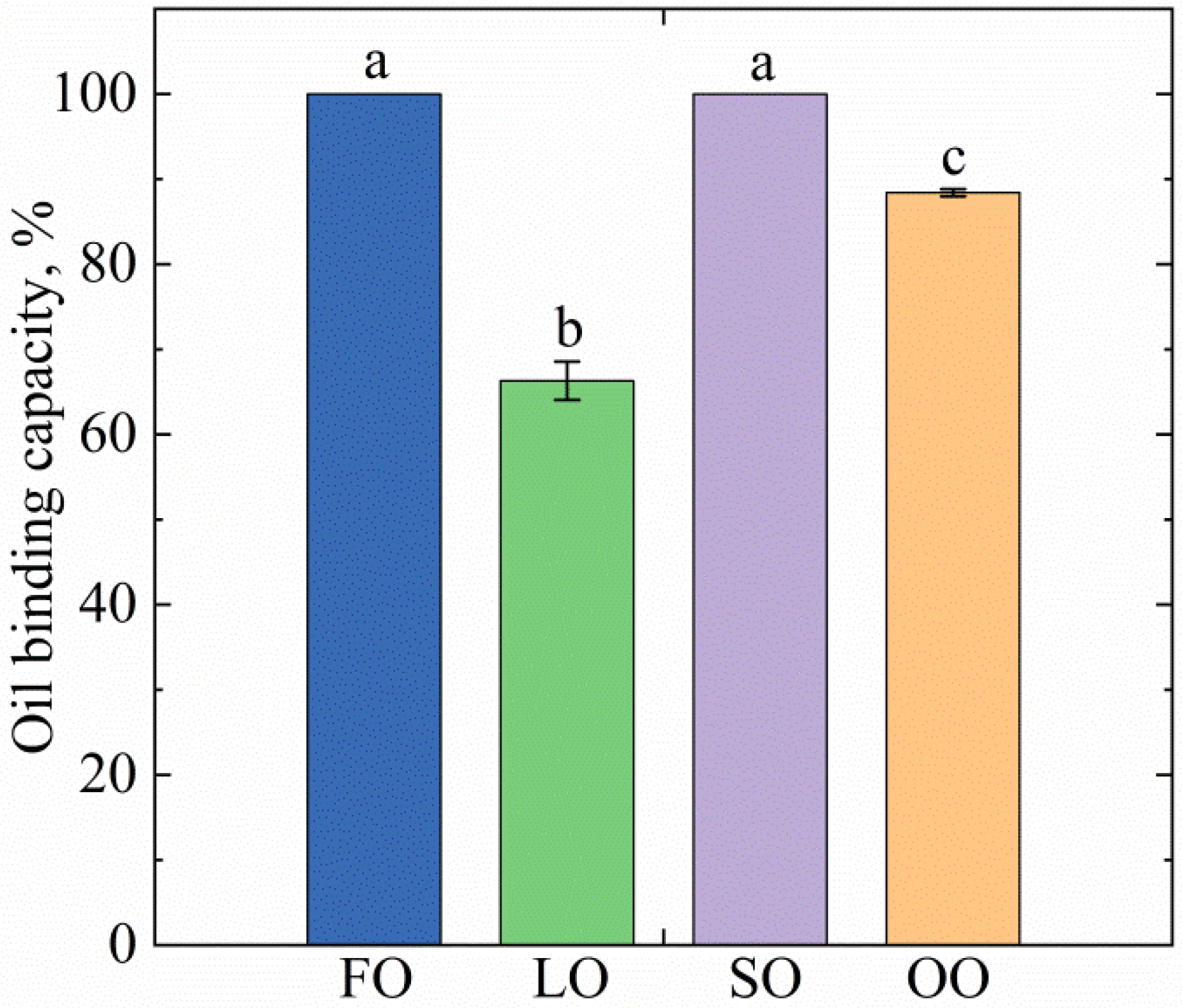
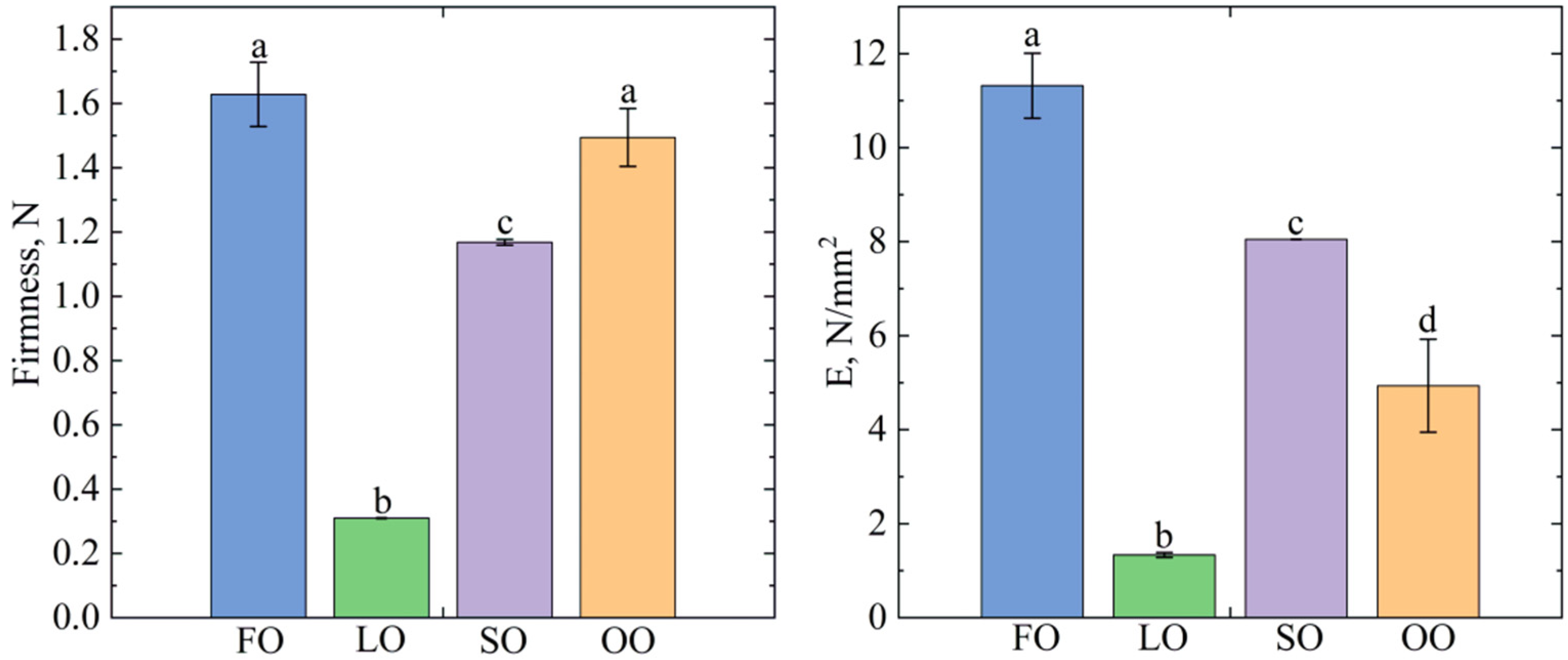
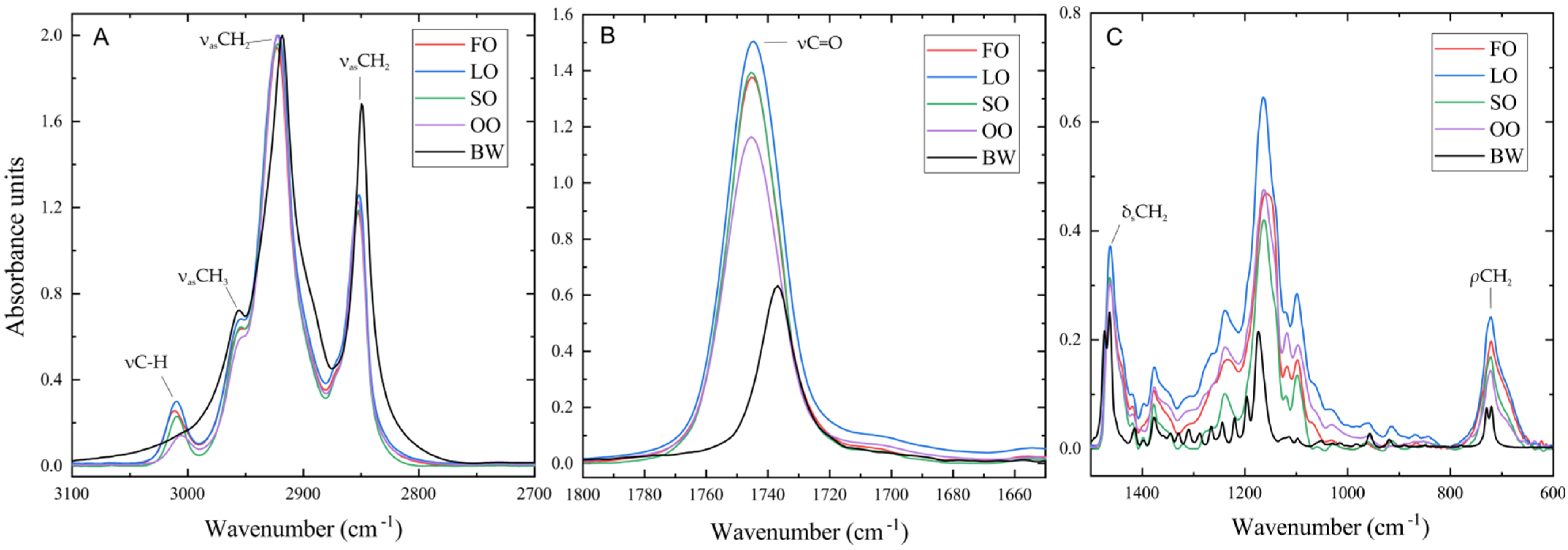
2.2. Oleogels Analysis
2.3. Correlational Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Edible Oil Analysis Methods
4.2.1. Fatty Acid Composition
4.2.2. Total Polar Materials (TPM)
4.2.3. Calculated Iodine Value
4.3. Oleogel Preparation
4.4. Oleogel Analysis
4.4.1. Microscopy
4.4.2. Oil binding Capacity (OBC)
4.4.3. Texture Analysis
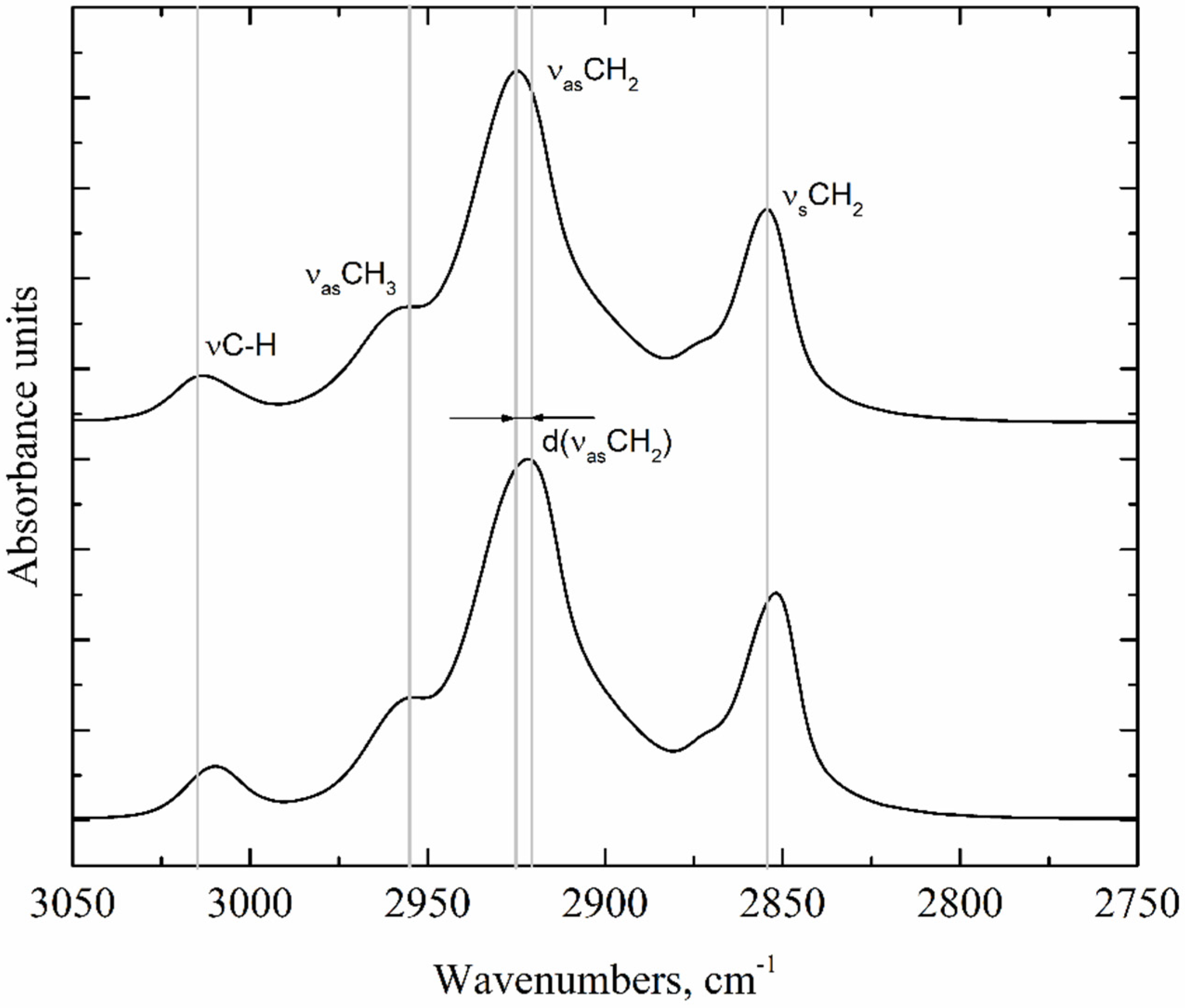
4.4.4. FTIR Spectroscopy
4.4.5. Differential Scanning Calorimetry (DSC)
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in food: A review of current and potential applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Co, E.D.; Marangoni, A.G. Oleogels: An Introduction. Edible Oleogels, 2nd ed.; AOCS Press: Boulder, IL, USA, 2018; pp. 1–29. [Google Scholar]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative study on the ability of different oleogelators to structure sunflower oil. Food Biophys. 2020, 15, 42–49. [Google Scholar] [CrossRef]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Cerqueira, M.A.; Purriños, L.; Pastrana, L.M.; Vicente, A.A.; Zapata, C.; Lorenzo, J.M. Evaluation of linseed oil oleogels to partially replace pork backfat in fermented sausages. J. Sci. Food Agric. 2020, 100, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, A.; Kopsahelis, N.; Freire, D.M.; Mandala, I.; Koutinas, A.A. Olive oil oleogel formulation using wax esters derived from soybean fatty acid distillate. Biomolecules 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zhang, T.; Hu, M.; Xue, Y.; Xue, C. Effects of oleogels prepared with fish oil and beeswax on the gelation behaviors of protein recovered from Alaska Pollock. LWT 2021, 137, 110423. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed oil in new application: Assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Yang, Y.; Drummond, C.J.; Conn, C.E. Effect of Crystallization State on the Gel Properties of Oleogels Based on β-sitosterol. Food Biophys. 2021, 16, 48–57. [Google Scholar] [CrossRef]
- Hwang, H.S.; Gillman, J.D.; Winkler-Moser, J.K.; Kim, S.; Singh, M.; Byars, J.A.; Evangelista, R.L. Properties of Oleogels Formed With High-Stearic Soybean Oils and Sunflower Wax. J. Am. Oil Chem. Soc. 2018, 95, 557–569. [Google Scholar] [CrossRef]
- CODEX-STAN 210 Codex Standards for Fats and Oils from Vegetable Sources; FAO/WHO: Rome, Italy, 1990.
- Frolova, Y.V.; Kochetkova, A.A.; Sobolev, R.V.; Vorobyeva, V.M.; Kodentsova, V.M. Oleogels as prospective nutritional ingredients of lipid nature. Vopr. Pitan. 2021, 90, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sarkisyan, V.; Sobolev, R.; Frolova, Y.; Malinkin, A.; Makarenko, M.; Kochetkova, A. Beeswax Fractions Used as Potential Oil Gelling Agents. J. Am. Oil Chem. Soc. 2021, 98, 281–296. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and External Factors Affecting the Crystallization, Gelation and Applicability of Wax-Based Oleogels in Food Industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Doan, C.D.; van de Walle, D.; Dewettinck, K.; Patel, A.R. Evaluating the Oil-Gelling Properties of Natural Waxes in Rice Bran Oil: Rheological, Thermal, and Microstructural Study. J. Am. Oil Chem. Soc. 2015, 92, 801–811. [Google Scholar] [CrossRef]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M.; Valoppi, F.; Barba, L.; Calligaris, S.; Nicoli, M.C. Potential Application of Pomegranate Seed Oil Oleogels Based on Monoglycerides, Beeswax and Propolis Wax as Partial Substitutes of Palm Oil in Functional Chocolate Spread. LWT 2017, 86, 523–529. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of Solvent Quality on the Mechanical Strength of Ethylcellulose Oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef]
- Adili, L.; Roufegarinejad, L.; Tabibiazar, M.; Hamishehkar, H.; Alizadeh, A. Development and characterization of reinforced ethyl cellulose based oleogel with adipic acid: Its application in cake and beef burger. LWT 2020, 126, 109277. [Google Scholar] [CrossRef]
- Calligaris, S.; Mirolo, G.; da Pieve, S.; Arrighetti, G.; Nicoli, M.C. Effect of Oil Type on Formation, Structure and Thermal Properties of γ-Oryzanol and β-Sitosterol-Based Organogels. Food Biophys. 2014, 9, 69–75. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Shen, M.; Xie, J.; Yang, J. Evaluation of trans fatty acids, carbonyl compounds and bioactive minor components in commercial linseed oils. Food Chem. 2022, 369, 130930. [Google Scholar] [CrossRef] [PubMed]
- Dell’agli, M.; Bosisio, E. Minor Polar Compounds of Olive Oil: Composition, Factors of Variability and Bioactivity. Stud. Nat. Prod. Chem. 2002, 27, 697–734. [Google Scholar]
- Giacintucci, V.; Di Mattia, C.D.; Sacchetti, G.; Flamminii, F.; Gravelle, A.J.; Baylis, B.; Dutcher, J.R.; Marangoni, A.G.; Pittia, P. Ethylcellulose oleogels with extra virgin olive oil: The role of oil minor components on microstructure and mechanical strength. Food Hydrocoll. 2018, 84, 508–514. [Google Scholar] [CrossRef]
- Bazina, N.; He, J. Analysis of Fatty Acid Profiles of Free Fatty Acids Generated in Deep-Frying Process. J. Food Sci. Technol. 2018, 55, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, Detoxification, Utilization, and Opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Frolova, Y.; Sobolev, R.; Kochetkova, A. Influence of Oil Combinations on the Structural Properties of Oleogels. E3S Web Conf. EDP Sci. 2021, 285, 05009. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. Plant wax crystals display platelet-like morphology. Food Struct. 2015, 3, 30–34. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, L.; Kang, S.; Jiang, S.; Pang, M. Influence of Wax Type on Characteristics of Oleogels from Camellia Oil and Medium Chain Triglycerides. Int. J. Food Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Palla, C.; de Vicente, J.; Carrín, M.E.; Gálvez Ruiz, M.J. Effects of Cooling Temperature Profiles on the Monoglycerides Oleogel Properties: A Rheo-Microscopy Study. Food Res. Int. 2019, 125, 108613. [Google Scholar] [CrossRef] [PubMed]
- Okuro, P.K.; Tavernier, I.; bin Sintang, M.D.; Skirtach, A.G.; Vicente, A.A.; Dewettinck, K.; Cunha, R.L. Synergistic Interactions between Lecithin and Fruit Wax in Oleogel Formation. Food Funct. 2018, 9, 1755–1767. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M. Properties and Stability of Hazelnut Oil Organogels with Beeswax and Monoglyceride. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1007–1017. [Google Scholar] [CrossRef]
- Öğütcü, M.; Arifoğlu, N.; Yilmaz, E. Storage Stability of Cod Liver Oil Organogels Formed with Beeswax and Carnauba Wax. Int. J. Food Sci. Technol. 2015, 50, 404–412. [Google Scholar] [CrossRef]
- Callau, M.; Sow-Kébé, K.; Nicolas-Morgantini, L.; Fameau, A.L. Effect of the ratio between behenyl alcohol and behenic acid on the oleogel properties. J. Colloid Interface Sci. 2020, 560, 874–884. [Google Scholar] [CrossRef]
- Choi, K.O.; Hwang, H.S.; Jeong, S.; Kim, S.; Lee, S. The thermal, rheological, and structural characterization of grapeseed oil oleogels structured with binary blends of oleogelator. J. Food Sci. 2020, 85, 3432–3441. [Google Scholar] [CrossRef]
- Do, V.H.; Mun, S.; Kim, Y.-L.; Rho, S.-J.; Park, K.H.; Kim, Y.-R. Novel Formulation of Low-Fat Spread Using Rice Starch Modified by 4-α-Glucanotransferase. Food Chem. 2016, 208, 132–141. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, H.; Liu, W.; Li, C.; Wang, S. The Effect of Cooling Rate on the Microstructure and Macroscopic Properties of Rice Bran Wax Oleogels. J. Oleo Sci. 2021, 70, 135–143. [Google Scholar] [CrossRef]
- Scharfe, M.; Ahmane, Y.; Seilert, J.; Keim, J.; Flöter, E. On the Effect of Minor Oil Components on β-Sitosterol/γ-oryzanol Oleogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1800487. [Google Scholar] [CrossRef]
- George, S. Infrared and Raman Characteristic Group Frequencies; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Cameron, D.G.; Gudgin, E.F.; Mantsch, H.H. Dependence of Acyl Chain Packing of Phospholipids on the Head Group and Acyl Chain Length. Biochemistry 1981, 20, 4496–4500. [Google Scholar] [CrossRef] [PubMed]
- Hondoh, H.; Ueno, S. Polymorphism of Edible Fat Crystals. Prog. Cryst. Growth Charact. Mater. 2016, 62, 398–399. [Google Scholar] [CrossRef]
- Pang, M.; Shi, Z.; Lei, Z.; Ge, Y.; Jiang, S.; Cao, L. Structure and Thermal Properties of Beeswax-Based Oleogels with Different Types of Vegetable Oil. Grasas Y Aceites 2020, 71, 380. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Fernández-Martín, F.; Rodríguez-Salas, L.; Ruiz-Capillas, C. Konjac gel fat analogue for use in meat products: Comparison with pork fats. Food Hydrocoll. 2012, 26, 63–72. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel Formation of Soybean Oil with Waxes. J. Am. Oil Chem. Soc. 2012, 89, 639–647. [Google Scholar] [CrossRef]
- Juárez, M.D.; Osawa, C.C.; Acuña, M.E.; Sammán, N.; Gonçalves, L.A.G. Degradation in Soybean Oil, Sunflower Oil and Partially Hydrogenated Fats after Food Frying, Monitored by Conventional and Unconventional Methods. Food Control 2011, 22, 1920–1927. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Topkafa, M.; Kara, H.; Sherazi, S.T.H. Evaluation of Fatty Acid Composition, Tocols Profile, and Oxidative Stability of Some Fully Refined Edible Oils. Int. J. Food Prop. 2015, 18, 2064–2076. [Google Scholar] [CrossRef]
- Ham, B.; Shelton, R.; Butler, B.; Thionville, P. Calculating the Lodine Value for Marine Oils from Fatty Acid Profiles. J. Am. Oil Chem. Soc. 1998, 75, 1445–1446. [Google Scholar] [CrossRef]
- Dàvila, E.; Parés, D. Structure of Heat-Induced Plasma Protein Gels Studied by Fractal and Lacunarity Analysis. Food Hydrocoll. 2007, 21, 147–153. [Google Scholar] [CrossRef]
- García-Armenta, E.; Picos-Corrales, L.A.; Gutiérrez-López, G.F.; Gutiérrez-Dorado, R.; Perales-Sánchez, J.X.K.; García-Pinilla, S.; Reynoso-García, F.; Martínez-Audelo, J.M.; Armenta-Manjarrez, M.A. Preparation of Surfactant-Free Emulsions Using Amaranth Starch Modified by Reactive Extrusion. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125550. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, M.; Zhang, X.; Hu, Y.; Han, M.; Xu, X.; Zhou, G. Effects of Inulin on the Gel Properties and Molecular Structure of Porcine Myosin: A Underlying Mechanisms Study. Food Hydrocoll. 2020, 108, 105974. [Google Scholar] [CrossRef]
- Haighton, A.J. The measurement of the hardness of margarine and fats with cone penetrometers. J. Am. Oil Chem. Soc. 1959, 36, 345–348. [Google Scholar] [CrossRef]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; Prđun, S.; Bubalo, D.; Gajger, I.T. An Approach for Routine Analytical Detection of Beeswax Adulteration Using FTIR-ATR Spectroscopy. J. Apic. Sci. 2015, 59, 37–49. [Google Scholar] [CrossRef] [Green Version]

| Fatty Acid Structure | OO | LO | FO | SO |
|---|---|---|---|---|
| 6:0 | - | - | - | |
| 8:0 | - | - | 0.02 ± 0.01 | - |
| 10:0 | - | - | 0.02 ± 0.01 | - |
| 12:0 | - | - | 0.04 ± 0.01 | 0.03 ± 0.00 |
| 13:0 | - | - | - | - |
| 14:0 | 0.03 ± 0.01 | 0.06 ± 0.01 | 3.85 ± 0.14 | 0.10 ± 0.02 |
| 15:0 | - | 0.03 ± 0.01 | 0.33 ± 0.11 | - |
| 16:0 | 12.56 ± 0.11 | 6.17 ± 0.14 | 9.66 ± 0.15 | 6.31 ± 0.31 |
| 16:1 | 0.15 ± 0.01 | 0.03 ± 0.01 | 0.37 ± 0.11 | 0.03 ± 0.01 |
| 16:1 9-c | 1.05 ± 0.12 | 0.12 ± 0.03 | 7.01 ± 0.21 | 0.12 ± 0.08 |
| 17:0 | 0.10 ± 0.02 | 0.09 ± 0.02 | 0.23 ± 0.05 | 0.05 ± 0.02 |
| 17:1 | 0.16 ± 0.04 | 0.05 ± 0.01 | 0.44 ± 0.05 | 0.04 ± 0.01 |
| 18:0 | 2.84 ± 0.10 | 4.32 ± 0.23 | 2.02 ± 0.10 | 3.54 ± 0.16 |
| 18:1 9-t | 0.21 ± 0.08 | - | 2.45 ± 0.20 | - |
| 18:1 9-c | 71.09 ± 0.47 | 20.76 ± 0.28 | 14.43 ± 0.27 | 27.31 ± 0.32 |
| 18:1 11-t | 2.28 ± 0.23 | 0.79 ± 0.13 | 4.26 ± 0.16 | 0.64 ± 0.07 |
| 18:2 9-c, 12-t | 0.04 ± 0.01 | 0.08 ± 0.02 | 0.06 ± 0.01 | 1.23 ± 0.14 |
| 18:2 9-t, 12-c | - | - | 0.03 ± 0.01 | 1.14 ± 0.08 |
| 18:2 | 8.08 ± 0.13 | 46.73 ± 0.32 | 1.83 ± 0.12 | 58.84 ± 0.48 |
| 18:3 ω-3 | 1.13 ± 0.21 | 20.43 ± 0.31 | 1.35 ± 0.17 | 0.29 ± 0.03 |
| 20:1 | 0.29 ± 0.07 | 0.34 ± 0.00 | 14.67 ± 0.21 | 0.30 ± 0.10 |
| 20:5 | - | - | 7.86 ± 0.19 | - |
| 22:0 | - | - | 0.04 ± 0.02 | 0.62 ± 0.11 |
| 22:1 | - | - | 10.91 ± 0.14 | - |
| 22:4 | - | - | 0.44 ± 0.05 | - |
| 22:5 | - | - | 1.51 ± 0.10 | - |
| 22:6 | - | - | 15.18 ± 0.24 | - |
| Total values | ||||
| SFA | 15.53 | 10.67 | 16.21 | 10.65 |
| UFA | 84.48 | 89.33 | 82.80 | 89.94 |
| MUFA | 75.23 | 22.06 | 54.54 | 28.44 |
| PUFA | 9.25 | 67.16 | 28.26 | 61.5 |
| cIV 1 | 85.33 | 160.38 | 164.18 | 137.02 |
| TPM | 5.5 ± 0.5 | 17.5 ± 0.5 | 4.0 ± 0.5 | 5.0 ± 0.5 |
| Oil Type in Oleogel | Lc, µm | D | A |
|---|---|---|---|
| FO | 7.1 ± 1.34 bcd | 2.82 ± 0.04 bc | 0.24 ± 0.05 bc |
| LO | 29.3 ± 4.01 acd | 2.49 ± 0.04 acd | 0.81 ± 0.05 acd |
| SO | 14.3 ± 1.74 abd | 2.75 ± 0.04 abd | 0.31 ± 0.05 abd |
| OO | 11.7 ± 1.72 abc | 2.81 ±0.04 bc | 0.24 ± 0.05 bc |
| Oil Type in Oleogel | YV (Nm−2 × 102) | Assessment |
|---|---|---|
| FO | 349.0 ± 2.3 bcd | Satisfactory plastic and spreadable |
| LO | 66.8 ± 7.3 acd | Very soft, not spreadable |
| SO | 250.5 ± 2.7 abd | Satisfactory plastic and spreadable |
| OO | 320.3 ± 1.5 abc | Satisfactory plastic and spreadable |
| Oil | State | Band Positions, cm−1 | |||
|---|---|---|---|---|---|
| νC-H | νasCH3 | νasCH2 | νsCH2 | ||
| Sunflower | Oil | 3008.938 | 2953.522 | 2924.939 | 2854.452 |
| Gel | 3009.08 | 2953.589 | 2920.267 | 2851.151 | |
| d(ν) | 0.1423 | 0.0662 | −4.672 | −3.3008 | |
| Fish | Oil | 3011.328 | 2953.151 | 2924.427 | 2853.931 |
| Gel | 3011.465 | 2953.003 | 2922.849 | 2852.524 | |
| d(ν) | 0.1363 | −0.1481 | −1.5778 | −1.4072 | |
| Linseed | Oil | 3009.586 | 2953.671 | 2925.178 | 2854.447 |
| Gel | 3009.637 | 2953.871 | 2923.337 | 2852.847 | |
| d(ν) | 0.0512 | 0.2003 | −1.8411 | −1.5997 | |
| Olive | Oil | 3005.632 | 2953.605 | 2924.073 | 2853.96 |
| Gel | 3005.573 | 2953.404 | 2921.91 | 2852.114 | |
| d(ν) 1 | −0.0588 | −0.2015 | −2.1633 | −1.8465 | |
| Sample | Heating | Cooling | ||||
|---|---|---|---|---|---|---|
| Ton | Tm | ∆Hm | Ton | Tc | ∆Hc | |
| FO | 36.80 ± 0.4 bcd | 50.65 ± 0.6 bd | −6.43 ± 0.5 | 48.48 ± 0.1 bcd | 46.52 ± 0.4 d | 4.76 ± 0.4 bd |
| LO | 34.84 ± 0.1 acd | 51.65 ± 0.9 acd | −6.26 ± 0.6 | 48.15 ± 0.0 acd | 46.53 ± 0.0 d | 4.22 ± 0.2 a |
| SO | 36.19 ± 0.3 ab | 50.48 ± 0.3 b | −6.36 ± 0.3 | 48.00 ± 0.1 abd | 46.52 ± 0.3 d | 4.58 ± 0.5 |
| OO | 36.04 ± 0.3 ab | 50.15 ± 0.2 ab | −6.21 ± 0.2 | 47.02 ± 0.2 abc | 45.36 ± 0.1 abc | 4.24 ± 0.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels. Gels 2022, 8, 48. https://doi.org/10.3390/gels8010048
Frolova Y, Sarkisyan V, Sobolev R, Makarenko M, Semin M, Kochetkova A. The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels. Gels. 2022; 8(1):48. https://doi.org/10.3390/gels8010048
Chicago/Turabian StyleFrolova, Yuliya, Varuzhan Sarkisyan, Roman Sobolev, Mariia Makarenko, Michael Semin, and Alla Kochetkova. 2022. "The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels" Gels 8, no. 1: 48. https://doi.org/10.3390/gels8010048
APA StyleFrolova, Y., Sarkisyan, V., Sobolev, R., Makarenko, M., Semin, M., & Kochetkova, A. (2022). The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels. Gels, 8(1), 48. https://doi.org/10.3390/gels8010048






