A Study of the Quantitative Relationship between Yield Strength and Crystal Size Distribution of Beeswax Oleogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructure Analysis
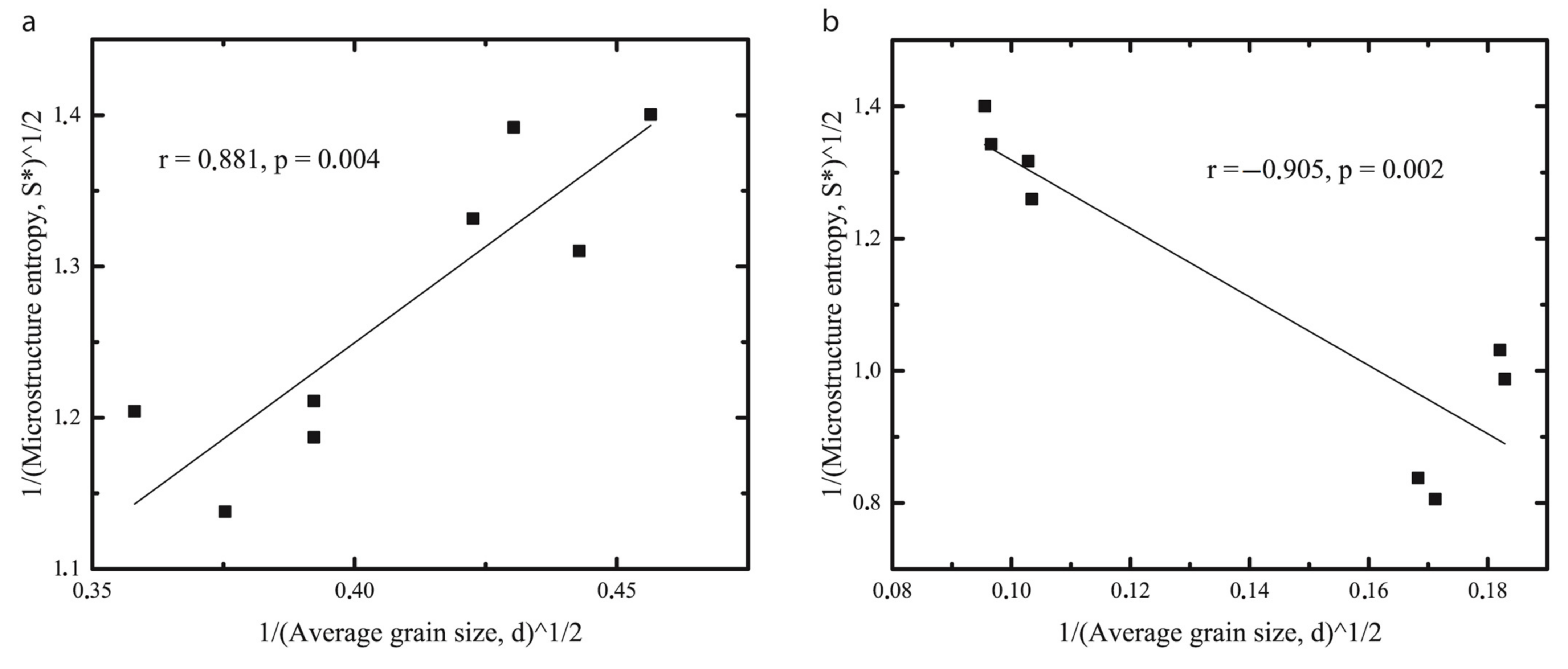
2.2. Microstructure Thermodynamic Parameters
2.3. Texture Properties of Oleogels
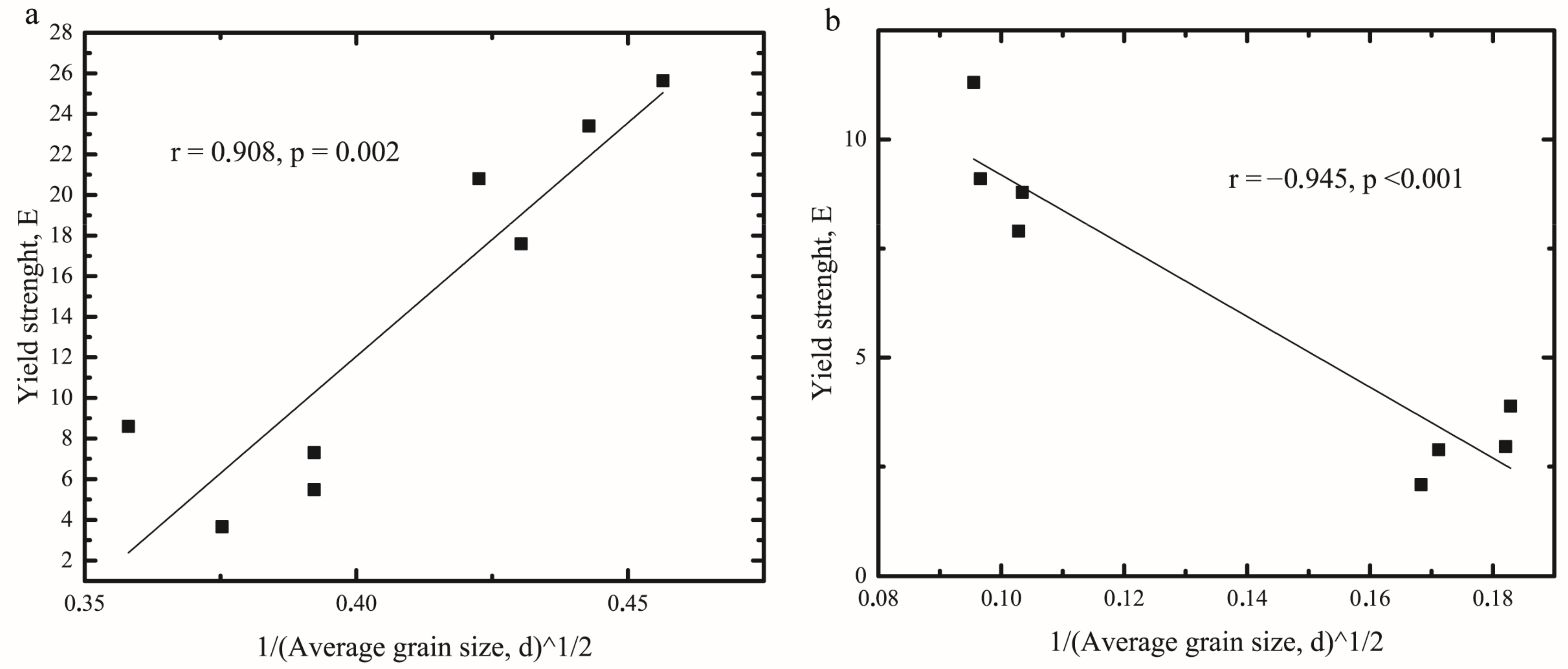
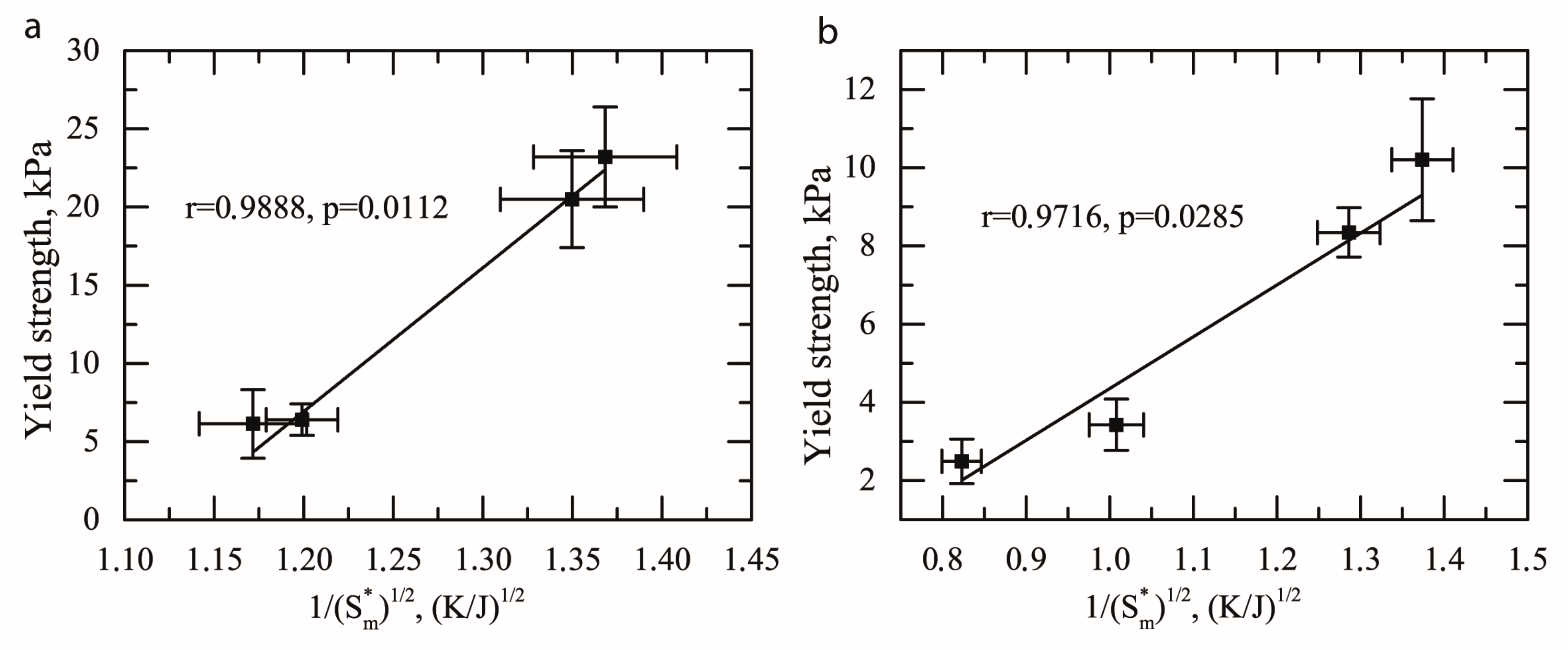
2.4. Hall-Petch Relation for Oleogels
3. Conclusions
4. Materials and Methods
4.1. Calculations
4.1.1. Crystal Size Distribution
4.1.2. Microstructure Thermodynamic Parameters
4.2. Materials
4.3. Hydrocarbons Separation
4.4. Oleogels Preparation
4.5. Texture Analysis
4.6. Microscopical Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Co, E.D.; Marangoni, A.G. Chapter 1–Oleogels: An Introduction. In Edible Oleogels, 2nd ed.; Marangoni, A.G., Garti, N., Eds.; AOCS Press: Champaign, IL, USA, 2018; pp. 1–29. ISBN 9780128142707. [Google Scholar]
- Hwang, H.S. A critical review on structures, health effects, oxidative stability, and sensory properties of oleogels. Biocatal. Agric. Biotechnol. 2020, 26, 101657. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; Vrieze, M.D.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Elnahas, H.H.; Abdou, S.M.; El-Zahed, H.; Abdeldaym, A. Structural, morphological and mechanical properties of gamma irradiated low density polyethylene/paraffin wax blends. Radiat. Phys. Chem. 2018, 151, 217–224. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and multi-component organogels: An overview from rheological perspective. Food Hydrocoll. 2021, 111, 106190. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel Formation of Soybean Oil with Waxes. J. Am. Oil Chem. Soc. 2012, 89, 639–647. [Google Scholar] [CrossRef]
- Szymańska, I.; Żbikowska, A.; Onacik-Gür, S. Candelilla wax-based oleogels versus palm oil: Evaluation of physical properties of innovative and conventional lipids using optical techniques. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, H.; Liu, W.; Li, C.; Wang, S. The Effect of Cooling Rate on the Microstructure and Macroscopic Properties of Rice Bran Wax Oleogels. J. Oleo Sci. 2021, 70, 135–143. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, Z.; Xue, C. Recent advances on food-grade oleogels: Fabrication, application and research trends. Crit. Rev. Food Sci. Nutr. 2021, 1–18. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Cordero, Z.C.; Knight, B.E.; Schuh, C.A. Six decades of the Hall–Petch effect—A survey of grain-size strengthening studies on pure metals. Int. Mater. Rev. 2016, 61, 495–512. [Google Scholar] [CrossRef]
- Hall, E.O. The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Soc. 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Kumar, K.S.; Swygenhoven, H.V.; Suresh, S. Mechanical behavior of nanocrystalline metals and alloys11The Golden Jubilee Issue—Selected topics in Materials Science and Engineering: Past, Present and Future, edited by S. Suresh. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Gong, N.; Misra, R.D.K.; Niu, G.; Wu, H. Microstructure entropy guided understanding of yield strength in alloy systems characterized by grain size distribution. Acta Mater. 2020, 195, 531–540. [Google Scholar] [CrossRef]
- Olson, I.A.; Shtukenberg, A.G.; Kahr, B.; Ward, M.D. Dislocations in molecular crystals. Rep. Prog. Phys. 2018, 81, 096501. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. Plant wax crystals display platelet-like morphology. Food Struct. 2015, 3, 30–34. [Google Scholar] [CrossRef]
- Leunissen, M.E.; Graswinckel, W.S.; van Enckevort, W.J.P.; Vlieg, E. Epitaxial Nucleation and Growth of n-Alkane Crystals on Graphite (0001). Cryst. Growth Des. 2004, 4, 361–367. [Google Scholar] [CrossRef]
- Co, E.D.; Marangoni, A.G. Fat Crystal Networks; Cryst. Growth Des. 2020, 20, 1628–1637. [Google Scholar] [CrossRef]
- Venkatesan, R.; Nagarajan, N.R.; Paso, K.; Yi, Y.B.; Sastry, A.M.; Fogler, H.S. The strength of paraffin gels formed under static and flow conditions. Chem. Eng. Sci. 2005, 60, 3587–3598. [Google Scholar] [CrossRef]
- Li, Y.; Bushby, A.J.; Dunstan, D.J. The Hall-Petch effect as a manifestation of the general size effect. Proc. A Math. Phys. Eng. Sci. 2016, 472, 20150890. [Google Scholar] [CrossRef]
- Khan, A.Q.; Brabers, M.; Delaey, L. The Hall-Petch relationship in cop-per-based martensites. Mater. Sci. Eng. 1974, 15, 263–274. [Google Scholar] [CrossRef]
- Sarkisyan, V.; Sobolev, R.; Frolova, Y.; Malinkin, A.; Makarenko, M.; Kochetkova, A. Beeswax Fractions Used as Potential Oil Gelling Agents. J. Am. Oil Chem. Soc. 2021, 98, 281–296. [Google Scholar] [CrossRef]
- Scattergood, R.O.; Koch, C.C. A modified-model for Hall-Petch behavior in nanocrystalline materials. Scr. Metall. Et Mater. 1992, 27, 1195–1200. [Google Scholar] [CrossRef]
- Masamura, R.A.; Hazzledine, P.M.; Liaw, P.K.; Lavernia, E.J. Yield stress of fine grained materials. Acta Metall. 1998, 46, 4527–4534. [Google Scholar] [CrossRef]
- Yamakov, V.; Wolf, D.; Phillpot, S.R.; Mukherjee, A.K.; Gleiter, H. Deformation-mechanism map for nanocrystalline metals by molecular dynamics simulation. Nat. Mater. 2004, 3, 43–47. [Google Scholar] [CrossRef]
- Fan, G.J.; Choo, H.; Liaw, P.K.; Lavernia, E.J. A model for the inverse Hall–Petch relation of nanocrystalline materials. Mater. Sci. Eng. A Struct. Mater. 2005, 409, 243–248. [Google Scholar] [CrossRef]
- Carlton, C.E.; Ferreira, P.J. What is behind the inverse Hall–Petch effect in nanocrystalline materials? Acta Mater. 2007, 55, 3749–3756. [Google Scholar] [CrossRef]
- Rohrer, G.S. Grain boundary energy anisotropy: A review. J. Mater. Sci. 2011, 46, 5881–5895. [Google Scholar] [CrossRef] [Green Version]
- Vedanti, P.; Wu, X.; Berdichevsky, V. Entropy decay during grain growth. Sci. Rep. 2020, 10, 11912. [Google Scholar] [CrossRef]
- Jaynes, E.T. Gibbs vs Boltzmann entropies. Am. J. Phys. 1965, 33, 391–398. [Google Scholar] [CrossRef]
- Berdichevsky, V.L. Entropy and temperature of microstructure in crystal plasticity. Int. J. Eng. Sci. 2018, 128, 24–30. [Google Scholar] [CrossRef]
- Berdichevsky, V.L. A continuum theory of edge dislocations. J. Mech. Phys. Solids 2017, 106, 95–132. [Google Scholar] [CrossRef]
- Mullins, W.W. Metal Surfaces: Structure, Energetics and Kinetics; American Society for Metals: Metals Park, OH, USA, 1963; pp. 17–66. [Google Scholar]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.J.; Scanlon, M.G.; Hartel, R.W.; Marangoni, A.G. Rheological Properties of Milkfat and Butter. J. Food Sci. 2001, 66, 1056–1071. [Google Scholar] [CrossRef]
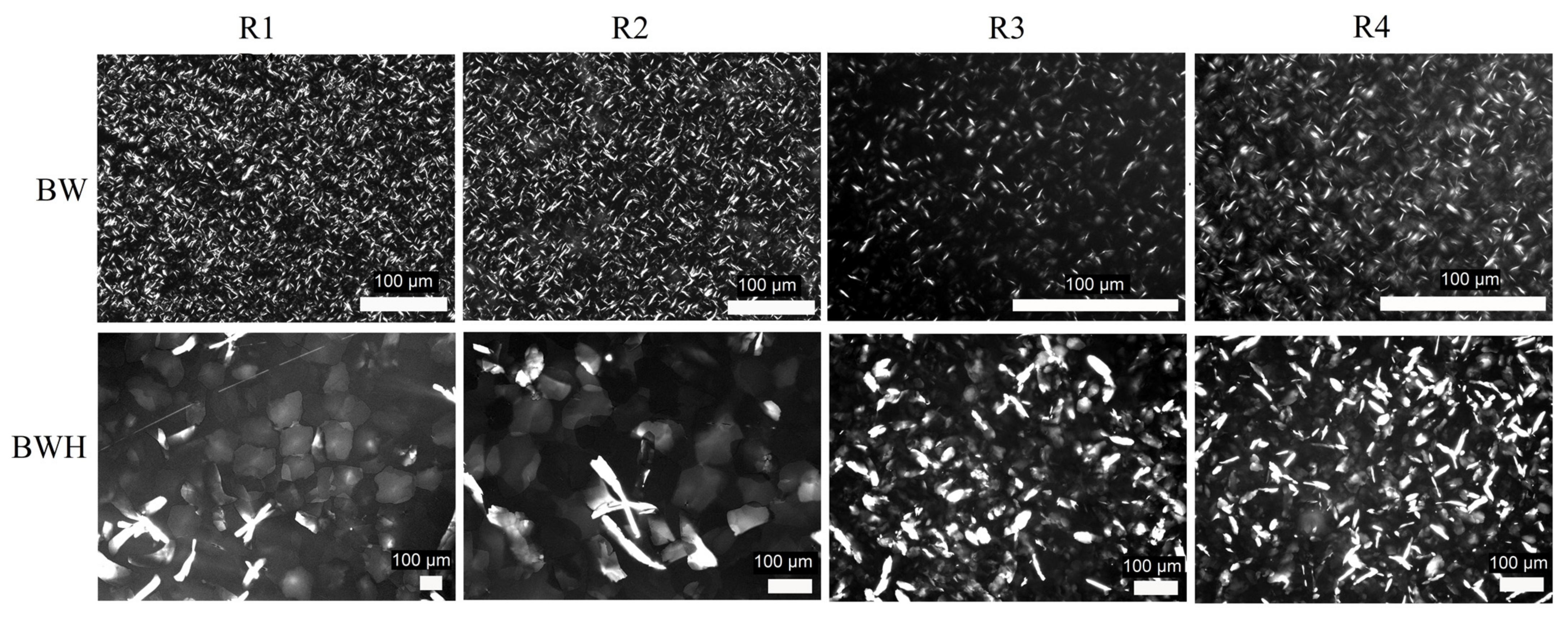
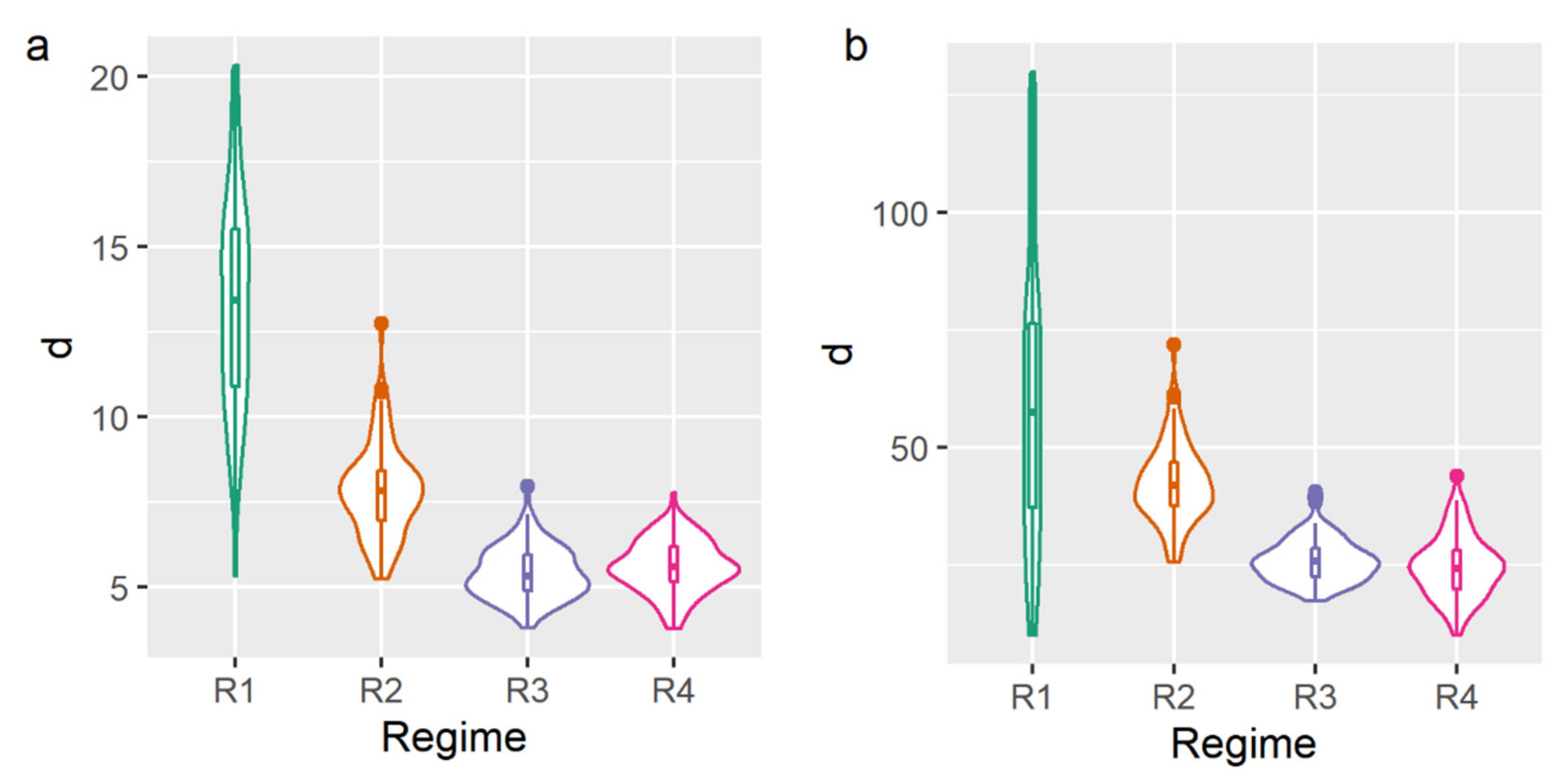

| Sample | Cooling Regime | x, μm | y, μm | z, μm |
|---|---|---|---|---|
| BW | R1 | 7.46 ± 0.51 | 2.24 ± 0.15 | 2.10 ± 0.20 c |
| R2 | 6.51 ± 0.01 | 1.95 ± 0.01 | 2.40 ± 0.40 c | |
| R3 | 5.25 ± 0.22 a | 1.57 ± 0.07 b | 0.80 ± 0.10 d | |
| R4 | 5.22 ± 0.54 a | 1.56 ± 0.16 b | 1.00 ± 0.10 d | |
| BWH | R1 | 108.39 ± 1.74 | 32.52 ± 0.52 | 30.1 ± 0.20 |
| R2 | 94.07 ± 0.79 | 28.22 ± 0.24 | 12.3 ± 0.30 | |
| R3 | 30.04 ± 0.18 | 9.01 ± 0.06 | 10.2 ± 0.10 e | |
| R4 | 34.7 ± 0.81 | 10.41 ± 0.24 | 10.1 ± 0.10 e |
| Sample | Cooling Regime | γs, J/m × 10−8 | Um, J | Tm, K | |
|---|---|---|---|---|---|
| BW | R1 | 0.73 ± 0.06 a | 7.66 ± 4.37 c | 0.048 ± 0.027 e | 0.066 ± 0.012 g |
| R2 | 0.70 ± 0.02 a | 7.99 ± 1.60 c | 0.053 ± 0.011 e | 0.076 ± 0.016 g | |
| R3 | 0.55 ± 0.04 b | 25.6 ± 5.13 d | 0.188 ± 0.038 f | 0.342 ± 0.011 h | |
| R4 | 0.54 ± 0.04 b | 29.0 ± 4.27 d | 0.213 ± 0.031 f | 0.394 ± 0.020 h | |
| BWH | R1 | 0.53 ± 0.03 | 12.8 ± 1.95 c | 0.050 ± 0.008 e | 0.094 ± 0.007 |
| R2 | 0.61 ± 0.04 | 10.4 ± 0.79 c | 0.041 ± 0.003 e | 0.067 ± 0.011 | |
| R3 | 0.99 ± 0.06 | 4.28 ± 0.82 d | 0.019 ± 0.004 f | 0.019 ± 0.009 | |
| R4 | 1.48 ± 0.08 | 3.11 ± 0.71 d | 0.013 ± 0.003 f | 0.008 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkisyan, V.; Sobolev, R.; Frolova, Y.; Vorobiova, I.; Kochetkova, A. A Study of the Quantitative Relationship between Yield Strength and Crystal Size Distribution of Beeswax Oleogels. Gels 2022, 8, 39. https://doi.org/10.3390/gels8010039
Sarkisyan V, Sobolev R, Frolova Y, Vorobiova I, Kochetkova A. A Study of the Quantitative Relationship between Yield Strength and Crystal Size Distribution of Beeswax Oleogels. Gels. 2022; 8(1):39. https://doi.org/10.3390/gels8010039
Chicago/Turabian StyleSarkisyan, Varuzhan, Roman Sobolev, Yuliya Frolova, Irina Vorobiova, and Alla Kochetkova. 2022. "A Study of the Quantitative Relationship between Yield Strength and Crystal Size Distribution of Beeswax Oleogels" Gels 8, no. 1: 39. https://doi.org/10.3390/gels8010039
APA StyleSarkisyan, V., Sobolev, R., Frolova, Y., Vorobiova, I., & Kochetkova, A. (2022). A Study of the Quantitative Relationship between Yield Strength and Crystal Size Distribution of Beeswax Oleogels. Gels, 8(1), 39. https://doi.org/10.3390/gels8010039






