Evaluating the Biocompatibility of an Injectable Wound Matrix in a Murine Model
Abstract
:1. Introduction
2. Results and Discussion
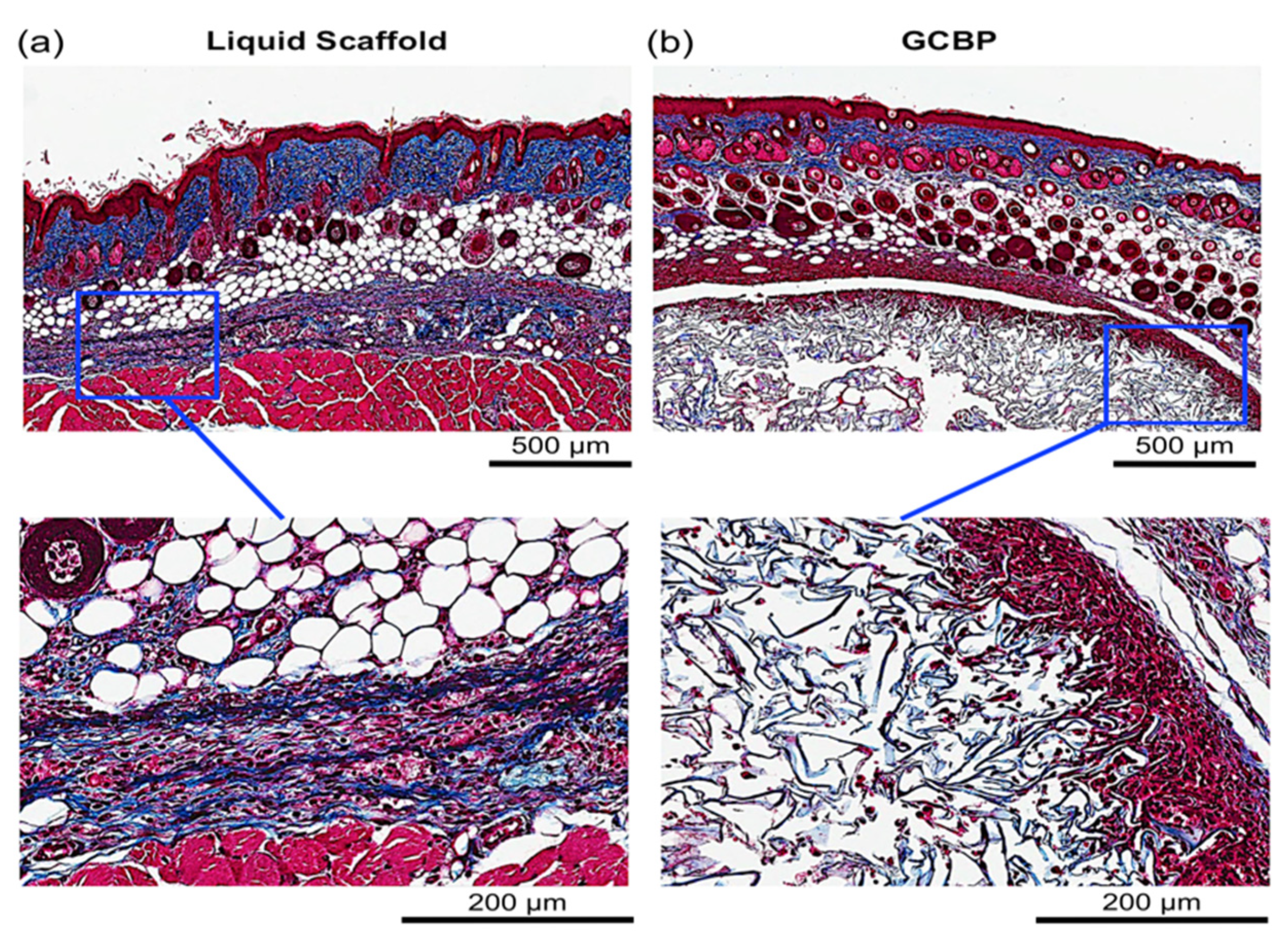
2.1. In Vivo Integration of Scaffolds
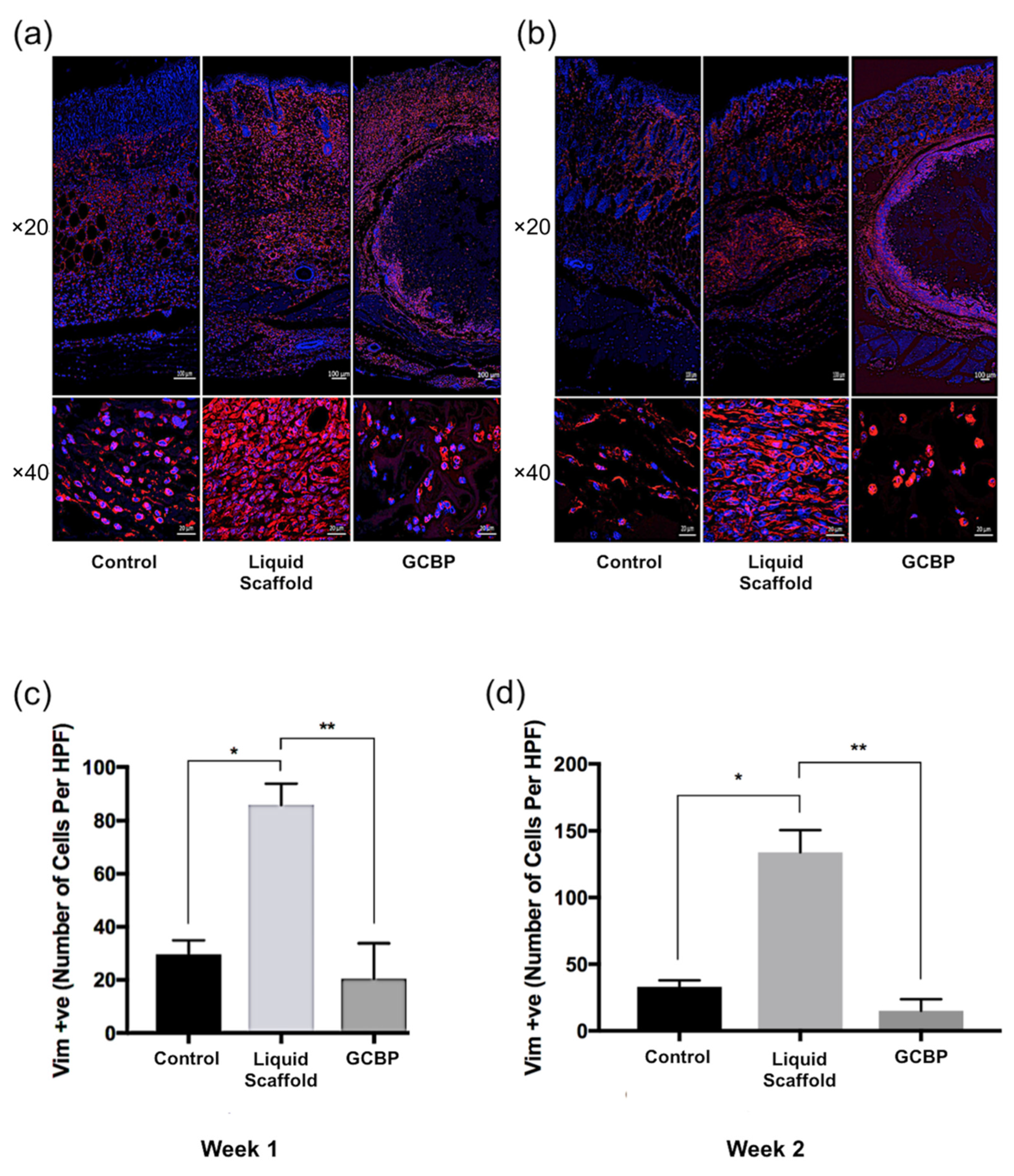
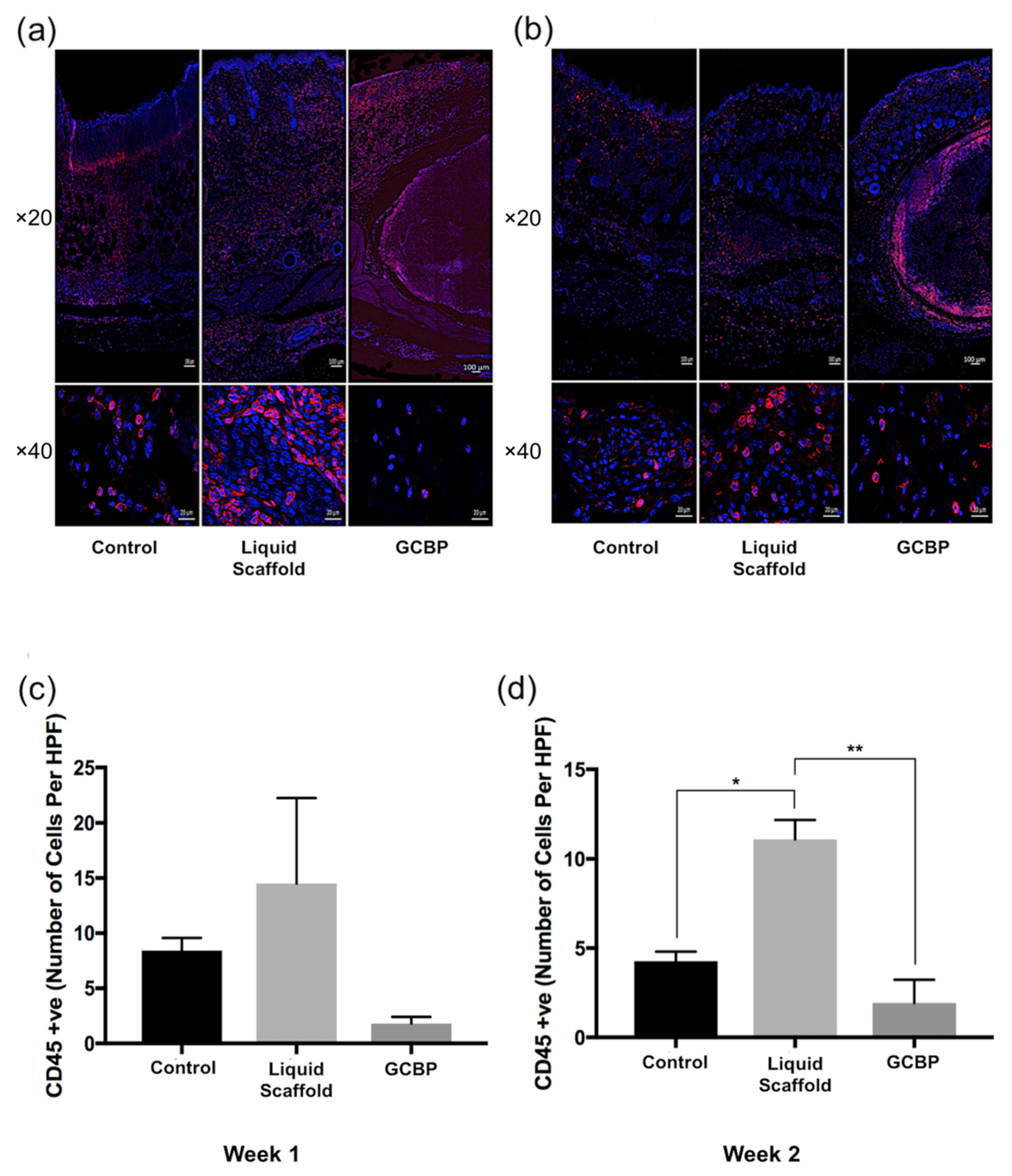
2.2. Immunofluorescence Staining and Quantification
2.3. Discussion
3. Conclusions
4. Materials and Methods
4.1. Preparation of Scaffolds
4.2. Animal Handling and Anesthesia
4.3. Surgical Procedure and Scaffolds Application
4.4. Histological Evaluation
4.5. Immunofluorescence Staining and Cellularity Quantification
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BSA | Bovine serum albumin |
| ECM | Extracellular matrix |
| FBR | Foreign body reaction |
| GAG | Glycosaminoglycan |
| GCBP | Granular collagen based product |
| H&E | Hematoxylin and Eosin |
| HPF | High power field |
| IF | Immunofluorescence |
| IgG | Immunoglobulin G |
| MT | Masson’s Trichrome |
| NGS | Normal goat serum |
| PBS | Phosphate buffered saline with Tween 20 |
| PVA | Polyvinyl alcohol |
| SEM | Standard error of the mean |
| VIM | Vimentin |
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular Pathogenesis of Chronic Wounds. Am. J. Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef]
- Park, H.; Copeland, C.; Henry, S.; Barbul, A. Complex Wounds and Their Management. Surg. Clin. N. Am. 2010, 90, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [Green Version]
- Rice, J.J.; Martino, M.; De Laporte, L.; Tortelli, F.; Briquez, P.S.; Hubbell, J. Engineering the Regenerative Microenvironment with Biomaterials. Adv. Healthc. Mater. 2012, 2, 57–71. [Google Scholar] [CrossRef]
- Ungerleider, J.L.; Johnson, T.D.; Hernandez, M.J.; Elhag, D.I.; Braden, R.L.; Dzieciatkowska, M.; Osborn, K.G.; Hansen, K.C.; Mahmud, E.; Christman, K.L. Extracellular Matrix Hydrogel Promotes Tissue Remodeling, Arteriogenesis, and Perfusion in a Rat Hindlimb Ischemia Model. JACC Basic Transl. Sci. 2016, 1, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hodgkinson, T.; Bayat, A. Ex Vivo evaluation of acellular and cellular collagen-glycosaminoglycan flowable matrices. Biomed. Mater. 2015, 10, 041001. [Google Scholar] [CrossRef] [PubMed]
- Später, T.; Frueh, F.S.; Menger, M.D.; Laschke, M.W. Potentials and limitations of Integra® flowable wound matrix seeded with adipose tissue-derived microvascular fragments. Eur. Cells Mater. 2017, 33, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, R.; Leung, V.; Chavez-Munoz, C.; Nabai, L.; Yang, H.; Ko, F.; Ghahary, A. A novel hydrogel-collagen composite improves functionality of an injectable extracellular matrix. Acta Biomater. 2011, 7, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, R.; Poormasjedi-Meibod, M.-S.; Chavez-Munoz, C.; Jalili, R.B.; Hossenini-Tabatabaei, A.; Ghahary, A. AnIn-SituForming Skin Substitute Improves Healing Outcome in a Hypertrophic Scar Model. Tissue Eng. Part A 2015, 21, 1085–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwell, R.; Chan, B.; Elliott, K.; Alnojeidi, H.; Ghahary, A. Polyvinyl alcohol-graft-polyethylene glycol hydrogels improve utility and biofunctionality of injectable collagen biomaterials. Biomed. Mater. 2016, 11, 035013. [Google Scholar] [CrossRef]
- Verly, M.; Mason, E.; Sheikh-Oleslami, S.; Jalili, R.; Russ, B.; Kilani, R.T.; Ghahary, A. A Pilot Trial Assessing the Feasibility and Efficacy of a Novel Powder for Rapid Wound Healing. Eur. J. Burn. Care 2021, 2, 238–248. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Brigido, S.A.; Schwartz, E.; McCarroll, R.; Hardin-Young, J. Use of an Acellular Flowable Dermal Replacement Scaffold on Lower Extremity Sinus Tract Wounds. Foot Ankle Spéc. 2009, 2, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Campitiello, F.; Della Corte, A.; Guerniero, R.; Pellino, G.; Canonico, S. Efficacy of a New Flowable Wound Matrix in Tunneled and Cavity Ulcers: A Preliminary Report. Wounds 2015, 27, 152–157. [Google Scholar] [PubMed]
- Keane, T.J.; Horejs, C.-M.; Stevens, M.M. Scarring vs. functional healing: Matrix-based strategies to regulate tissue repair. Adv. Drug Deliv. Rev. 2018, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena, L.; Requena, C.; Christensen, L.; Zimmermann, U.S.; Kutzner, H.; Cerroni, L. Adverse reactions to injectable soft tissue fillers. J. Am. Acad. Dermatol. 2011, 64, 1–34. [Google Scholar] [CrossRef]
- Badylak, S.F. Decellularized Allogeneic and Xenogeneic Tissue as a Bioscaffold for Regenerative Medicine: Factors that Influence the Host Response. Ann. Biomed. Eng. 2014, 42, 1517–1527. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, A.H.; Stamer, D.; Kyriakides, T. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef]
- Später, T.; Frueh, F.S.; Metzger, W.; Menger, M.D.; Laschke, M.W. In Vivo biocompatibility, vascularization, and incorporation of Integra® dermal regenerative template and flowable wound matrix. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 52–60. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnojeidi, H.; Kilani, R.T.; Ghahary, A. Evaluating the Biocompatibility of an Injectable Wound Matrix in a Murine Model. Gels 2022, 8, 49. https://doi.org/10.3390/gels8010049
Alnojeidi H, Kilani RT, Ghahary A. Evaluating the Biocompatibility of an Injectable Wound Matrix in a Murine Model. Gels. 2022; 8(1):49. https://doi.org/10.3390/gels8010049
Chicago/Turabian StyleAlnojeidi, Hatem, Ruhangiz Taghi Kilani, and Aziz Ghahary. 2022. "Evaluating the Biocompatibility of an Injectable Wound Matrix in a Murine Model" Gels 8, no. 1: 49. https://doi.org/10.3390/gels8010049
APA StyleAlnojeidi, H., Kilani, R. T., & Ghahary, A. (2022). Evaluating the Biocompatibility of an Injectable Wound Matrix in a Murine Model. Gels, 8(1), 49. https://doi.org/10.3390/gels8010049





