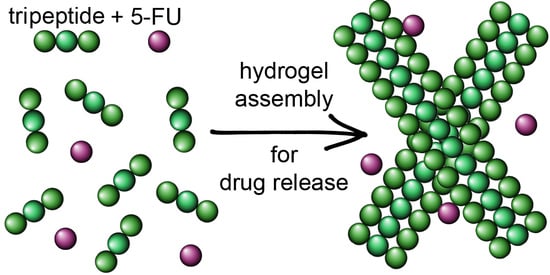
Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil
Abstract
1. Introduction
2. Results and Discussion
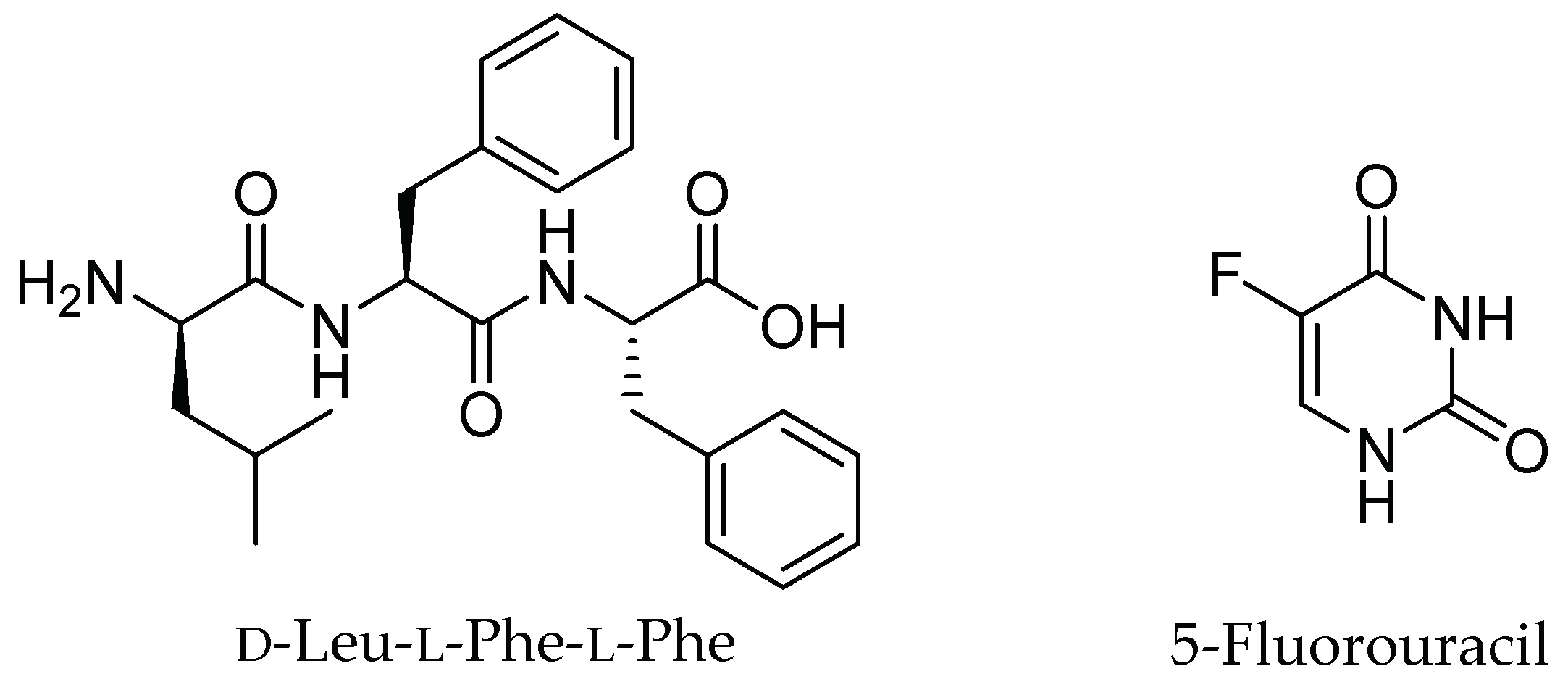
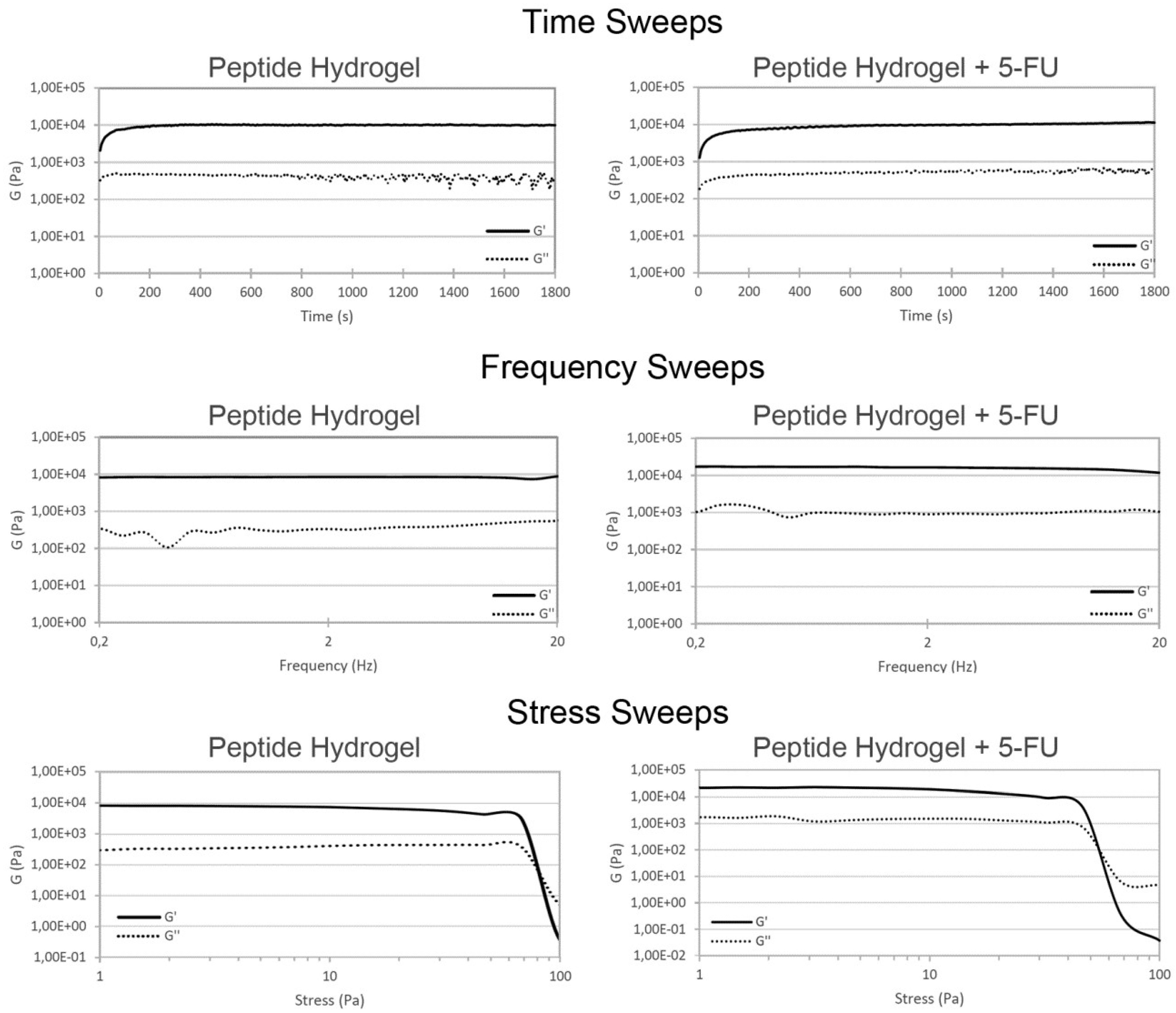
2.1. Hydrogel Characterization
2.2. Nanostructure Morphology
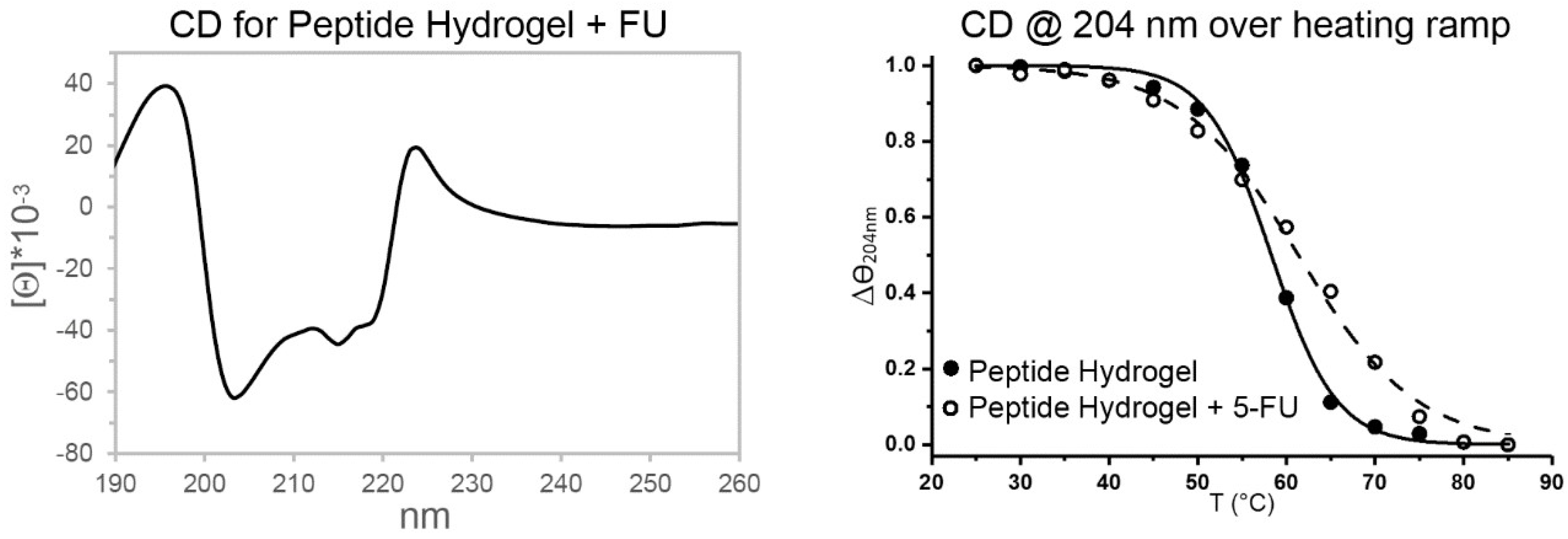
2.3. Spectroscopic Study of Peptide Conformation
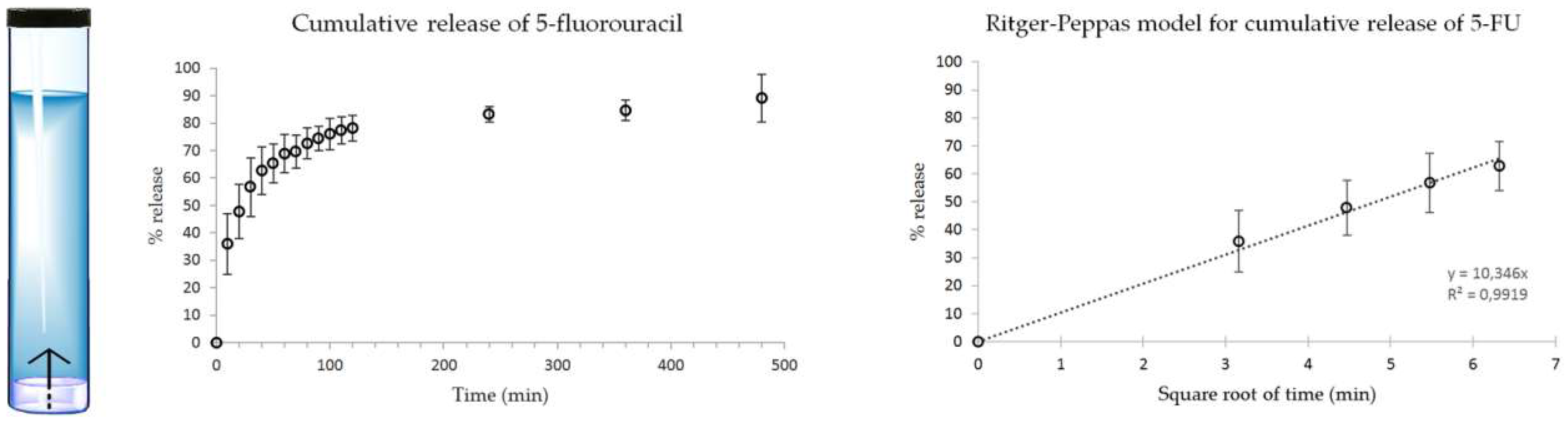
2.4. Drug Release Studies
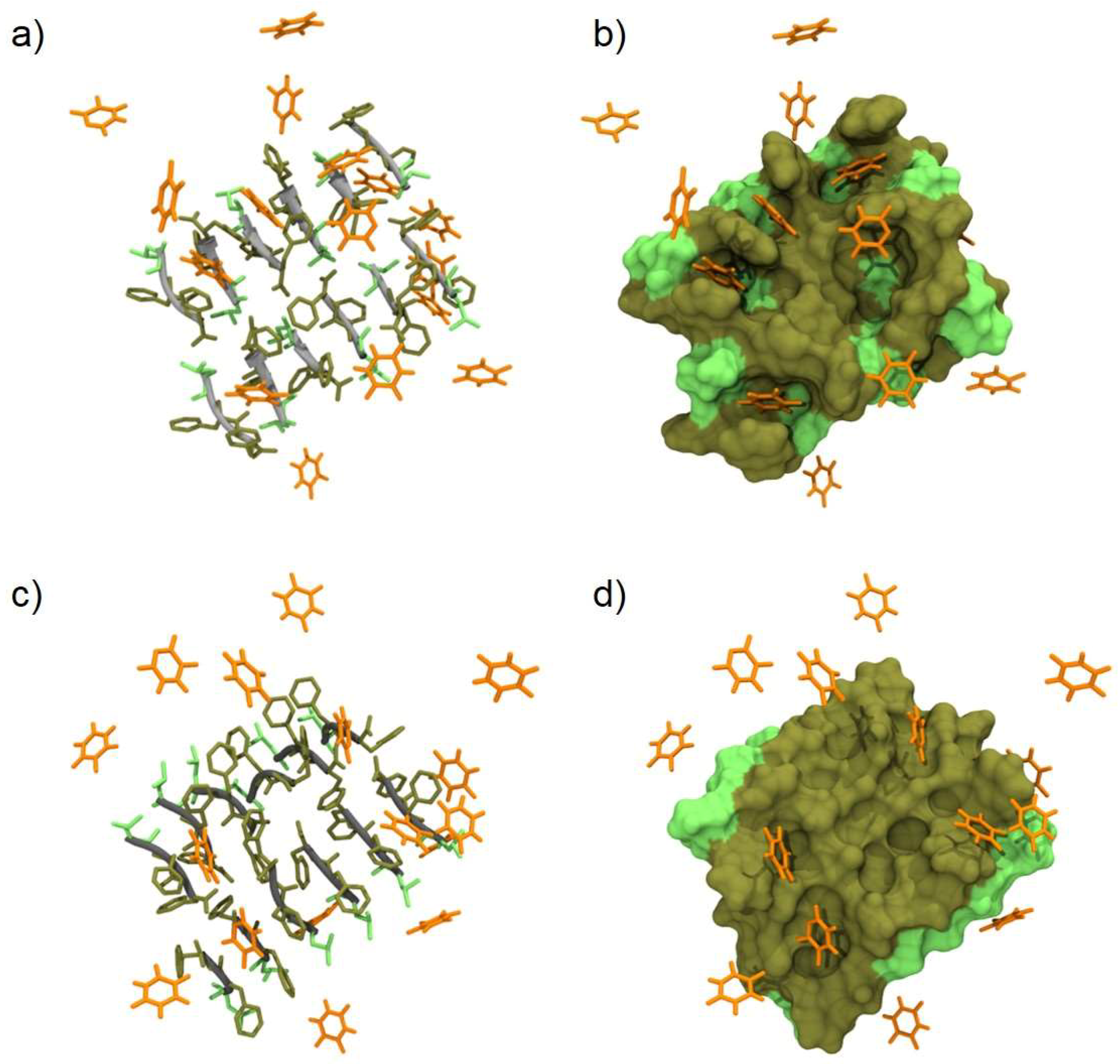
2.5. Molecular Modeling of d-Leu-Phe-Phe/5-FU Interaction
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis and Purification
4.3. Hydrogel Preparation
4.4. Oscillatory Rheometry
4.5. Transmission Electron Microscopy
4.6. Circular Dichroism
4.7. Fourier-Transformed Infrared Spectroscopy
4.8. Thioflavin T Fluorescence Assay
4.9. Drug Release Assay
4.10. Molecular Modeling
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R. A the Hallmark of Cancer. Cell 2000, 60, 319–326. [Google Scholar] [CrossRef]
- Parnell, C.; Woll, P.J. Principles of cancer chemotherapy. Found. Years 2005, 1, 18–22. [Google Scholar] [CrossRef]
- Rampling, R.; James, A.; Papanastassiou, V. The present and future management of malignant brain tumours: Surgery, radiotherapy, chemotherapy. Neurol. Pract. 2004, 75, 24–30. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2018; American Cancer Society: Atlanta, GA, USA, 2018. [Google Scholar]
- Jain, V.; Jain, S.; Mahajan, S.C. Nanomedicines Based Drug Delivery Systems for Anti-Cancer Targeting and Treatment. Curr. Drug Deliv. 2015, 12, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Slingerland, M.; Guchelaar, H.J.; Gelderblom, H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov. Today 2012, 17, 160–166. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Muzzalupo, R.; Trapasso, E.; Picci, N.; Fresta, M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int. J. Pharm. 2008, 353, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mosallaei, N.; Jaafari, M.R.; Hanafi-Bojd, M.Y.; Golmohammadzadeh, S.; Malaekeh-Nikouei, B. Docetaxel-loaded solid lipid nanoparticles: Preparation, characterization, in vitro, and in vivo evaluations. J. Pharm. Sci. 2013, 102, 1994–2004. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Cirillo, G.; Iemma, F.; Puoci, F.; Parisi, O.I.; Curcio, M.; Spizzirri, U.G.; Picci, N. Imprinted hydrophilic nanospheres as drug delivery systems for 5-fluorouracil sustained release. J. Drug Target. 2009, 17, 72–77. [Google Scholar] [CrossRef]
- Chen, J.; Shao, R.; Zhang, X.D.; Chen, C. Applications of nanotechnology for melanoma treatment, diagnosis, and theranostics. Int. J. Nanomed. 2013, 8, 2677–2688. [Google Scholar] [CrossRef]
- Naderi, N.; Madani, S.Y.; Ferguson, E.; Mosahebi, A.; Seifalian, A.M. Carbon nanotubes in the diagnosis and treatment of malignant melanoma. Anticancer. Agents Med. Chem. 2013, 13, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Melchionna, M.; Prato, M. Carbon nanostructures for nanomedicine: Opportunities and challenges. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 190–195. [Google Scholar] [CrossRef]
- Lima-Tenório, M.K.; Gómez Pineda, E.A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Deliv. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.; Saldías, C.; Díaz Díaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef]
- Loo, Y.; Zhang, S.; Hauser, C.A.E. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol. Adv. 2012, 30, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomater. 2014, 10, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Liang, L.; Chen, C.S.; Lu, B.; Wang, N.L.; Jiang, F.G.; Zhang, X.Z.; Zhuo, R.X. Peptide hydrogel as an intraocular drug delivery system for inhibition of postoperative scarring formation. ACS Appl. Mater. Interfaces 2010, 2, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Styan, K.; Marchesan, S. The unexpected advantages of using d-amino acids for peptide self-assembly into nanostructured hydrogels for medicine. Curr. Top. Med. Chem. 2016, 16, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Styan, K.E.; Easton, C.D.; Waddington, L.; Vargiu, A.V. Higher and lower supramolecular orders for the design of self-assembled heterochiral tripeptide hydrogel biomaterials. J. Mater. Chem. B 2015, 3, 8123–8132. [Google Scholar] [CrossRef]
- Liang, G.; Yang, Z.; Zhang, R.; Li, L.; Fan, Y.; Kuang, Y.; Gao, Y.; Wang, T.; Lu, W.W.; Xu, B. Supramolecular hydrogel of a d-amino acid dipeptide for controlled drug release in vivo. Langmuir 2009, 25, 8419–8422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. d-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J. Am. Chem. Soc. 2013, 135, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Kurbasic, M.; Romano, C.; Garcia, A.; Kralj, S.; Marchesan, S. Assembly of a Tripeptide and Anti-Inflammatory Drugs into Supramolecular Hydrogels for Sustained Release. Gels 2017, 3, 29. [Google Scholar] [CrossRef]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; McLean, K.M.; Forsythe, J.S.; Hartley, P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 22, 237–251. [Google Scholar] [CrossRef]
- Leelakanok, N.; Geary, S.; Salem, A. Fabrication and Use of Poly(d,l-lactide-co-glycolide)-Based Formulations Designed for Modified Release of 5-Fluorouracil. J. Pharm. Sci. 2018, 107, 513–528. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Kralj, S.; Kurbasic, M.; Urban, M.; Marchesan, S. Luminescent supramolecular hydrogels from a tripeptide and nitrogen-doped carbon nanodots. Beilstein J. Nanotechnol. 2017, 8, 1553–1562. [Google Scholar] [CrossRef]
- Garcia, A.M.; Iglesias, D.; Parisi, E.; Styan, K.E.; Waddington, L.J.; Deganutti, C.; De Zorzi, R.; Grassi, M.; Melchionna, M.; Vargiu, A.V.; et al. Chirality Effects on Peptide Self-Assembly Unraveled from Molecules to Materials. Chem 2018, 4, 1862–1875. [Google Scholar] [CrossRef]
- Di Carlo, M.G.; Minicozzi, V.; Foderà, V.; Militello, V.; Vetri, V.; Morante, S.; Leone, M. Thioflavin T templates amyloid β(1-40) conformation and aggregation pathway. Biophys. Chem. 2015, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Amdursky, N.; Erez, Y.; Huppert, D. Molecular rotors: What lies behind the high sensitivity of the thioflavin-T fluorescent marker. Acc. Chem. Res. 2012, 45, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Buur, A.; Bundgaard, H. Prodrugs of 5-fluorouracil. III. Hydrolysis kinetics in aqueous solution and biological media, lipophilicity and solubility of various 1-carbamoyl derivatives of 5-fluorouracil. Int. J. Pharm. 1985, 23, 209–222. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR Fundamentals and Applications in Chemistry and Biology, Volume 1. Hydrophobic, Electronic and Steric Constants. J. Am. Chem. Soc. 1996, 118, 10678. [Google Scholar]
- Sangster, J. LOGKOW A Databank of Evaluated Octanol-Water Partition Coefficients; GDF Darabanks Bulletin; 1997; Volume 1, Available online: http://www.gdfdatabanks.ro/documente/sangster.PDF (accessed on 1 December 2018).
- Takács-Novák, K.; Józan, M.; Hermecz, I.; Szász, G. Lipophilicity of antibacterial fluoroquinolones. Int. J. Pharm. 1992, 79, 89–96. [Google Scholar] [CrossRef]
- Yuan, C.; Li, S.; Zou, Q.; Ren, Y.; Yan, X. Multiscale simulations for understanding the evolution and mechanism of hierarchical peptide self-assembly. Phys. Chem. Chem. Phys. 2017, 19, 23614–23631. [Google Scholar] [CrossRef]
- Manandhar, A.; Kang, M.; Chakraborty, K.; Tang, P.K.; Loverde, S.M. Molecular simulations of peptide amphiphiles. Org. Biomol. Chem. 2017, 15, 7993–8005. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Botello-Smith, W.; Cerutti, D.S.; Cheatham, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; et al. AMBER 18. University of California: San Francisco, CA, USA, 2018. Available online: http://ambermd.org/AmberTools.php (accessed on 1 December 2018).
- Humphries, M.J. The molecular basis and specificity of integrin-ligand interactions. J. Cell Sci. 1990, 97 Pt 4, 585–592. [Google Scholar]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Marson, D.; Laurini, E.; Posocco, P.; Fermeglia, M.; Pricl, S. Cationic carbosilane dendrimers and oligonucleotide binding: An energetic affair. Nanoscale 2015, 7, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Vanquelef, E.; Simon, S.; Marquant, G.; Garcia, E.; Klimerak, G.; Delepine, J.C.; Cieplak, P.; Dupradeau, F.Y.R.E.D. Server: A web service for deriving RESP and ESP charges and building force field libraries for new molecules and molecular fragments. Nucleic Acids Res. 2011, 39, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Waddington, L.; Easton, C.D.; Winkler, D.A.; Goodall, L.; Forsythe, J.; Hartley, P.G. Unzipping the role of chirality in nanoscale self-assembly of tripeptide hydrogels. Nanoscale 2012, 4, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Pastor, R.W.; Brooks, B.R.; Szabo, A. An analysis of the accuracy of langevin and molecular dynamics algorithms. Mol. Phys. 1988, 65, 1409–1419. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, E.; Garcia, A.M.; Marson, D.; Posocco, P.; Marchesan, S. Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil. Gels 2019, 5, 5. https://doi.org/10.3390/gels5010005
Parisi E, Garcia AM, Marson D, Posocco P, Marchesan S. Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil. Gels. 2019; 5(1):5. https://doi.org/10.3390/gels5010005
Chicago/Turabian StyleParisi, Evelina, Ana M. Garcia, Domenico Marson, Paola Posocco, and Silvia Marchesan. 2019. "Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil" Gels 5, no. 1: 5. https://doi.org/10.3390/gels5010005
APA StyleParisi, E., Garcia, A. M., Marson, D., Posocco, P., & Marchesan, S. (2019). Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil. Gels, 5(1), 5. https://doi.org/10.3390/gels5010005