Influence of Structure-Directing Additives on the Properties of Poly(methylsilsesquioxane) Aerogel-Like Materials
Abstract
1. Introduction
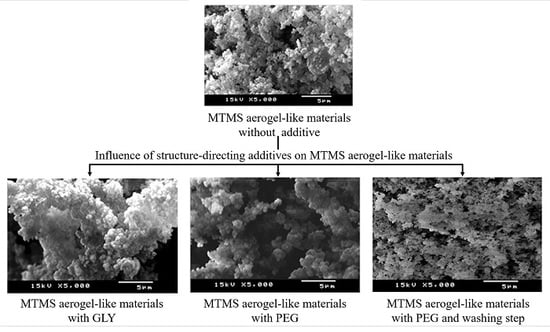
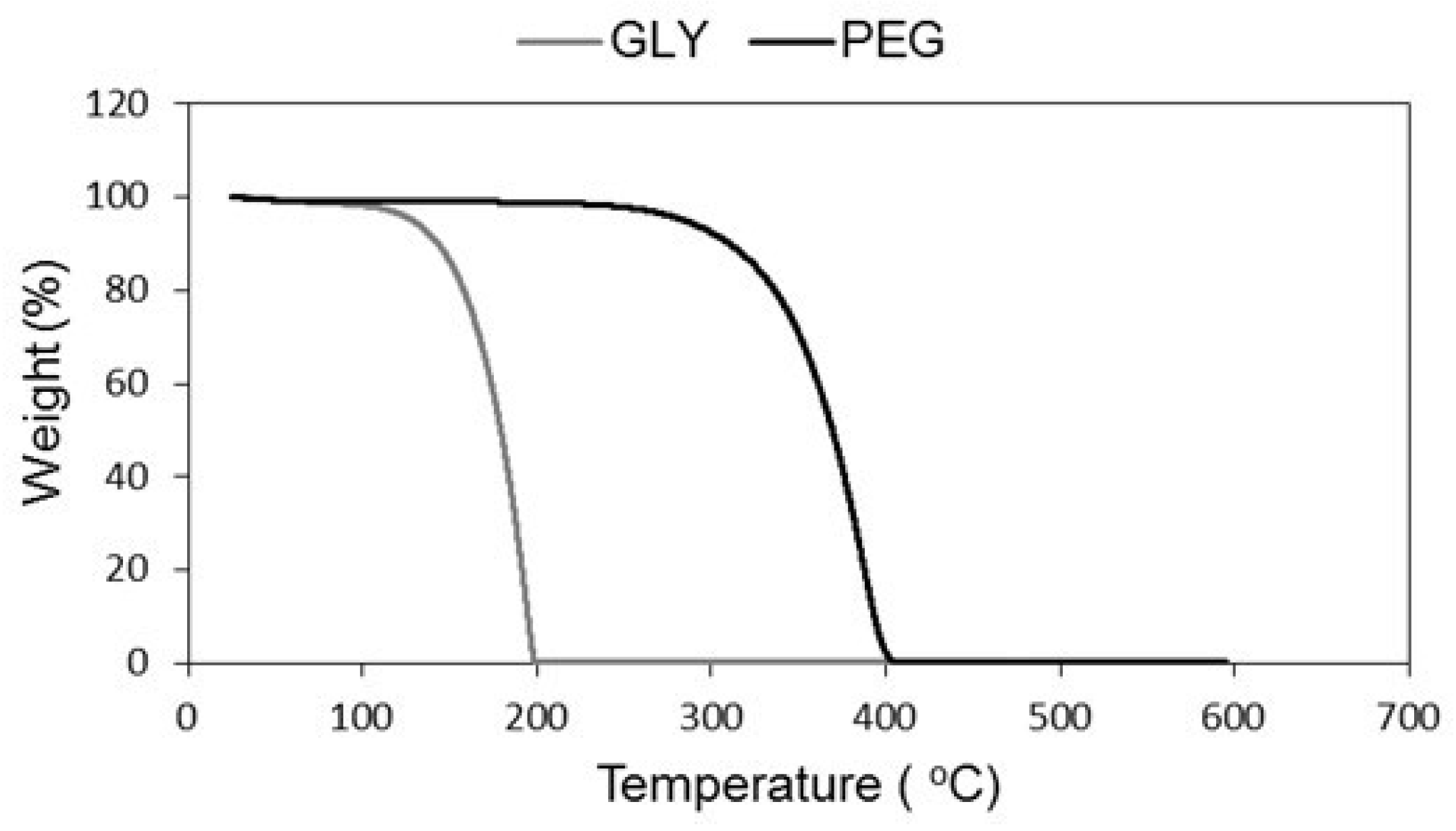
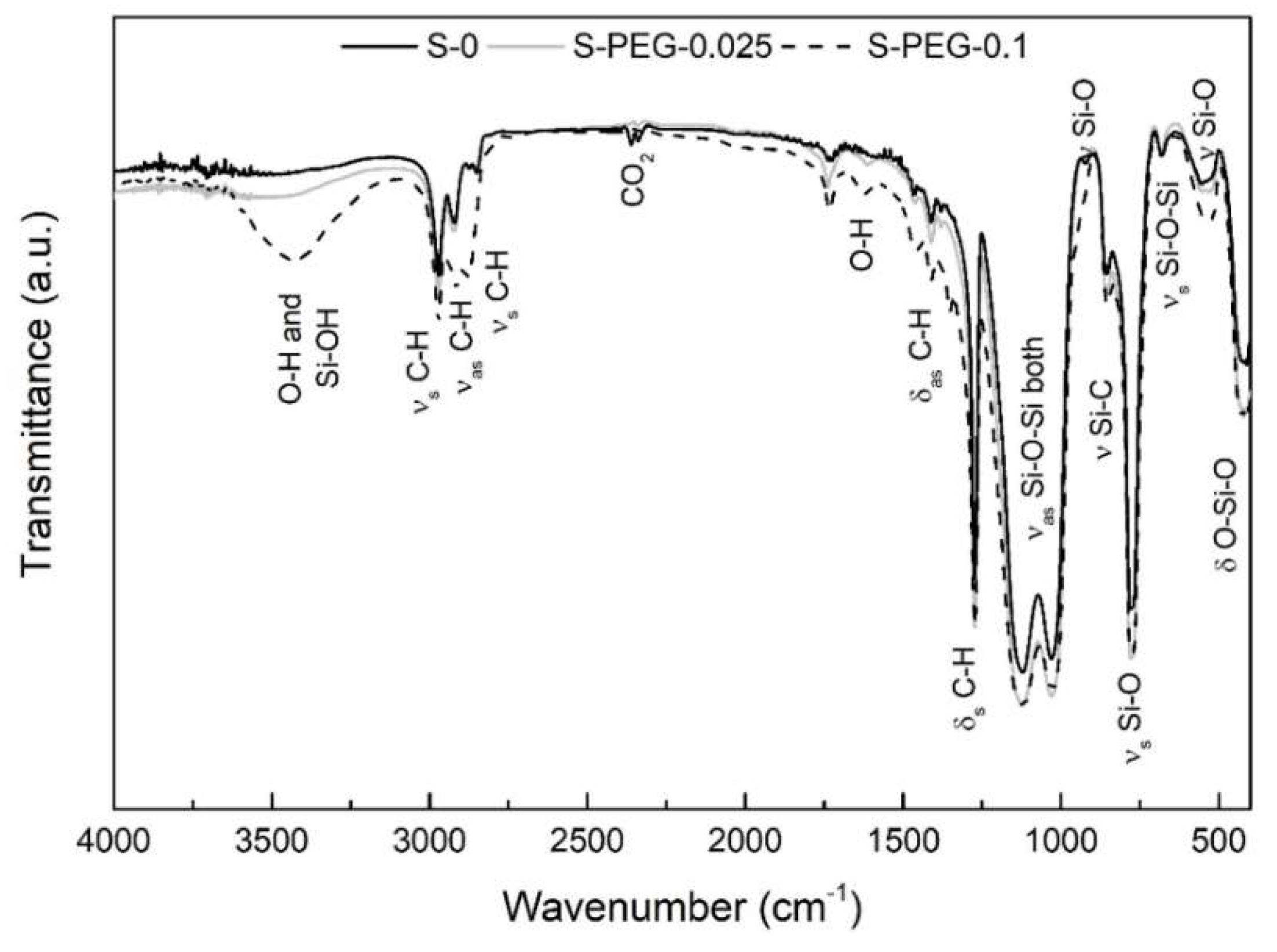
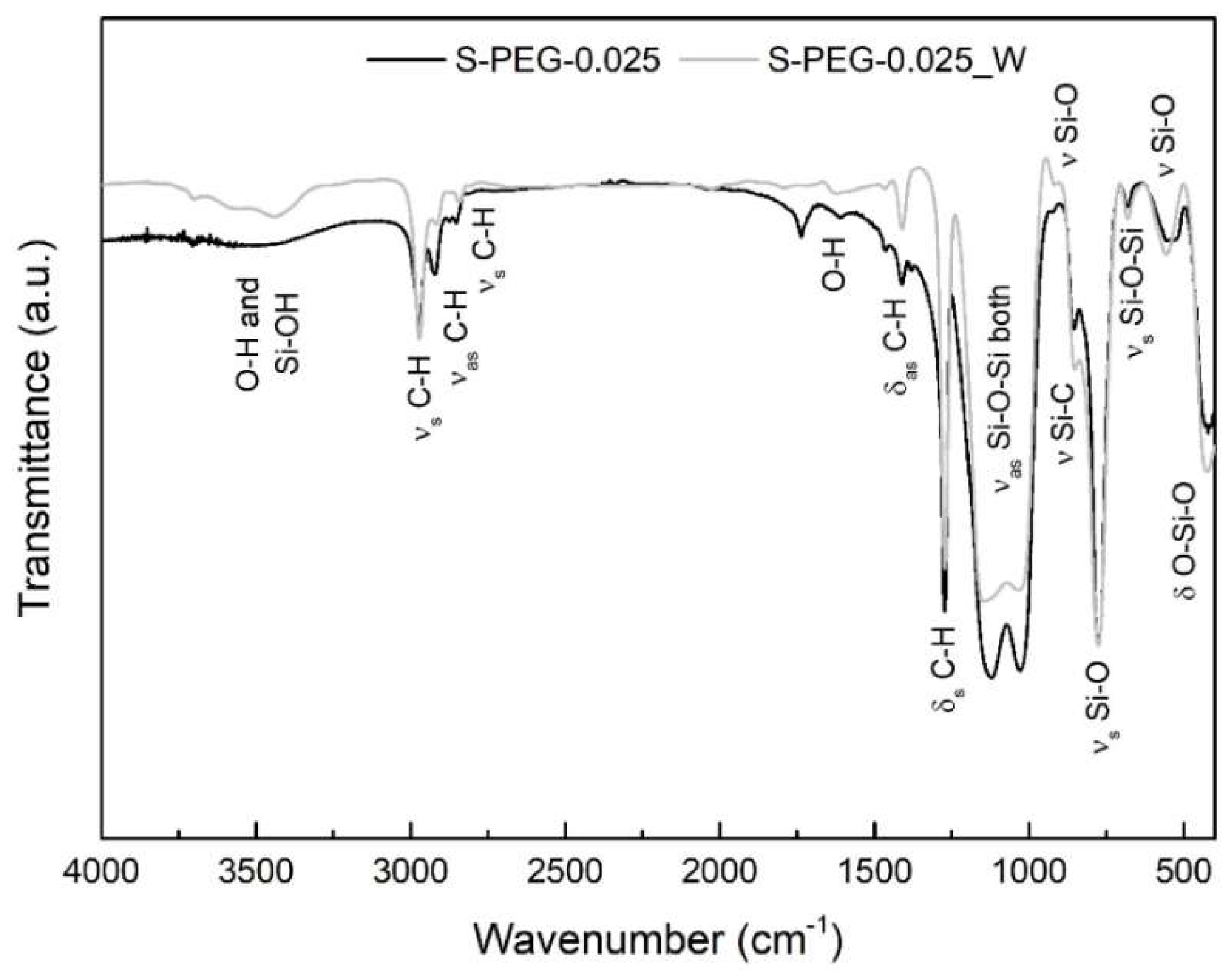
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Silica Aerogel-Like Materials
4.3. Characterization
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Rao, A.V.; Bhagat, S.D.; Hirashima, H.; Pajonk, G.M. Synthesis of flexible silica aerogels using methyltrimethoxysilane (MTMS) precursor. J. Colloid Interface Sci. 2006, 300, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Oh, C.-S.; Kim, Y.-H.; Ahn, Y.-S.; Yeo, J.-G. Methyltrimethoxysilane based monolithic silica aerogels via ambient pressure drying. Microporous Mesoporous Mater. 2007, 100, 350–355. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar] [CrossRef]
- Hegde, N.D.; Rao, A.V. Physical properties of methyltrimethoxysilane based elastic silica aerogels prepared by the two-stage sol-gel process. J. Mater. Sci. 2007, 42, 6965–6971. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Rao, A.V. Effect of post-treatment (gel aging) on the properties of methyltrimethoxysilane based silica aerogels prepared by two-step sol-gel process. J. Sol-Gel Sci. Technol. 2009, 49, 53–59. [Google Scholar] [CrossRef]
- Durães, L.; Ochoa, M.; Rocha, N.; Patrício, R.; Duarte, N.; Redondo, V.; Portugal, A. Effect of the Drying Conditions on the Microstructure of Silica Based Xerogels and Aerogels. J. Nanosci. Nanotechnol. 2012, 12, 6828–6834. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Murakata, T.; Suzuki, T.; Ohgawara, T. Control of pore size distribution of silica gel through sol-gel process using water soluble polymers as additives. J. Mater. Sci. 1990, 25, 4880–4885. [Google Scholar] [CrossRef]
- Sun, J.; Fan, W.; Wu, D.; Sun, Y. Structure Control of SiO2 Sol-Gels via Addition of PEG. In Studies in Surface Science and Catalysis; Delmon, B., Jacobs, P.A., Maggi, R., Martens, J.A., Grange, P., Poncelet, G., Eds.; Elsevier: New York, NY, USA, 1998; Volume 118, pp. 617–624. [Google Scholar]
- Rao, A.V.; Kulkarni, M.M. Effect of glycerol additive on physical properties of hydrophobic silica aerogels. Mater. Chem. Phys. 2003, 77, 819–825. [Google Scholar] [CrossRef]
- Kim, C.E.; Yoon, J.S.; Hwang, H.J. Synthesis of nanoporous silica aerogel by ambient pressure drying. J. Sol-Gel Sci. Technol. 2009, 49, 47. [Google Scholar] [CrossRef]
- Gorbunova, O.V.; Baklanova, O.N.; Gulyaeva, T.I.; Trenikhin, M.V.; Drozdov, V.A. Poly(ethylene glycol) as structure directing agent in sol-gel synthesis of amorphous silica. Microporous Mesoporous Mater. 2014, 190, 146–151. [Google Scholar] [CrossRef]
- Durães, L.; Maia, A.; Portugal, A. Effect of additives on the properties of silica based aerogels synthesized from methyltrimethoxysilane (MTMS). J. Supercrit. Fluids 2015, 106, 85–92. [Google Scholar] [CrossRef]
- Martin, J.; Hosticka, B.; Lattimer, C.; Norris, P.M. Mechanical and acoustical properties as a function of PEG concentration in macroporous silica gels. J. Non-Cryst. Solids 2001, 285, 222–229. [Google Scholar] [CrossRef]
- Rosa-Fox, N.; Esquivais, L.; Zarzycki, J. Silica sonogel with drying control chemical additives. J. Mater. Sci. Lett. 1991, 10, 1237–1242. [Google Scholar] [CrossRef]
- Rao, A.V.; Pajonk, G.M.; Haranath, D.; Wagh, P.B. Effect of glycerol on monolithicity, density, microhardness and sintering temperature of TMOS silica aerogels. Microporous Mater. 1997, 12, 63–69. [Google Scholar] [CrossRef]
- Haranath, D.; Rao, A.V.; Wagh, P.B. Influence of DCCAs on Optical Transmittance and Porosity Properties of TMOS Silica Aerogels. J. Porous Mater. 1999, 6, 55–62. [Google Scholar] [CrossRef]
- Ma, X.; Sun, H.; Yu, P. A novel way for preparing high surface area silica monolith with bimodal pore structure. J. Mater. Sci. 2008, 43, 887–891. [Google Scholar] [CrossRef]
- Gómez-Siurana, A.; Marcilla, A.; Beltrán, M.; Berenguer, D.; Martínez-Castellanos, I.; Menargues, S. TGA/FTIR study of tobacco and glycerol–tobacco mixtures. Thermochim. Acta 2013, 573, 146–157. [Google Scholar] [CrossRef]
- Perotti, G.F.; Kijchavengkul, T.; Auras, R.A.; Constantino, V.R. Nanocomposites Based on Cassava Starch and Chitosan-Modified Clay: Physico-Mechanical Properties and Biodegradability in Simulated Compost Soil. J. Brazil. Chem. Soc. 2017, 28, 649–658. [Google Scholar] [CrossRef]
- Afonso, S.; Matias, T.; Ochoa, M.; Portugal, A.; Durães, L. Thermal Stability of Hydrophobic Xerogels and Aerogels Synthesized from MTMS. In Proceedings of the Seminar on Aerogels—Properties-Manufacture Applications, ENSIC-Nancy, Nancy, France, 6–7 December 2012; pp. 99–110. [Google Scholar]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Becker, H.G.O.; Herold, B.J.; Rauter, A.P. Organikum: Química Orgânica Experimental, 2nd ed.; Fundação Calouste Gulbenkian: Lisboa, Portugal, 1997; p. 1053. [Google Scholar]
- Amand, L.E.; Tullin, C.J. The Theory Behind FTIR Analysis; University of Technology GOteborg: Gothenburg, Sweden, 1996; p. 15. [Google Scholar]
- Malfait, W.J.; Zhao, S.; Verel, R.; Iswar, S.; Rentsch, D.; Fener, R.; Zhang, Y.; Milow, B.; Koebel, M.M. Surface Chemistry of Hydrophobic Silica Aerogels. Chem. Mater. 2015, 27, 6737–6745. [Google Scholar] [CrossRef]
- He, S.; Chen, X. Flexible silica aerogel based on methyltrimethoxysilane with improved mechanical property. J. Non-Cryst. Solids 2017, 463, 6–11. [Google Scholar] [CrossRef]
- Vareda, J.P.; Maximiano, P.; Cunha, L.P.; Ferreira, A.F.; Simões, P.N.; Durães, L. Effect of different types of surfactants on the microstructure of methyltrimethoxysilane-derived silica aerogels: A combined experimental and computational approach. J. Colloid Interface Sci. 2018, 512, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Reichenauer, G.; Scherer, G.W. Effects upon Nitrogen Sorption Analysis in Aerogels. J. Colloid Interface Sci. 2001, 236, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Reichenauer, G.; Scherer, G.W. Nitrogen sorption in aerogels. J. Non-Cryst. Solids 2001, 285, 167–174. [Google Scholar] [CrossRef]
- Iswar, S.; Malfait, W.J.; Balog, S.; Winnefeld, F.; Lattuada, M.; Koebel, M.M. Effect of aging on silica aerogel properties. Microporous Mesoporous Mater. 2017, 241, 293–302. [Google Scholar] [CrossRef]
- Feng, J.; Le, D.; Nguyen, S.T.; Tan Chin Nien, V.; Jewell, D.; Duong, H.M. Silica cellulose hybrid aerogels for thermal and acoustic insulation applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 298–305. [Google Scholar] [CrossRef]
- Sequeira, S.; Evtuguin, D.V.; Portugal, I. Preparation and properties of cellulose/silica hybrid composites. Polym. Compos. 2009, 30, 1275–1282. [Google Scholar] [CrossRef]

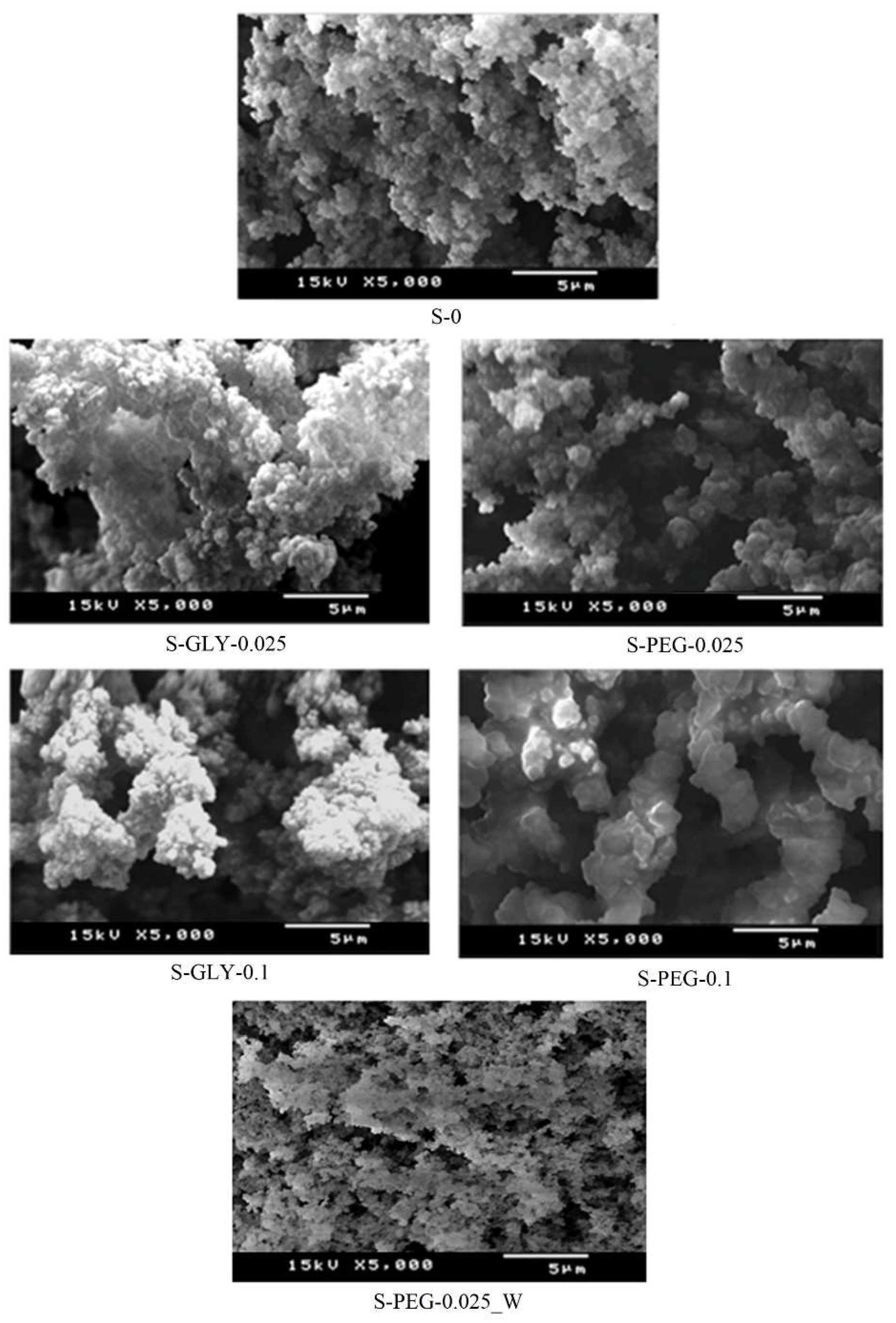

| Chemical System a,b | Drying Method c | Thermal Post-Treatments | Main Conclusions d | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Precursor(s) | Solvent | Water | Catalyst | Additive(s) | ||||
| TEOS 5 g | - | 30 g | HCl pH = 1.8 NH4OH pH = 8.2 | PEG (up to 37.5 wt%) Other: PVA, PAA, PEI, proteins | APD | 500–800 °C | - PEG had little influence on silica particle growth of the sol and led to a decrease of the specific surface area of the final material; - PEG influenced the (meso)pores size distribution. | [10] (1990) |
| TMOS or TEOS 1 | - | 4–10 | HNO3 pH = 1.5 | GLY or PEG 1 to 5 Other: FA | APD | 300 °C 400 °C 500–1100 °C | - PEG and GLY lead to a substantial increase of gelation time and with these additives the produced materials were not monolithic. | [17] (1991) |
| TMOS 1 | MeOH 6–12 | 4–8 | NH4OH 0.0036–0.1 | GLY 0.33–1 | SCD | 260–1025 °C | - GLY/TMOS molar ratio above 1.1 resulted in cracked samples, while 0.83 was the best ratio to obtain monoliths; - Larger pore radii were obtained when the post-treatment temperature increases from 260 to 650 °C. | [18] (1997) |
| TEOS 1 | EtOH 40 | 2 | NH4OH 0.0005 | PEG Up to 10 mol% | APD or vacuum | - | - PEG allows a controlled texture; - The presence of PEG in SiO2 sols led to an increase in the particle size and then the formation of secondary particles with ring-like structures with short-order. | [11] (1998) |
| TMOS 1 | MeOH 12 | 4 | NH4OH 0.0036 | GLY 0.2–0.8 Other: DMF, FA, Oxalic acid | SCD | - | - GLY leads to an increase in the specific surface area; - GLY is a suitable DCCA to produce monolithic aerogels. | [19] (1999) |
| TEOS 1 | - | 33 | HNO3 0.02 KOH 0.035 | PEG 2.5–10.2 mg/mL of sol. | SCD with CO2 | - | - With the increase of PEG concentration in the precursor system, the specific surface area decreased due to an increase in pore size; - Small concentrations of PEG increase the mechanical strength of the solid matrix. | [16] (2001) |
| TMOS/MTMS 1/0.7 | MeOH 12 | 4 | NH4OH 0.0036 | GLY 0–0.2 | SCD | - | - The lowest density and pores shrinkage were obtained for a GLY/TMOS molar ratio of 0.025. | [12] (2003) |
| TMOS 11 mL | - | See catalyst | Acid (acetic + citric) Aqueous solution pH = 5 | PEG 2.45 g GLY solution | APD | 550 °C | - PEG, together with citric acid, showed a control in the particle aggregation and internal structure; - Immersing the wet gel in a glycerol solution allows obtaining a crack free monolith. | [20] (2008) |
| Sodium Silicate 144 mL | - | 525 mL | NH4OH pH = 4 | GLY 3–5 wt% | APD | - | - Addition of GLY gives a more homogeneous microstructure; - GLY retards surface modification and solvent exchange; - The aerogel obtained with GLY maintained a relatively low bulk density compared with the aerogels aged in mixed ethanol/TEOS solution. | [13] (2008) |
| TEOS 0.5–1 mL/min | - | See catalyst | HCl 1.7 N | PEG 0.01–100 mmol/L | APD | 600 °C | - Depending on the combination of molecular weight and concentration of the PEG solution, microporous and mesoporous silica materials can be obtained; - Texture of the produced silica is strongly correlated with polymer solution rheology. | [14] (2014) |
| MTMS 1 | MeOH 35 | See catalyst | Oxalic acid 4 NH4OH 4 | PEG 0.01 Other: BTMSH and ODS | SCD with CO2 | - | - PEG provides some uniformity to the porous network; - The addition of PEG leads to samples with lower densities, thermal conductivities and modulus. | [15] (2015) |
| Sample | MTMS:CH3OH:Acidic Water:Basic Water:Additive (Molar Ratio) | Bulk Density a (kg/m3) | Skeletal Density b (kg/m3) | Porosity (%) |
|---|---|---|---|---|
| S-0 | 1:35:4:4:0 | 79.9 ± 5.8 | 1223.5 ± 140.1 | 93.4 |
| S-GLY-0.025 | 1:35:4:4:0.025 | 76.1 ± 3.3 | 1120.2 ± 59.7 | 93.2 |
| S-GLY-0.05 | 1:35:4:4:0.05 | 79.9 ± 5.6 | 831.5 ± 36.0 | 90.4 |
| S-GLY-0.075 | 1:35:4:4:0.075 | 83.4 ± 5.4 | 852.3 ± 21.3 | 90.2 |
| S-GLY-0.1 | 1:35:4:4:0.1 | 84.2 ± 4.1 | 723.0 ± 8.0 | 88.4 |
| S-PEG-0.025 | 1:35:4:4:0.025 | 72.7 ± 2.3 | 1543.3 ± 31.7 | 95.3 |
| S-PEG-0.05 | 1:35:4:4:0.05 | 89.1 ± 6.7 | 1303.6 ± 79.2 | 93.2 |
| S-PEG-0.075 | 1:35:4:4:0.075 | 89.4 ± 8.0 | 1319.2 ± 52.8 | 93.2 |
| S-PEG-0.1 | 1:35:4:4:0.1 | 97.9 ± 11.9 | 1333.3 ± 265.7 | 92.7 |
| S-PEG-0.025_W | 1:35:4:4:0.025 | 46.1 ± 3.8 | 1684.8 ± 105.9 | 97.3 |
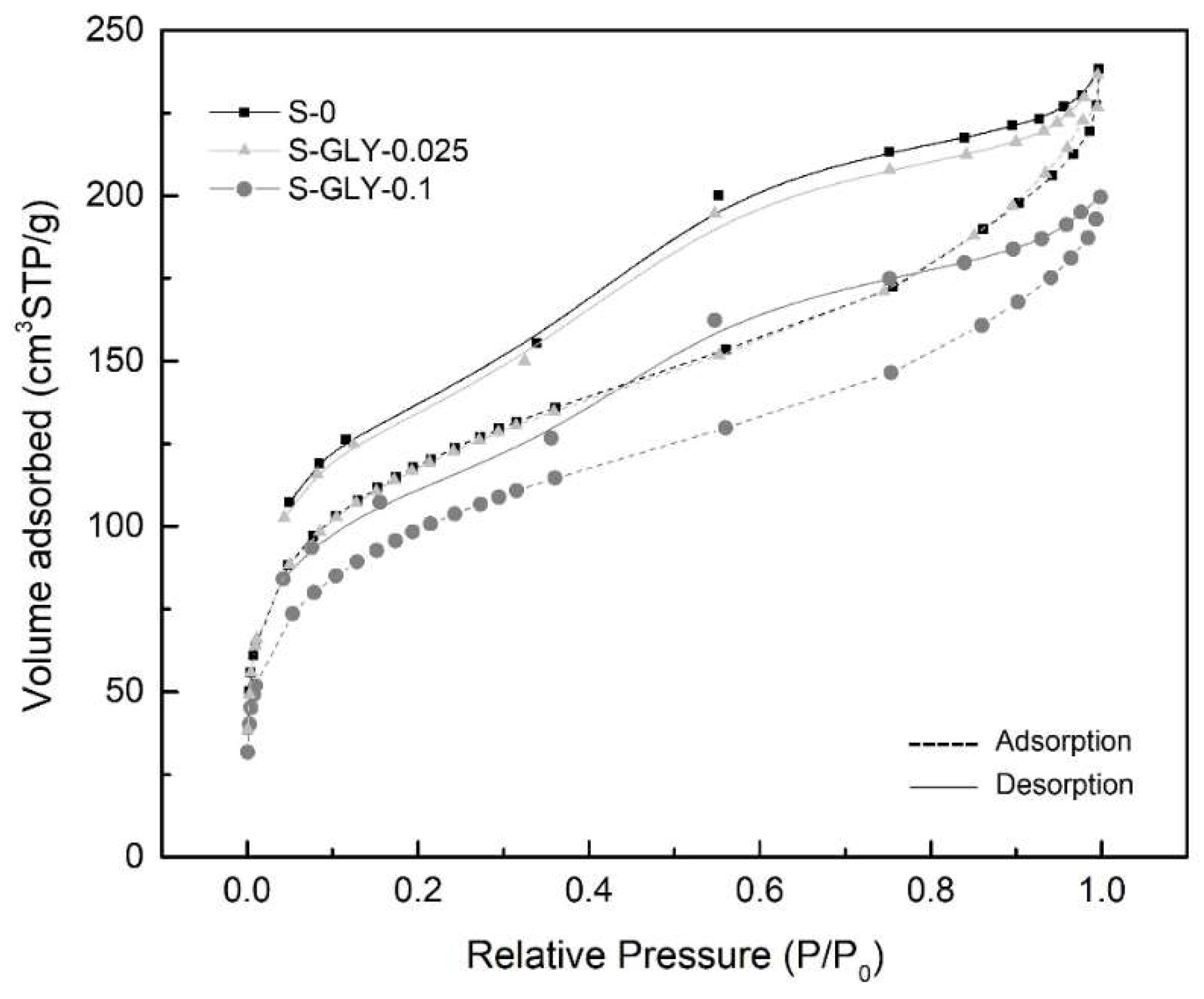
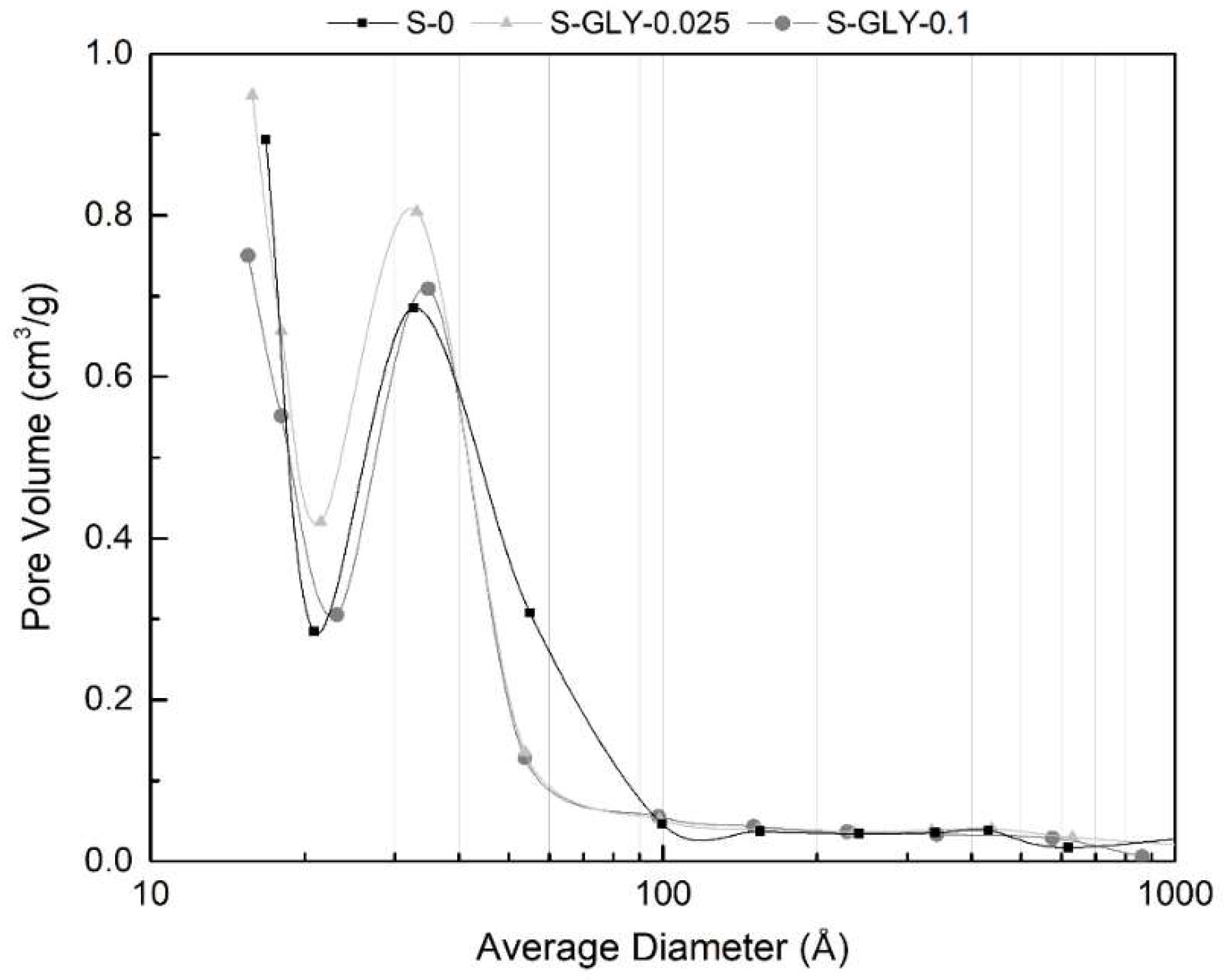
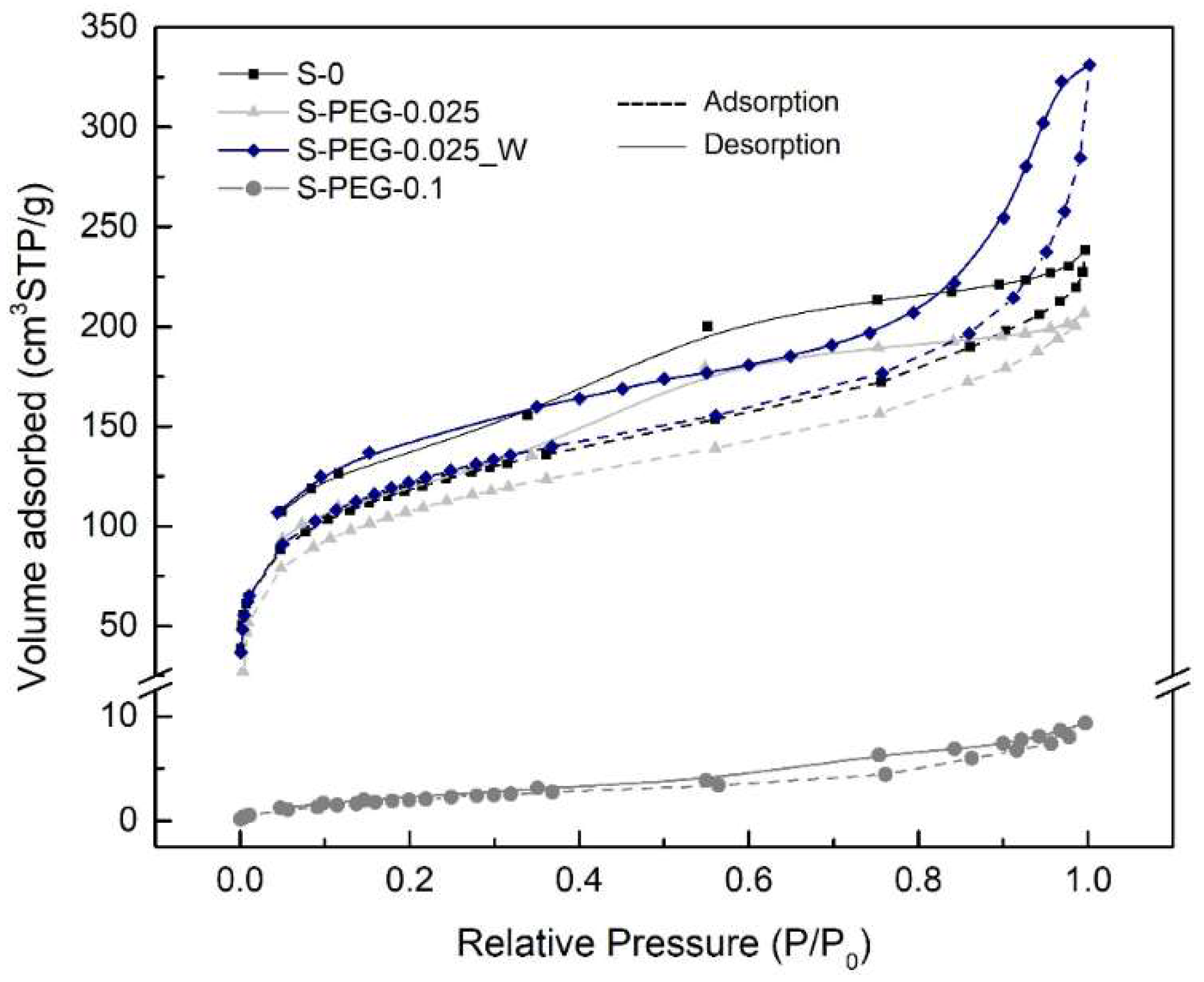
| Sample | BET Specific Surface Area a (m2/g) | BJH-Desorption Pore Volume (cm3/g) | BJH-Desorption Aver. Pore Size b (Å) | Calculated Pore Volume c (cm3/g) | Calculated Pore Size d (nm) |
|---|---|---|---|---|---|
| S-0 | 400.3 ± 10.5 | 0.418 | 30.3 | 11.7 ± 1.0 | 116.8 ± 6.9 |
| S-GLY-0.025 | 408.2 ± 7.2 | 0.417 | 28.2 | 12.3 ± 0.6 | 120.0 ± 3.9 |
| S-GLY-0.1 | 347.0 ± 5.7 | 0.349 | 28.8 | 10.5 ± 0.6 | 121.0 ±4.8 |
| S-PEG-0.025 | 374.5 ± 6.9 | 0.366 | 28.5 | 13.1 ± 0.4 | 140.0 ± 2.2 |
| S-PEG-0.1 | 8.74 ± 0.14 | 0.015 | 39.4 | 9.5 ± 1.4 | 4331.6 ± 567.3 |
| S-PEG-0.025_W | 421.0 ± 8.8 | 0.574 | 37.3 | 21.1 ± 1.8 | 200.46 ± 13.2 |
| Sample | Contact Angle a (°) | Thermal Conductivity a (mW·m−1·K−1) |
|---|---|---|
| S-0 | 141.3 ± 1.7 | 38.65 ± 0.22 |
| S-GLY-0.025 | 136.8 ± 0.9 | 38.28 ± 0.21 |
| S-GLY-0.1 | 129.3 ± 3.1 | 39.98 ± 0.03 |
| S-PEG-0.025 | 147.1 ± 6.0 | 39.01 ± 0.21 |
| S-PEG-0.075 b | 134.5 ± 4.9 | 44.73 ± 0.26 |
| S-PEG-0.025_W | 146.6 ± 4.7 | 35.22 ± 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa, M.; Lamy-Mendes, A.; Maia, A.; Portugal, A.; Durães, L. Influence of Structure-Directing Additives on the Properties of Poly(methylsilsesquioxane) Aerogel-Like Materials. Gels 2019, 5, 6. https://doi.org/10.3390/gels5010006
Ochoa M, Lamy-Mendes A, Maia A, Portugal A, Durães L. Influence of Structure-Directing Additives on the Properties of Poly(methylsilsesquioxane) Aerogel-Like Materials. Gels. 2019; 5(1):6. https://doi.org/10.3390/gels5010006
Chicago/Turabian StyleOchoa, Marta, Alyne Lamy-Mendes, Ana Maia, António Portugal, and Luísa Durães. 2019. "Influence of Structure-Directing Additives on the Properties of Poly(methylsilsesquioxane) Aerogel-Like Materials" Gels 5, no. 1: 6. https://doi.org/10.3390/gels5010006
APA StyleOchoa, M., Lamy-Mendes, A., Maia, A., Portugal, A., & Durães, L. (2019). Influence of Structure-Directing Additives on the Properties of Poly(methylsilsesquioxane) Aerogel-Like Materials. Gels, 5(1), 6. https://doi.org/10.3390/gels5010006