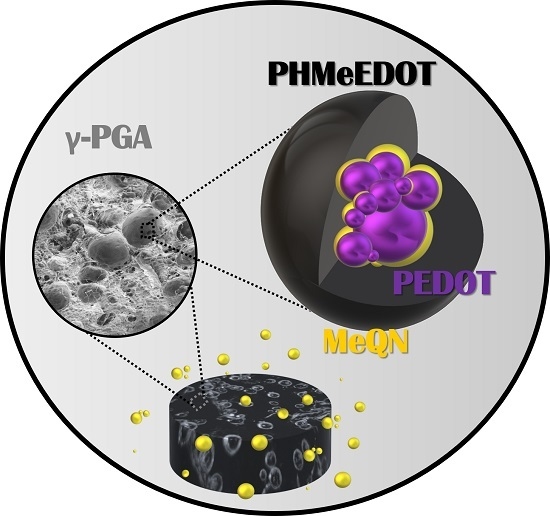
Assembly of Conducting Polymer and Biohydrogel for the Release and Real-Time Monitoring of Vitamin K3
Abstract
:1. Introduction
2. Results and Discussion
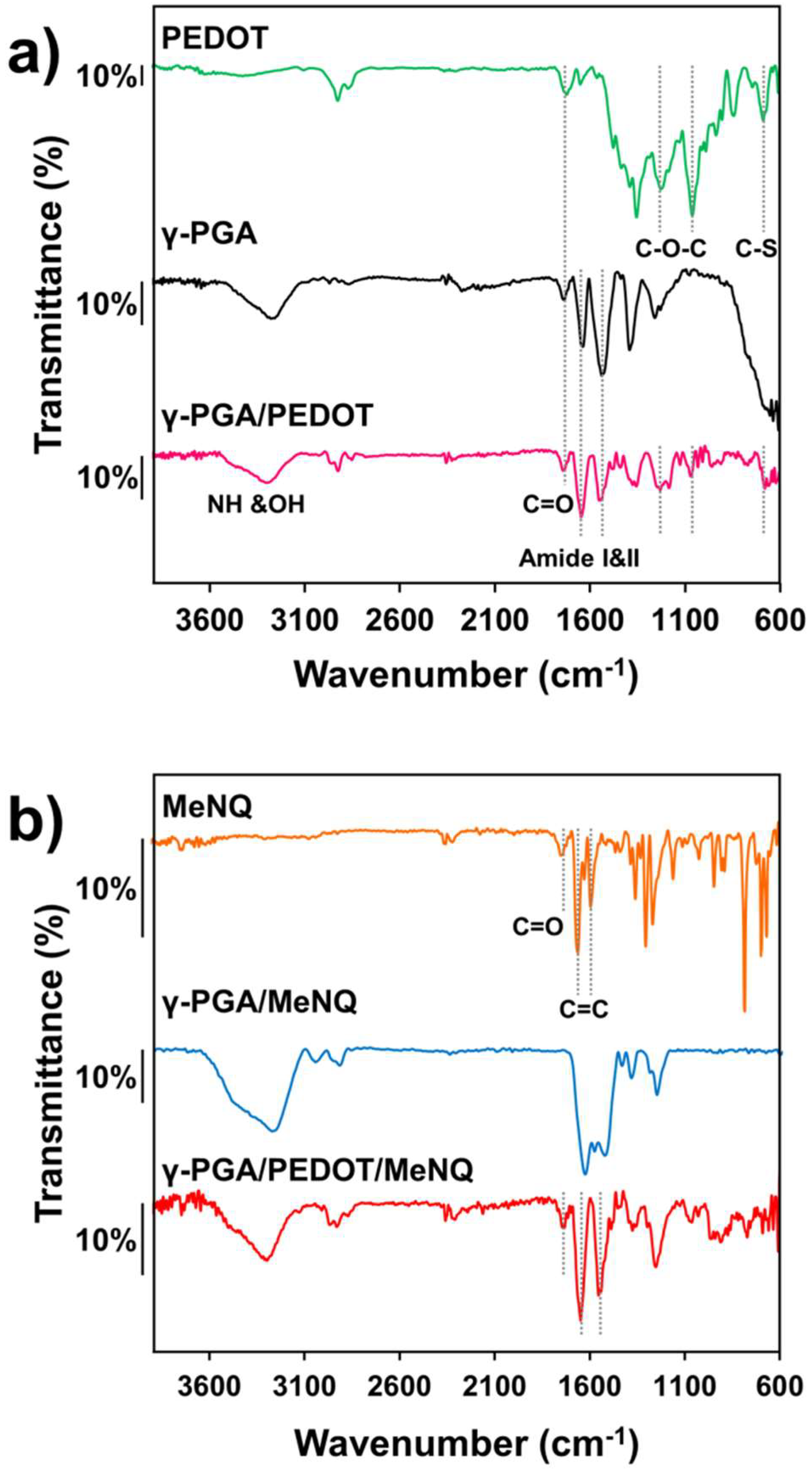
2.1. Loading of PEDOT NPs into γ-PGA Hydrogels
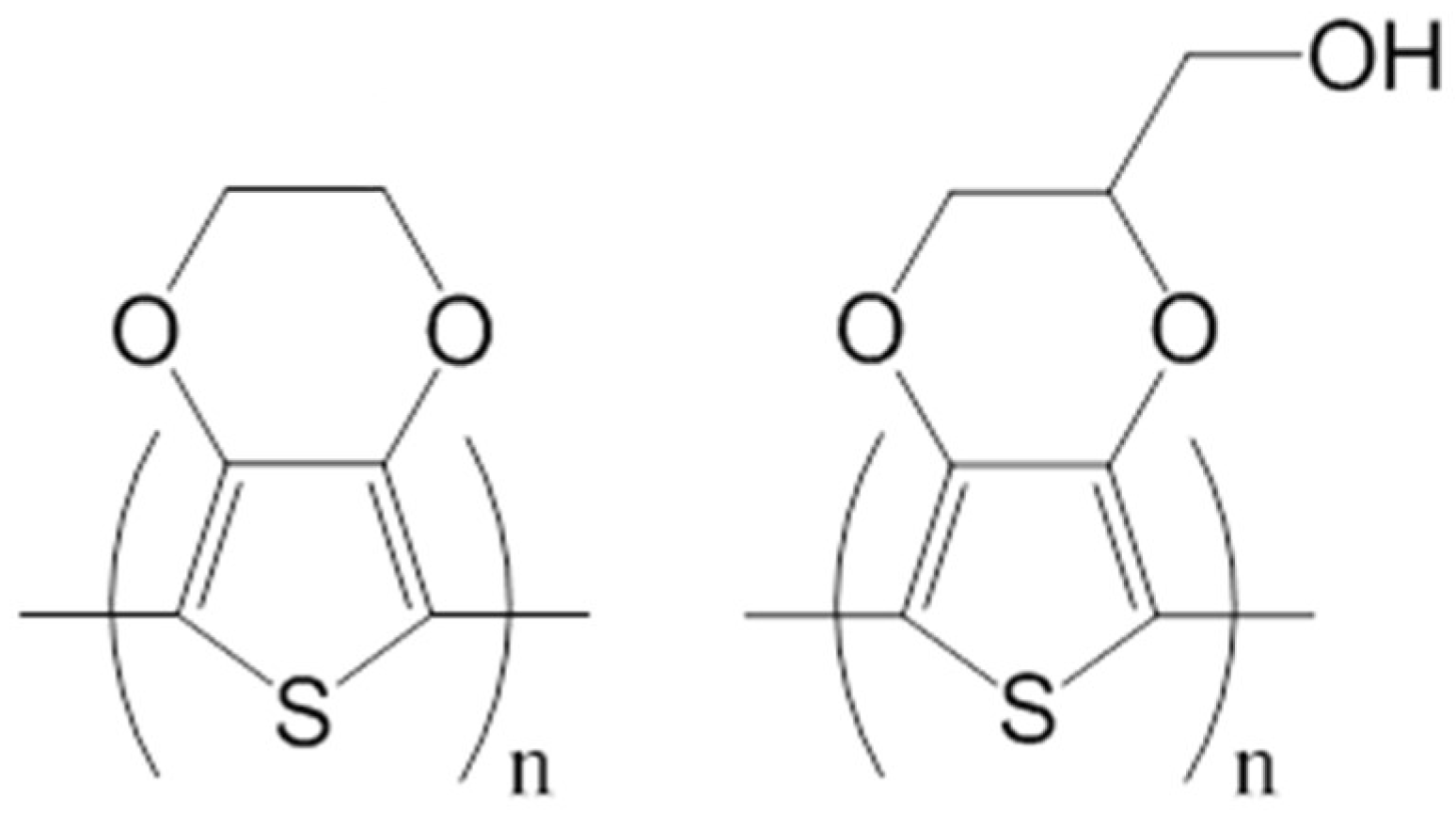
2.2. Characterization of Electroactive and Flexible Electrodes Prepared through PHMeEDOT Polymerization
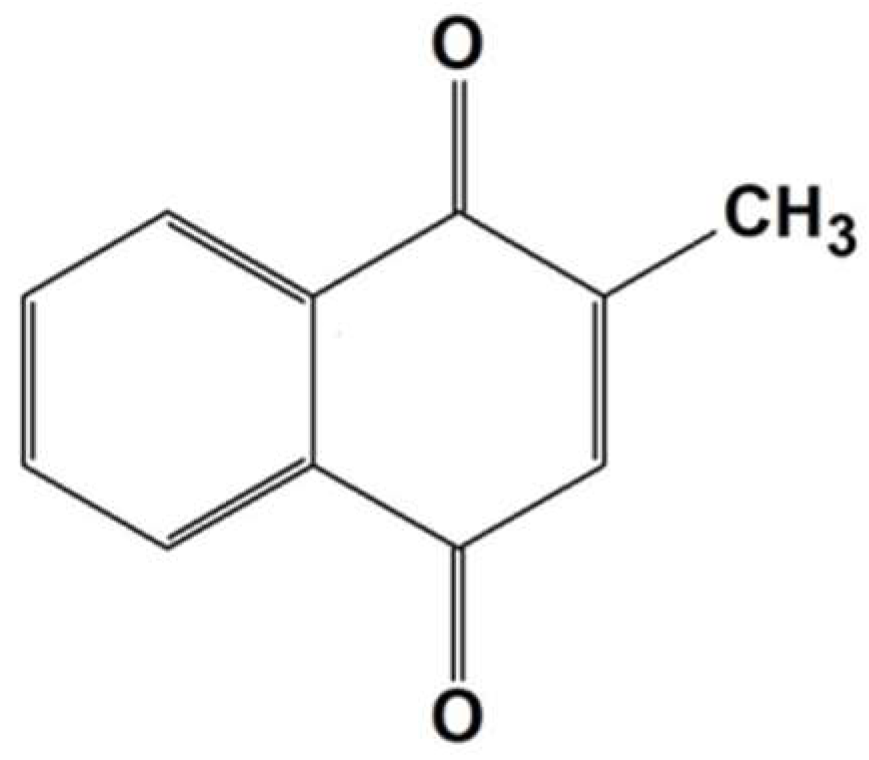
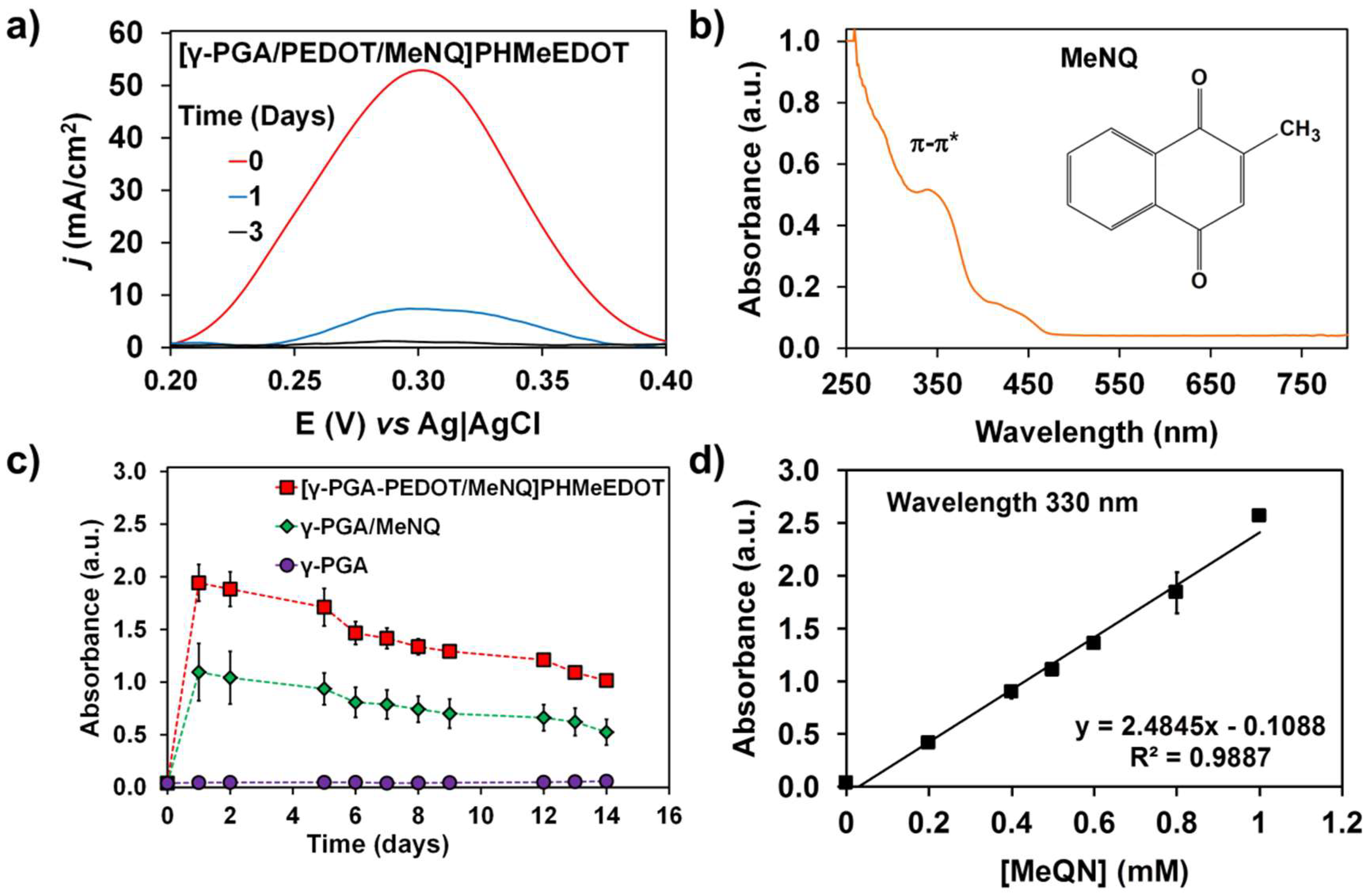
2.3. Release and Detection of MeNQ
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PEDOT NPs
4.3. Synthesis of the γ-PGA Hydrogel
4.4. Preparation of γ-PGA/PEDOT
4.5. Preparation of [γ-PGA/PEDOT]PHMeEDOT Composites
4.6. Preparation of MeNQ-Containing Hydrogels
4.7. Characterization
4.8. Release and Electrochemical Detection of MeNQ
Author Contributions
Funding
Conflicts of Interest
References
- Le Goff, G.C.; Srinivas, R.L.; Hill, W.A.; Doyle, P.S. Hydrogel microparticles for biosensing. Eur. Polym. J. 2015, 72, 386–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.; Jang, E.; Park, J.; Koh, W.G. Development of microfluidic devices incorporating non-spherical hydrogel microparticles for protein-based bioassay. Microfluid Nanofluidics 2008, 5, 703–710. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.; Jo, W.H. Preparation of hydrogel nanoparticles by atom transfer radical polymerization of N-isopropylacrylamide in aqueous media using PEG macro-initiator. Polymer 2005, 46, 2836–2840. [Google Scholar] [CrossRef]
- Na, K.; Park, K.-H.; Kim, S.W.; Bae, Y.H. Self-assembled hydrogel nanoparticles from curlan derivatives: Characterization, anti-cancer drug delivery and interaction with a hepatoma cell line (Hep G2). J. Control. Release 2000, 69, 225–236. [Google Scholar] [CrossRef]
- Ye, Y.; Mao, Y. Vapor-based synthesis of ultrathin hydrogel coatings for thermo-responsive nanovalves. J. Mater. Chem. 2011, 21, 7946–7952. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xiong, L.; Wang, H.; Zha, G.; Du, H.; Li, X.; Shen, Z. Sustained drug release from an ultrathin hydrogel film. Polym. Chem. 2015, 6, 7097–7099. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007, 19, 2696–2701. [Google Scholar] [CrossRef]
- Jung, I.Y.; Kim, J.S.; Choi, B.R.; Lee, K.; Lee, H. Hydrogel Based Biosensors for In Vitro Diagnostics of Biochemicals, Proteins, and Genes. Adv. Healthc. Mater. 2017, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, Y.; Markland, P.; Yang, V.C. Poly(glutamic acid) poly(ethylene glycol) hydrogels prepared by photoinduced polymerization: Synthesis, characterization, and preliminary release studies of protein drugs. J. Biomed. Mater. Res. 2002, 62, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Thorne, C.B.; Gomez, C.G.; Noyes, H.E.; Housewright, R.D. Production of glutamyl polypeptide by Bacillus subtilis. J. Bacteriol. 1954, 68, 307–315. [Google Scholar] [PubMed]
- Shih, I.L.; Van, Y.T. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Bajaj, I.; Singhal, R. Poly (glutamic acid)—An emerging biopolymer of commercial interest. Bioresour. Technol. 2011, 102, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Madrigal, M.M.; Edo, M.G.; Díaz, A.; Puiggalí, J.; Alemán, C. Poly-γ-glutamic Acid Hydrogels as Electrolyte for Poly(3,4-ethylenedioxythiophene)-Based Supercapacitors. J. Phys. Chem. C 2017, 121, 3182–3193. [Google Scholar] [CrossRef]
- Matsusaki, M.; Serizawa, T.; Kishida, A.; Akashi, M. Novel functional biodegradable polymer. III. The construction of poly(γ-glutamic acid)-sulfonate hydrogel with fibroblast growth factor-2 activity. J. Biomed. Mater. Res. Part A 2005, 73, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, G.; Giménez, A.; Díaz, A.; Puiggalí, A.; Alemán, C. Dual-functionalization device for therapy through dopamine release and monitoring. Macromol. Biosci. 2018, 18, 1800014. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W. Porous-structured conductive polypyrrole cell scaffolds. Bull. Korean Chem. Soc. 2014, 35, 293–296. [Google Scholar] [CrossRef]
- Zhao, Q.; Jamal, R.; Zhang, L.; Wang, M.; Abdiryim, T. The structure and properties of PEDOT synthesized by template-free solution method. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Deshpande, S.D.; Yun, S.; Li, Q. A Comparative Study of Conductive Polypyrrole and Polyaniline Coatings on Electro-Active Papers. Polym. J. 2006, 38, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Moschou, E.A.; Peteu, S.F.; Bachas, L.G.; Madou, M.J.; Daunert, S. Artificial muscle material with fast electroactuation under neutral pH conditions. Chem. Mater. 2004, 16, 2499–2502. [Google Scholar] [CrossRef]
- Stoyanov, H.; Kollosche, M.; Risse, S.; Waché, R.; Kofod, G. Soft conductive elastomer materials for stretchable electronics and voltage controlled artificial muscles. Adv. Mater. 2013, 25, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Puiggalí-Jou, A.; Micheletti, P.; Estrany, F.; del Valle, L.J.; Alemán, C. Electrostimulated Release of Neutral Drugs from Polythiophene Nanoparticles: Smart Regulation of Drug–Polymer Interactions. Adv. Healthc. Mater. 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Lavan, D.A.; Burdick, J.A.; Chen, C.Y.; Liang, E.; Langer, R. Electrically controlled drug delivery from biotin-doped conductive polypyrrole. Adv. Mater. 2006, 18, 577–581. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Lee, S.; Lee, S.B. Conductive polymer nanotube patch for fast and controlled ex vivo transdermal drug delivery. Nanomedicine 2014, 9, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Tarabella, G.; Balducci, A.G.; Coppedè, N.; Marasso, S.; D’Angelo, P.; Barbieri, S.; Cocuzza, M.; Colombo, P.; Sonvico, F.; Mosca, R.; Iannotta, S. Liposome sensing and monitoring by organic electrochemical transistors integrated in microfluidics. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4374–4380. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, G.; Casanovas, J.; Redondo, E.; Armelin, E.; Alemán, C. A rational design for the selective detection of dopamine using conducting polymers. Phys. Chem. Chem. Phys. 2014, 16, 7850–7861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, B.G.; Bendrea, A.D.; Cianga, L.; Armelin, E.; Del Valle, L.J.; Cianga, I.; Alemán, C. The biocompatible polythiophene-g-polycaprolactone copolymer as an efficient dopamine sensor platform. Polym. Chem. 2017, 8, 6112–6122. [Google Scholar] [CrossRef]
- Molina, B.G.; Cianga, L.; Bendrea, A.D.; Cianga, I.; Del Valle, L.J.; Estrany, F.; Alemán, C.; Armelin, E. Amphiphilic polypyrrole-poly(Schiff base) copolymers with poly(ethylene glycol) side chains: Synthesis, properties and applications. Polym. Chem. 2018, 9, 4218–4232. [Google Scholar] [CrossRef]
- Bendrea, A.D.; Fabregat, G.; Cianga, L.; Estrany, F.; Del Valle, L.J.; Cianga, I.; Alemán, C. Hybrid materials consisting of an all-conjugated polythiophene backbone and grafted hydrophilic poly(ethylene glycol) chains. Polym. Chem. 2013, 4, 2709–2723. [Google Scholar] [CrossRef]
- Acharya, B.R.; Choudhury, D.; Das, A.; Chakrabarti, G. Vitamin K3 disrupts the microtubule networks by binding to tubulin: A novel mechanism of its antiproliferative activity. Biochemistry 2009, 48, 6963–6974. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Fritz-Wolf, K.; Winzer, A.; Kühner, S.; Little, S.; Yardley, V.; Vezin, H.; Palfey, B.; Schirmer, R.; Davioud-Charvet, E. A fluoro analogue of the menadione derivative 6-[2′-(3′-methyl)-1′,4′-naphthoquinolyl]hexanoic acid is a suicide substrate of glutathione reductase. crystal structure of the alkylated human enzyme. J. Am. Chem. Soc. 2006, 128, 10784–10794. [Google Scholar] [CrossRef] [PubMed]
- Sirisha, V.L.; Sinha, M.; S’Souza, J.S. Menadione-induced caspase-dependent programmed cell death in the green chlorophyte Chlamydomonas reinhardtii. J. Physiol. 2014, 50, 587–601. [Google Scholar] [CrossRef]
- Zampol, M.A.; Barros, M.H. Melatonin improves survival and respiratory activity of yeast cells challenged by alpha-synuclein and menadione. Yeast 2018, 35, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Choi, S.J.; Moon, J.Y.; Kim, H.S. alpha-Syntrophin is involved in the survival signaling pathway in myoblasts under menadione-induced oxidative stress. Exp. Cell Res. 2016, 344, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.V.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel menadione hybrids: Synthesis, anticancer activity, and cell-based studies. Chem. Biol. Drug Des. 2018, 91, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Han, M.G.; Kim, S.Y.; Oh, S.G.; Im, S.S. Poly(3,4-ethylenedioxythiophene) nanoparticles prepared in aqueous DBSA solutions. Synth. Met. 2004, 141, 293–299. [Google Scholar] [CrossRef]
- Ha, Y.H.; Nikolov, N.; Pollack, S.K.; Mastrangelo, J.; Martin, B.D.; Shashidhar, R. Towards a transparent, highly conductive poly (3,4-ethylenedioxythiophene). Adv. Funct. Mater. 2004, 14, 615–622. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Cichy, M.; Ruszkowski, P.; Turczyn, R.; Jarosz, T.; Zak, J.K.; Lapkowski, M.; Bednarczyk-Cwynar, B. Betulin-loaded PEDOT films for regional chemotherapy. Mater. Sci. Eng. C 2017, 73, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Saborío, M.C.G.; Lanzalaco, S.; Fabregat, G.; Puiggalí, J.; Estrany, F.; Alemán, C. Flexible Electrodes for Supercapacitors Based on the Supramolecular Assembly of Biohydrogel and Conducting Polymer. J. Phys. Chem. C 2018, 122, 1078–1090. [Google Scholar] [CrossRef]
- Mac, M.; Wirz, J. Salt effects on the reactions of radical ion pairs formed by electron transfer quenching of triplet 2-methyl-1,4-naphthoquinone by amines. Optical flash photolysis and step-scan FTIR investigations. Photochem. Photobiol. Sci. 2002, 1, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Khan, Z.H. Ab initio and semiempirical study of structure and electronic spectra of hydroxy substituted naphthoquinones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Zielenkiewicz, W.; Terekhova, I.V.; Kozbial, M.; Poznanski, J.; Kumeev, R.S. Inclusion of menadione with cyclodextrins studied by calorimetry and spectroscopic methods. J. Phys. Org. Chem. 2007, 20, 656–661. [Google Scholar] [CrossRef]
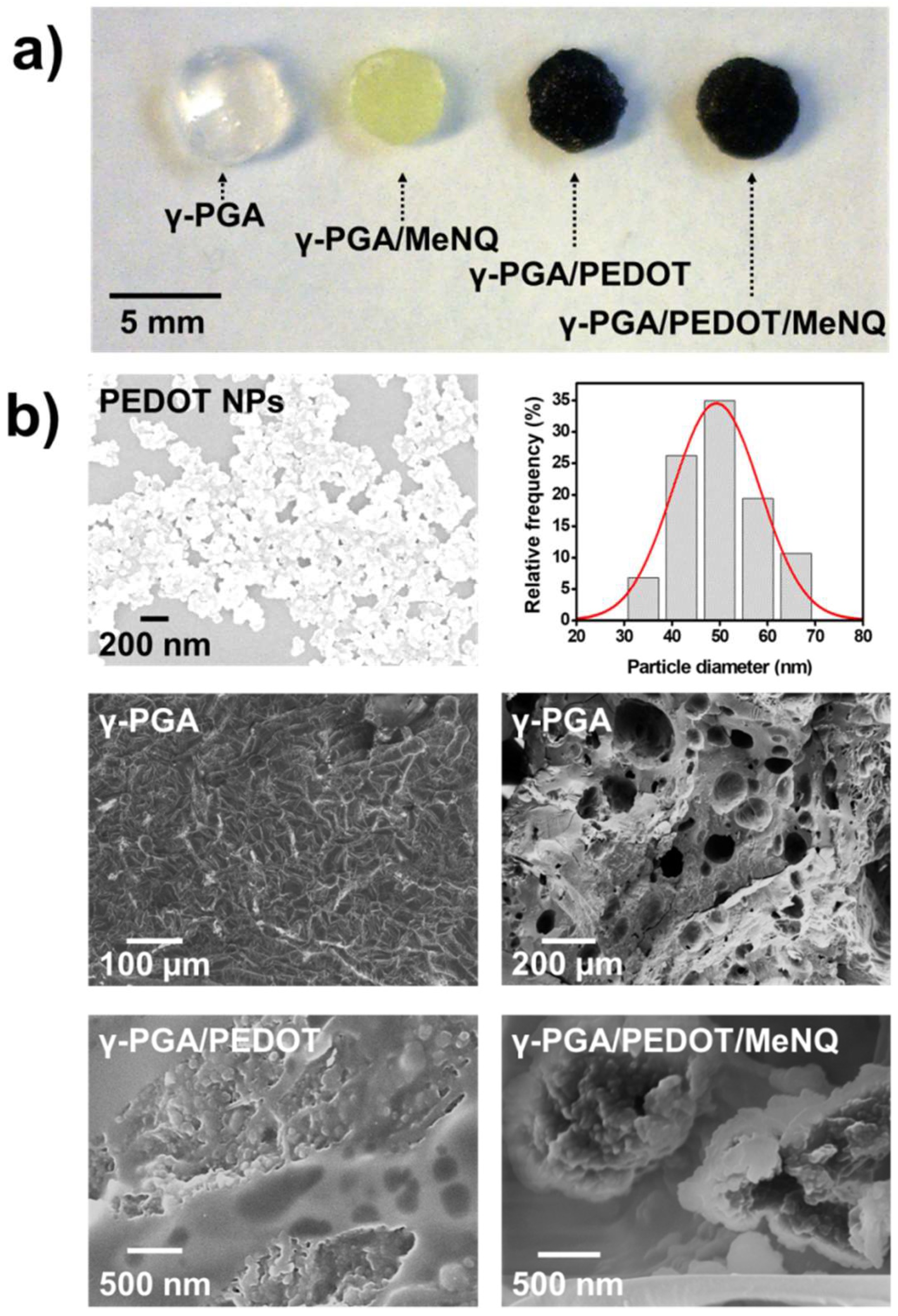
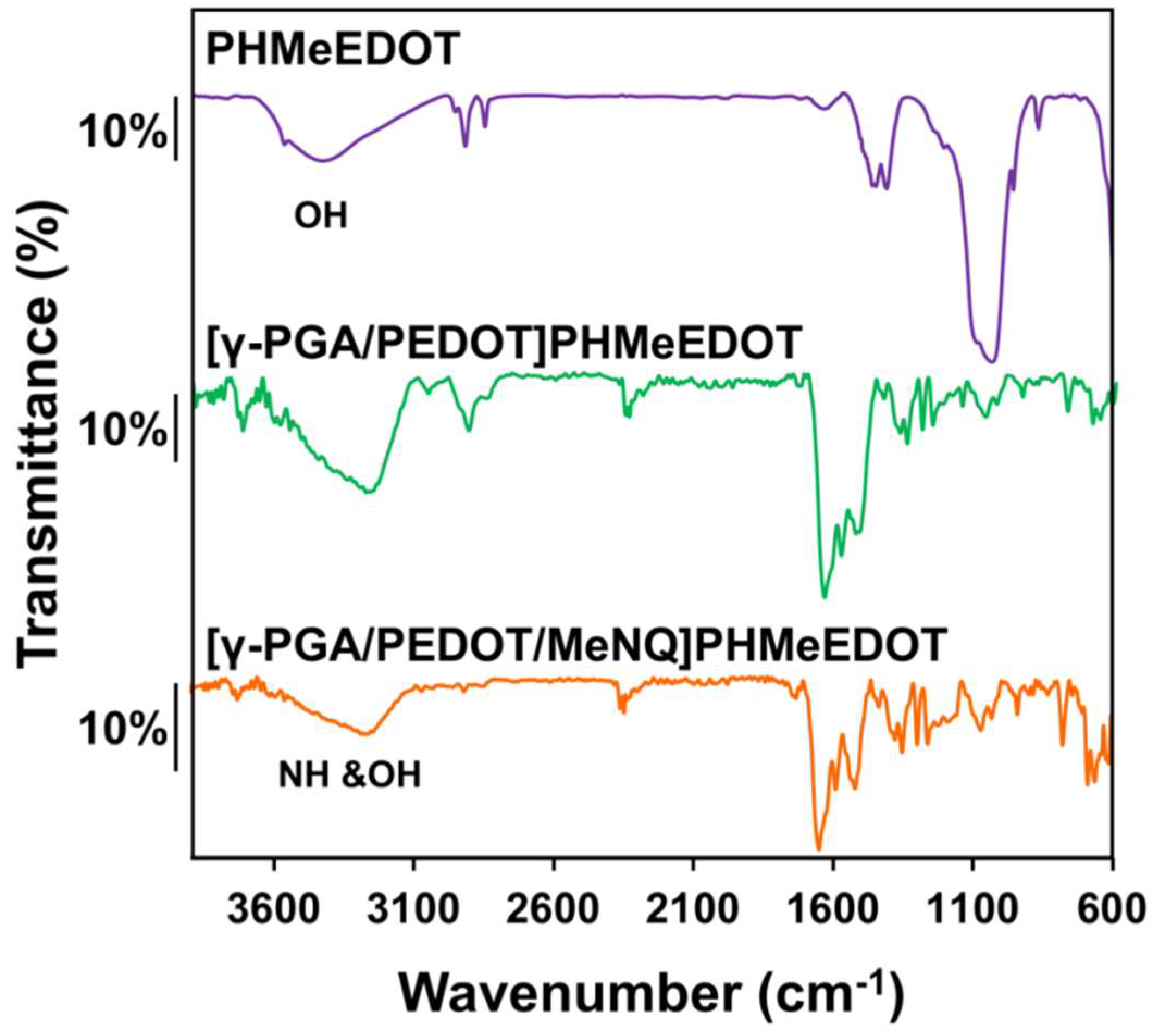
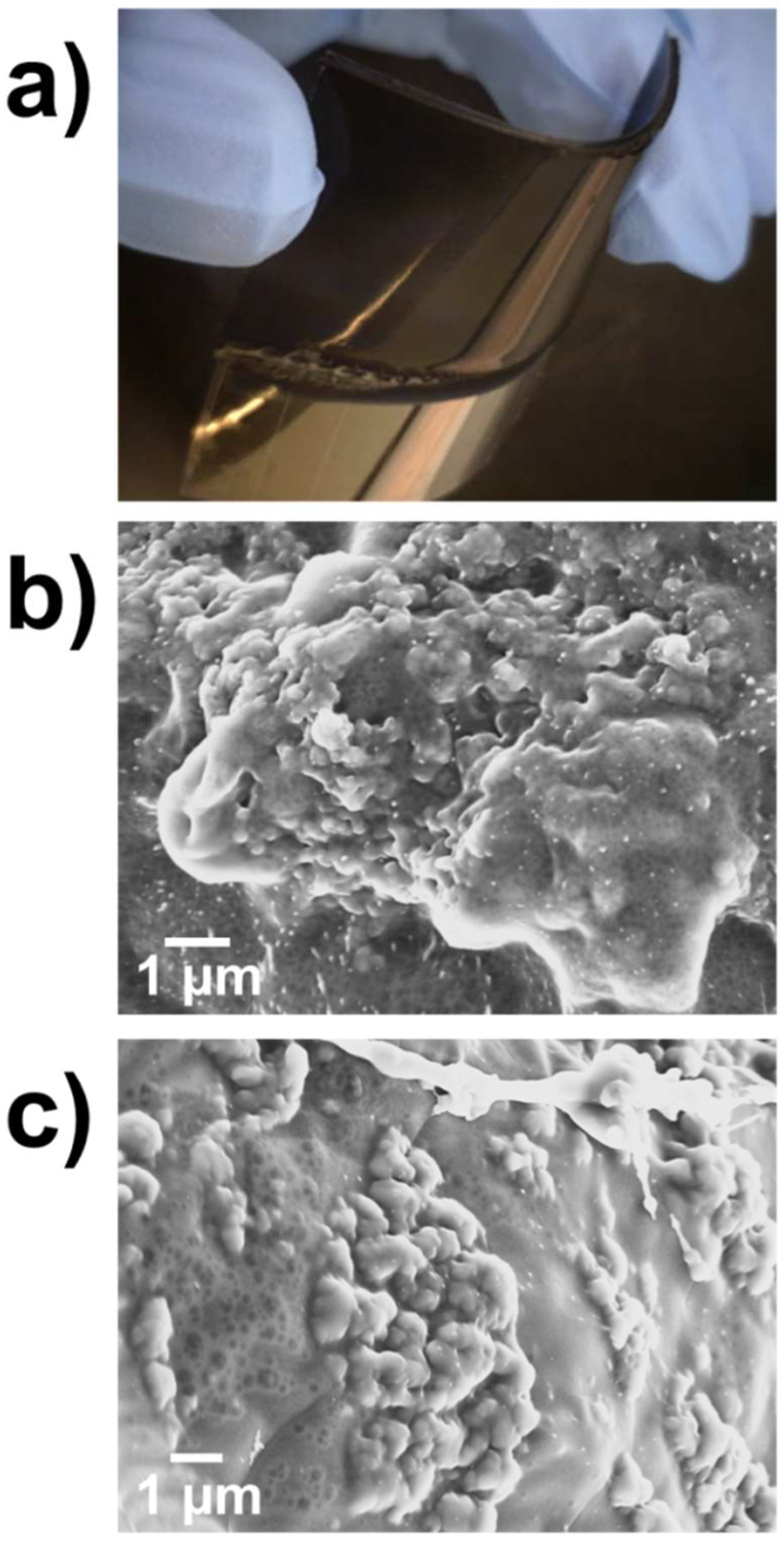
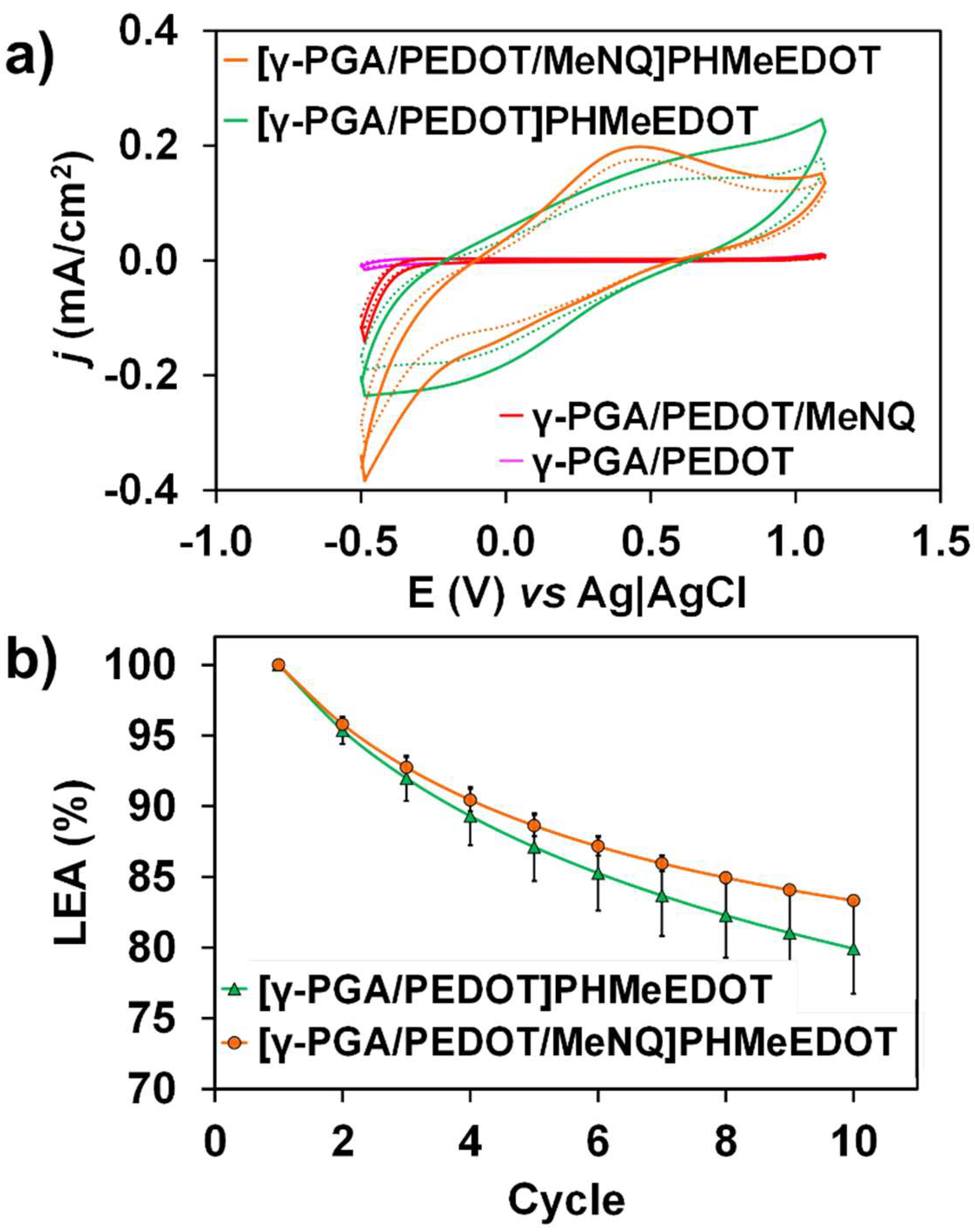
| Sample | SR (%) |
|---|---|
| γ-PGA | 408 ± 170 |
| γ-PGA/PEDOT | 488 ± 254 |
| [γ-PGA/PEDOT]PHMeEDOT | 922 ± 92 |
| γ-PGA/MeNQ | 624 ± 81 |
| γ-PGA/PEDOT/MeNQ | 932 ± 375 |
| [γ-PGA/PEDOTMeNQ]PHMeEDOT | 1711 ± 82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, B.G.; Domínguez, E.; Armelin, E.; Alemán, C. Assembly of Conducting Polymer and Biohydrogel for the Release and Real-Time Monitoring of Vitamin K3. Gels 2018, 4, 86. https://doi.org/10.3390/gels4040086
Molina BG, Domínguez E, Armelin E, Alemán C. Assembly of Conducting Polymer and Biohydrogel for the Release and Real-Time Monitoring of Vitamin K3. Gels. 2018; 4(4):86. https://doi.org/10.3390/gels4040086
Chicago/Turabian StyleMolina, Brenda G., Eva Domínguez, Elaine Armelin, and Carlos Alemán. 2018. "Assembly of Conducting Polymer and Biohydrogel for the Release and Real-Time Monitoring of Vitamin K3" Gels 4, no. 4: 86. https://doi.org/10.3390/gels4040086
APA StyleMolina, B. G., Domínguez, E., Armelin, E., & Alemán, C. (2018). Assembly of Conducting Polymer and Biohydrogel for the Release and Real-Time Monitoring of Vitamin K3. Gels, 4(4), 86. https://doi.org/10.3390/gels4040086