Curdlan-Induced Significant Enhancement of Lipid Oxidation Control and Gelling Properties of Low-Salt Marine Surimi Gel Containing Transglutaminase and Lysine
Abstract
1. Introduction
2. Results and Discussion
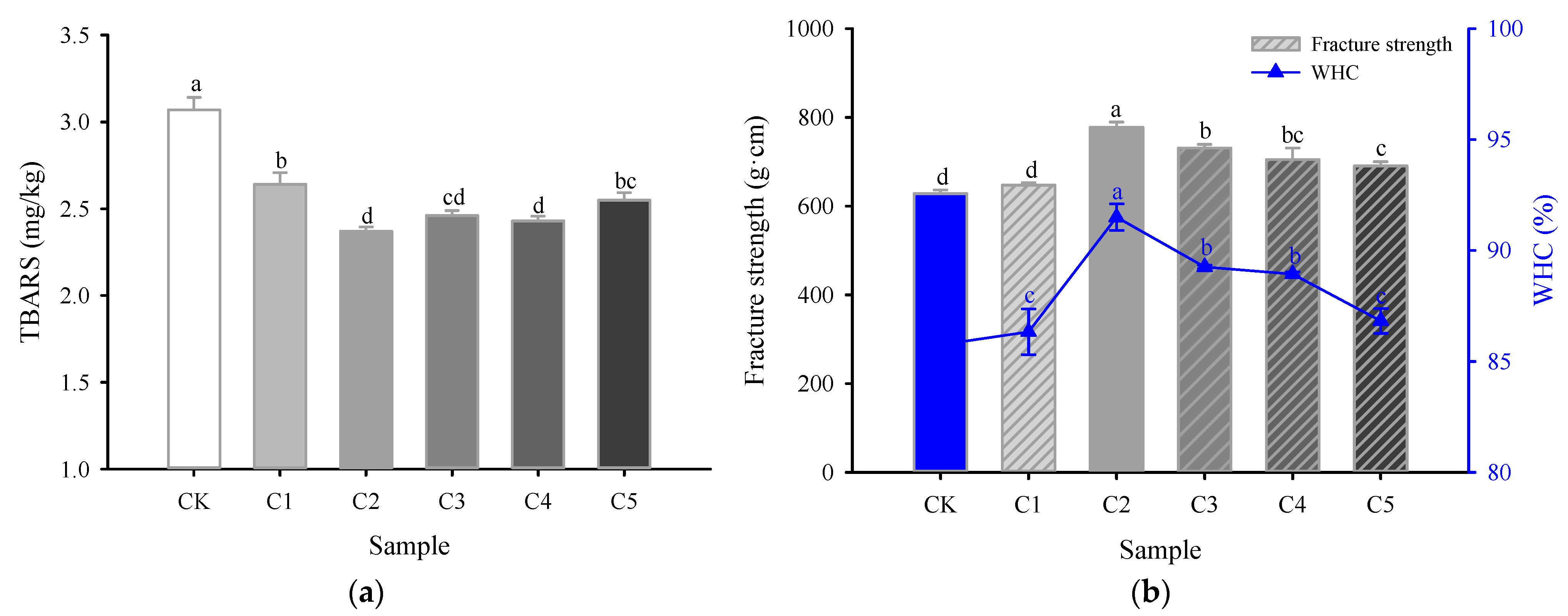
2.1. Lipid Oxidation
2.2. Fracture Strength and Water-Holding Capacity
2.3. TPA
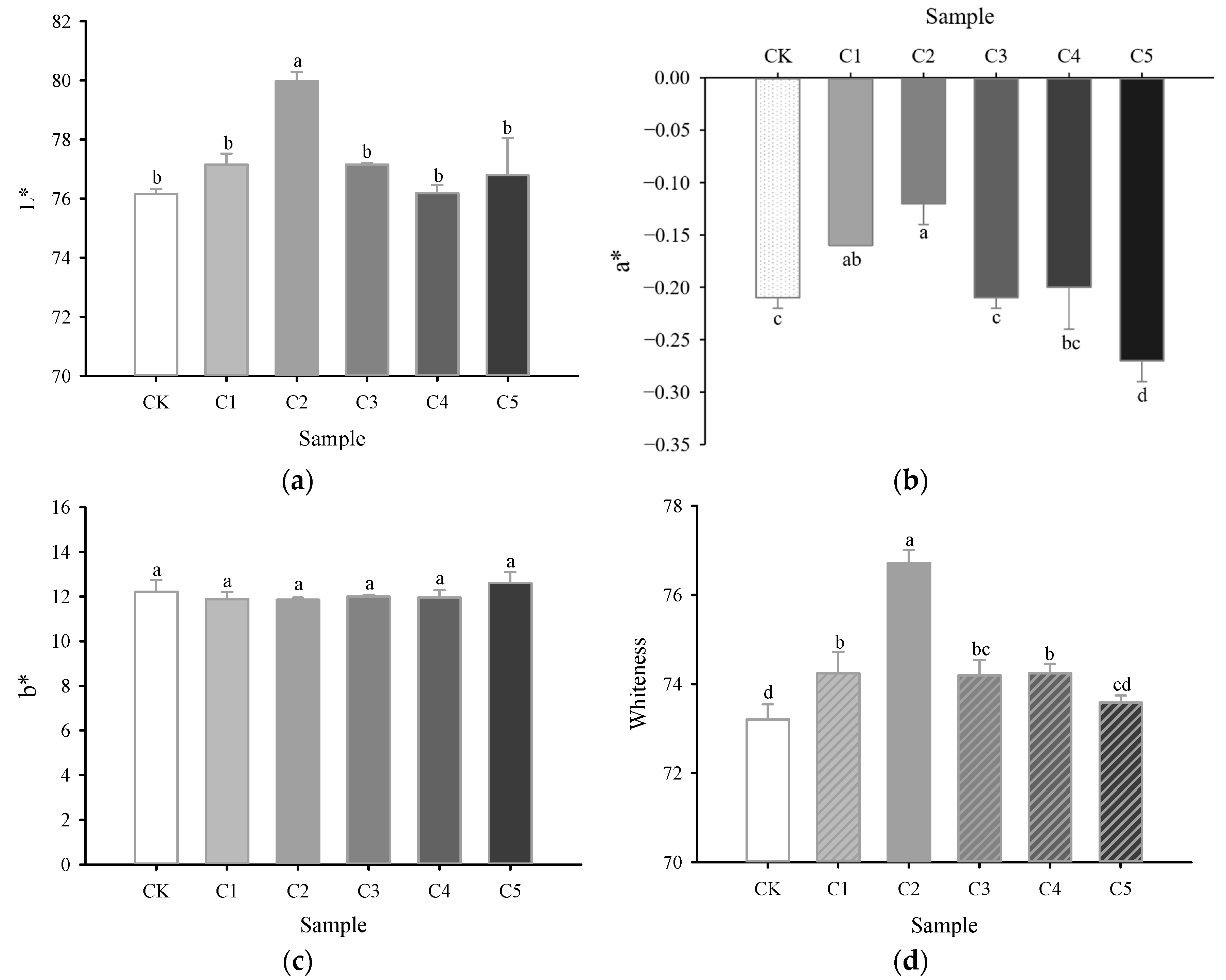
2.4. Color
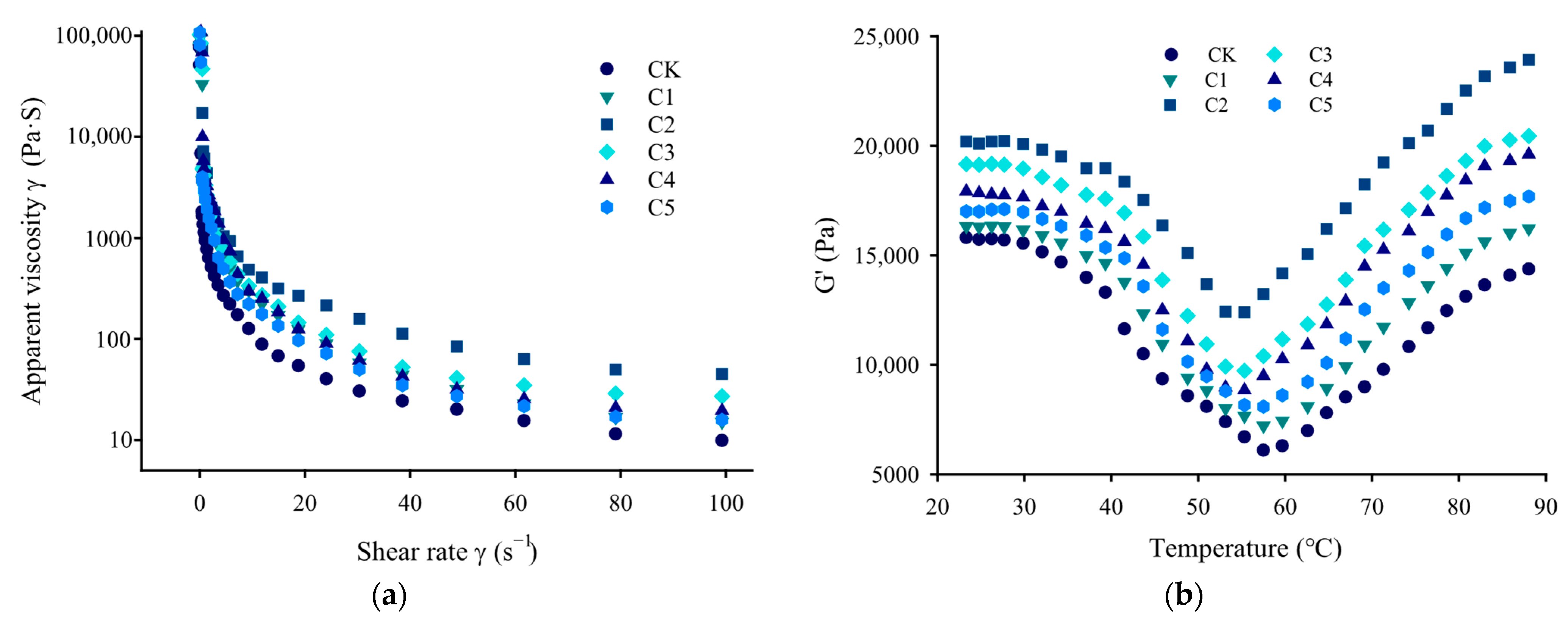
2.5. Rheological Properties
2.5.1. Steady-State Shear Properties
2.5.2. Dynamic Elastic Properties During Gelation
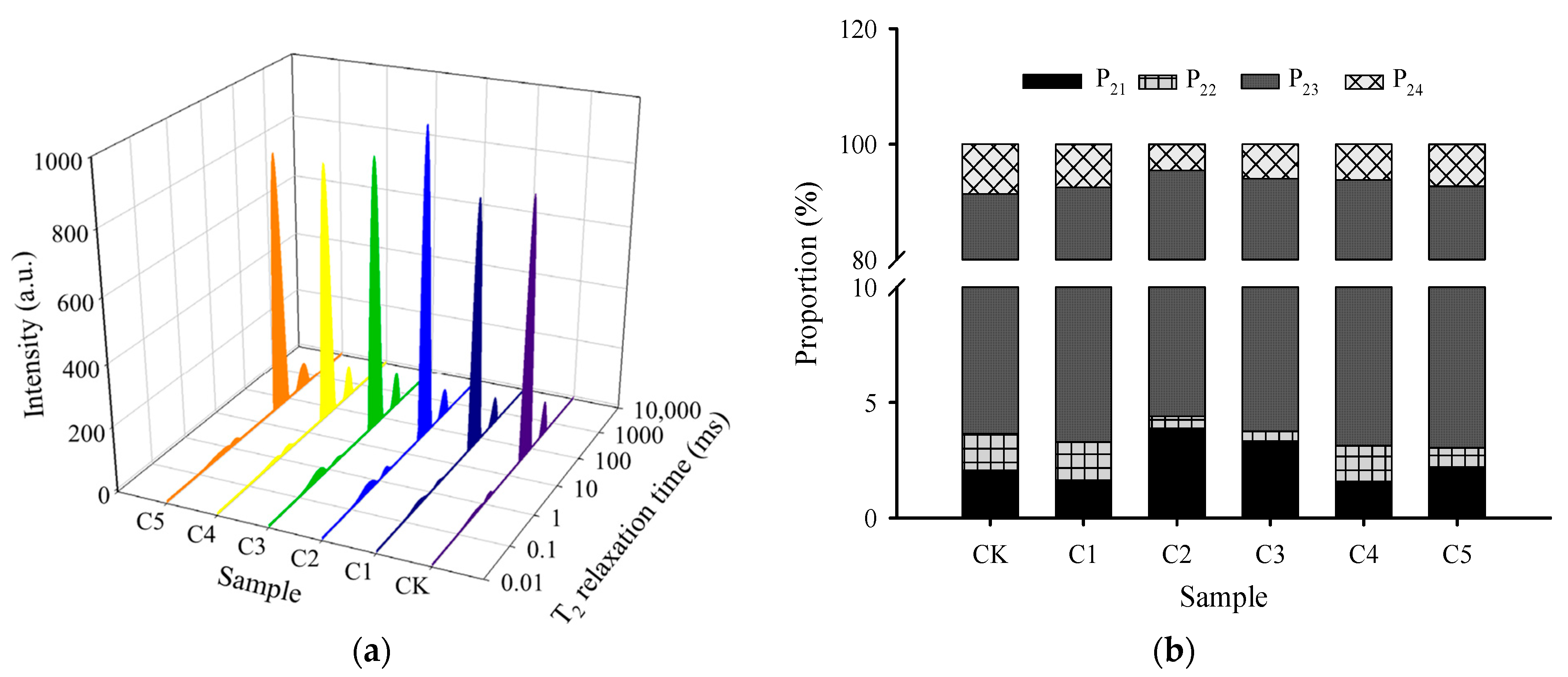
2.6. LF–NMR Relaxation
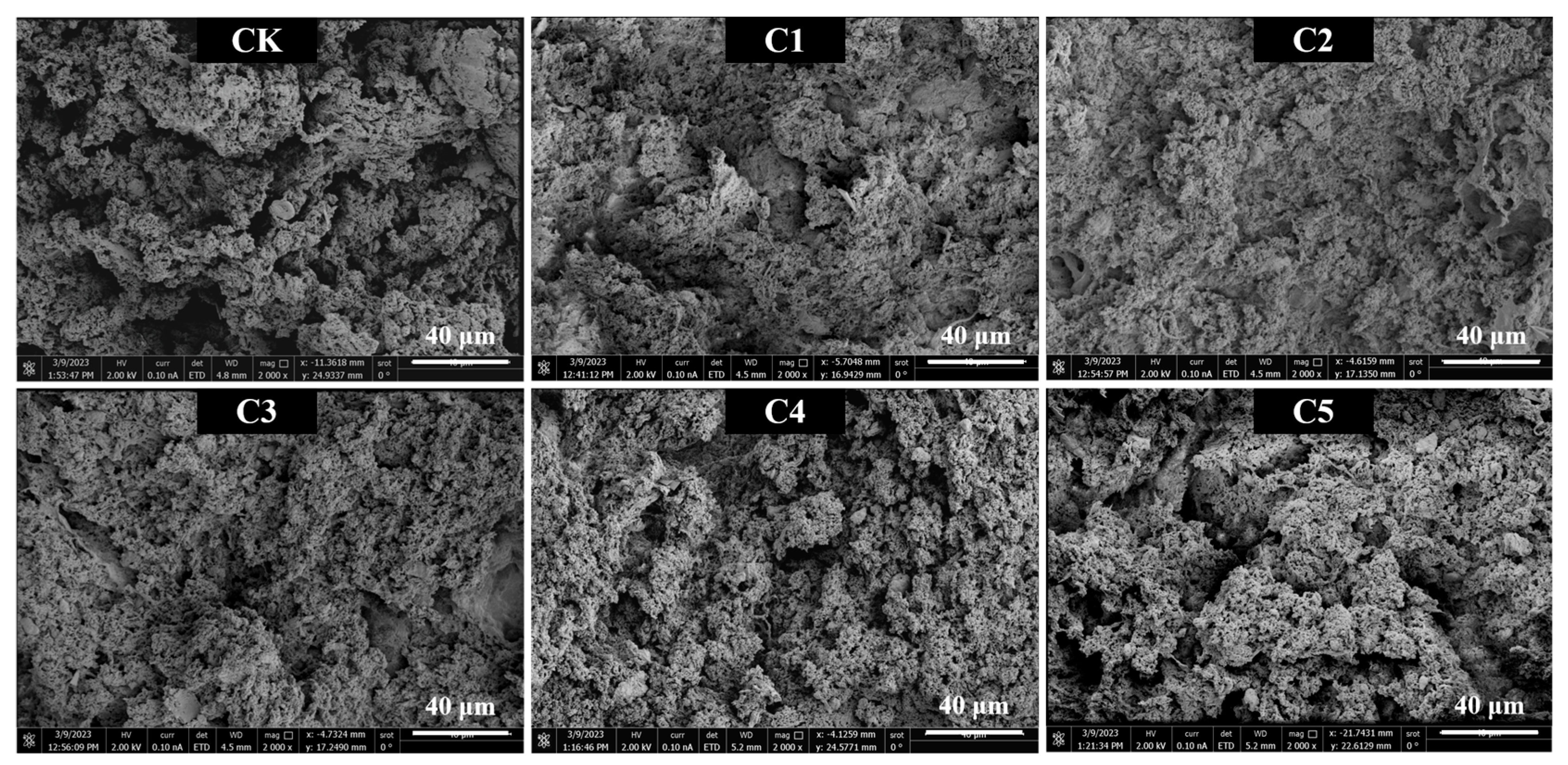
2.7. Microstructure
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Mixed Surimi Samples
4.3. Evaluation of Lipid Oxidation
4.4. Determination of Fracture Strength
4.5. Determination of Water-Holding Capacity (WHC)
4.6. Texture Profile Analysis (TPA)
4.7. Color
4.8. Rheological Properties
4.9. Low-Field Nuclear Magnetic Resonance (LF–NMR)
4.10. Scanning Electron Microscopy (SEM)
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Xiong, J.; Li, P.; Zhang, Y.; Li, F.; Yin, T.; Huang, Q. Dual enhancement effects of different yeast extract on gel properties and saltiness perception of low-salt surimi gel from silver carp. Food Hydrocoll. 2024, 152, 109925. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhuang, D.; Ye, J.R.; Guo, Y.J.; Zhu, J. Modulation of sodium distribution and digestion properties in low salt myofibrillar protein gel incorporating γ-polyglutamic acid and transglutaminase: Conformation and microstructure. Food Hydrocoll. 2024, 153, 110007. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Wang, J.; Wang, Y.; Jin, G.; Teng, W.; Geng, F.; Cao, J. Myofibrillar protein denaturation/oxidation in freezing-thawing impair the heat-induced gelation: Mechanisms and control technologies. Trends Food Sci. Technol. 2023, 138, 655–670. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Zheng, B.; Li, J.W.; Cheong, K.L.; Li, R.; Chen, J.P.; Liu, X.F.; Jia, X.J.; Song, B.B.; Wang, Z.; et al. Emulsion-filled surimi gel: A promising approach for enhancing gel properties, water holding capacity, and flavor. Trends Food Sci. Technol. 2024, 152, 104663. [Google Scholar] [CrossRef]
- Mi, H.B.; Liang, S.Y.; Chen, J.X.; Li, X.P.; Li, J.R. Effect of starch-based emulsion with different amylose content on the gel properties of Nemipterus virgatus surimi. Int. J. Biol. Macromol. 2024, 259, 129183. [Google Scholar] [CrossRef]
- Mente, A.; O'Donnell, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Ah, S.T.L.; Wei, L.; Diaz, R.; et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: A community-level prospective epidemiological cohort study. Lancet 2018, 392, 496–506. [Google Scholar] [CrossRef]
- Lan, H.J.; Chen, L.; Wang, Y.T.; Lu, M.X.; Chen, B.Y.; Ai, C.; Teng, H. Effect of к-carrageenan on saltiness perception and texture characteristic related to salt release in low-salt surimi. Int. J. Biol. Macromol. 2023, 253, 126852. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Lu, M.; Ai, C.; Cao, H.; Xiao, J.; Zhong, S.; Teng, H. Effect of cellulose on gel properties of heat-induced low-salt surimi gels: Physicochemical characteristics, water distribution and microstructure. Food Chem. X 2023, 19, 100820. [Google Scholar] [CrossRef]
- Liang, F.; Lin, L.; He, T.; Zhou, X.; Jiang, S.; Lu, J. Effect of transglutaminase on gel properties of surimi and precocious Chinese mitten crab (Eriocheir sinensis) meat. Food Hydrocoll. 2020, 98, 105261. [Google Scholar] [CrossRef]
- Lu, M.X.; Zhang, C.; Chen, B.Y.; Ai, C.; Chen, L.; Teng, H. Improvement of gelation properties of Penaeus vannamei surimi by magnetic field-assisted freezing in combination with curdlan. Int. J. Biol. Macromol. 2024, 257, 128323. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, Y.; Ye, T.; Jiang, S.; Lin, L.; Lu, J. Effect of ultrasound assisted treatment and microwave combined with water bath heating on gel properties of surimi-crabmeat mixed gels. LWT 2020, 133, 110098. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Zhou, G. Covalent chemical modification of myofibrillar proteins to improve their gelation properties: A systematic review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 924–959. [Google Scholar] [CrossRef]
- Cao, Y.; Li, B.; Fan, X.; Wang, J.; Zhu, Z.; Huang, J.; Xiong, Y. Synergistic recovery and enhancement of gelling properties of oxidatively damaged myofibrillar protein by L-lysine and transglutaminase. Food Chem. 2021, 358, 129860. [Google Scholar] [CrossRef]
- Cando, D.; Borderías, A.J.; Moreno, H.M. Combined effect of amino acids and microbial transglutaminase on gelation of low salt surimi content under high pressure processing. Innov. Food Sci. Emerg. Technol. 2016, 36, 10–17. [Google Scholar] [CrossRef]
- Mirarab Razi, S.; Bagheri, H.; Mohammadian, M.; Mirarab-Razi, V.; Rashidinejad, A. Gelation properties of egg white proteins: A review. Food Rev. Int. 2023, 40, 2751–2774. [Google Scholar] [CrossRef]
- Choi, M.; Choi, H.W.; Jo, M.; Hahn, J.; Choi, Y.J. High-set curdlan emulsion gel fortified by transglutaminase: A promising animal fat substitute with precisely simulated texture and thermal stability of animal fat. Food Hydrocoll. 2024, 154, 110063. [Google Scholar] [CrossRef]
- Hu, W.M.; Xu, X.D.; Wang, X.Y.; Ma, T.H.; Li, Y.T.; Qin, X.L.; Wei, J.B.; Chen, S. Effect of curdlan on the gel properties and interactions of whey protein isolate gels. Int. J. Biol. Macromol. 2024, 277, 134161. [Google Scholar] [CrossRef]
- Xu, Y.N.; Liang, X.; Kong, B.H.; Sun, F.D.; Xia, X.F.; Zhang, H.W.; Liu, Q.; Cao, C.A. Evaluating the effect of thermo-reversible and thermo-irreversible curdlan gels on the gelling properties and in vitro digestibility of myofibrillar protein gels under low-salt condition. Food Res. Int. 2024, 181, 114115. [Google Scholar] [CrossRef]
- Zhu, S.C.; Wang, Y.Y.; Ding, Y.C.; Xiang, X.W.; Yang, Q.; Wei, Z.P.; Song, H.; Liu, S.L.; Zhou, X.X. Improved texture properties and toughening mechanisms of surimi gels by double network strategies. Food Hydrocoll. 2024, 152, 109900. [Google Scholar] [CrossRef]
- Li, Y.; Kong, L.; Zhang, X.; Wen, R.; Peng, X. Protection of whey polypeptide on the lipid oxidation, color, and textural stability of frozen–thawed spanish mackerel surimi. Foods 2023, 12, 4464. [Google Scholar] [CrossRef]
- Huang, J.; Yin, T.; Xiong, S.; Huang, Q. Effect of refrigeration and reheating on the lipid oxidation and volatile compounds in silver carp surimi gels. J. Sci. Food Agric. 2025, 105, 1147–1158. [Google Scholar] [CrossRef]
- Cui, J.; Cao, J.; Zeng, S.; Ge, J.; Li, P.; Li, C. Comprehensive evaluation of lipidomics profiles in golden threadfin bream (Nemipterus virgatus) and its by-products using UHPLC-Q-exactive Orbitrap-MS. LWT 2022, 165, 113690. [Google Scholar] [CrossRef]
- Yin, T.; Park, J.W. Comprehensive review: By-products from surimi production and better utilization. Food Sci. Biotechnol. 2023, 32, 1957–1980. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Liu, W.J.; Yuan, C.H.; Morioka, K.; Chen, S.; Liu, D.; Ye, X. Enhancement of the gelation properties of hairtail (Trichiurus haumela) muscle protein with curdlan and transglutaminase. Food Chem. 2015, 176, 115–122. [Google Scholar] [CrossRef]
- Zhang, M.; Chai, Y.; Li, F.; Bao, Y. Effect of pleurotus eryngii on the characteristics of pork patties during freezing and thawing cycles. Foods 2024, 13, 501. [Google Scholar] [CrossRef]
- Petcharat, T.; Benjakul, S. Effect of gellan incorporation on gel properties of bigeye snapper surimi. Food Hydrocoll. 2018, 77, 746–753. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, G.; Zhang, W. Effects of regenerated cellulose fiber on the characteristics of myofibrillar protein gels. Carbohyd. Polym. 2019, 209, 276–281. [Google Scholar] [CrossRef]
- Liang, Y.; Qu, Z.T.; Liu, M.; Zhu, M.F.; Zhang, X.; Wang, L.; Jia, F.; Zhan, X.B.; Wang, J.S. Further interpretation of the strengthening effect of curdlan on frozen cooked noodles quality during frozen storage: Studies on water state and properties. Food Chem. 2021, 346, 128908. [Google Scholar] [CrossRef]
- Mi, H.B.; Li, Y.; Wang, C.; Yi, S.M.; Li, X.P.; Li, J.R. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, L.F.; Ren, Z.Y.; Hao, G.X.; Chen, J.; Weng, W. Effects of emulsified lard and TGase on gel properties of threadfin bream (Nemipterus virgatus) surimi. LWT 2021, 146, 111513. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Fu, R.J.; Li, J. Effects of the β-glucan, curdlan, on the fermentation performance, microstructure, rheological and textural properties of set yogurt. LWT 2020, 128, 109449. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Teng, H. Phase behavior of the gelation process of myofibrillar protein-curdlan blended system: Discussion based on rheology and gel properties. Food Chem. 2024, 437, 137839. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Liang, X.; Zhang, J.M.; Kong, B.H.; Shi, P.R.; Cao, C.N.; Zhang, H.W.; Liu, Q.; Zhang, Y.M. Effects of transglutaminase coupled with κ-carrageenan on the rheological behaviours, gel properties and microstructures of meat batters. Food Hydrocoll. 2024, 146, 109265. [Google Scholar] [CrossRef]
- Li, Q.; Yi, S.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Different thermal treatment methods and TGase addition affect gel quality and flavour characteristics of Decapterus maruadsi surimi products. Foods 2022, 11, 66. [Google Scholar] [CrossRef]
- Li, C.L.; Peng, A.; He, L.C.; Ma, S.; Wu, W.; Yang, H.; Sun, X.; Zeng, Q.; Jin, G.; Zhang, J.; et al. Emulsifying properties development of pork myofibrillar and sacroplasmic protein irradiated at different dose: A combined FT-IR spectroscopy and low-field NMR study. Food Chem. 2018, 252, 108–114. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, C.; Xu, X.L.; Zeng, X.M.; Xing, T. Ultrasound combined with carrageenan and curdlan addition improved the gelation properties of low-salt chicken meat paste. LWT 2022, 172, 114230. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G. The effects of three polysaccharides on the gelation properties of myofibrillar protein: Phase behaviour and moisture stability. Meat Sci. 2020, 170, 108228. [Google Scholar] [CrossRef]
- Huang, C.Y.; Blecker, C.; Wei, X.R.; Xie, X.R.; Li, S.B.; Chen, L.; Zhang, D.Q. Effects of different plant polysaccharides as fat substitutes on the gel properties, microstructure and digestion characteristics of myofibrillar protein. Food Hydrocoll. 2024, 150, 109717. [Google Scholar] [CrossRef]
- Luo, X.Y.; Huang, K.; Niu, Y.X.; Zhang, X.; An, Y.Q.; Liu, R.; Xiong, S.B.; Hu, Y. Effects of freezing methods on physicochemical properties, protein/fat oxidation and odor characteristics of surimi gels with different cross-linking degrees. Food Chem. 2024, 432, 137268. [Google Scholar] [CrossRef]
- Ma, W.H.; Yuan, F.; Feng, L.; Wang, J.K.; Sun, Y.J.; Cao, Y.G.; Huang, J.R. ε-Polylysine-mediated enhancement of the structural stability and gelling properties of myofibrillar protein under oxidative stress. Int. J. Biol. Macromol. 2022, 220, 1114–1123. [Google Scholar] [CrossRef]
- He, Y.J.; Chen, L.; Wang, H.; Zhang, C.; Ma, L.Y.; Teng, H. Effect of insoluble dietary fiber on physicochemical and digestive properties of tilapia surimi gel: Interactions, performance and mechanisms. Food Hydrocoll. 2025, 167, 111410. [Google Scholar] [CrossRef]
| Sample | Hardness (g) | Springiness | Cohesiveness | Chewiness (g) | Resilience |
|---|---|---|---|---|---|
| CK | 2186.28 ± 166.61 e | 0.86 ± 0.01 ab | 0.72 ± 0.01 a | 1382.40 ± 51.96 e | 0.40 ± 0.01 ab |
| C1 | 2280.34 ± 97.16 d | 0.86 ± 0.02 ab | 0.72 ± 0.00 a | 1436.89 ± 63.61 de | 0.39 ± 0.00 bc |
| C2 | 2903.45 ± 91.94 a | 0.88 ± 0.01 a | 0.72 ± 0.01 a | 1781.44 ± 43.94 a | 0.41 ± 0.01 a |
| C3 | 2672.97 ± 126.53 b | 0.87 ± 0.03 ab | 0.73 ± 0.01 a | 1677.84 ± 70.76 b | 0.40 ± 0.01 ab |
| C4 | 2681.43 ± 85.23 b | 0.86 ± 0.01 ab | 0.71 ± 0.03 ab | 1568.83 ± 72.02 bc | 0.38 ± 0.00 cd |
| C5 | 2396.71 ± 73.01 c | 0.85 ± 0.04 b | 0.69 ± 0.02 b | 1510.73 ± 86.14 cd | 0.37 ± 0.00 d |
| Sample | T21 (ms) | T22 (ms) | T23 (ms) | T24 (ms) |
|---|---|---|---|---|
| CK | 0.425 ± 0.077 ab | 1.956 ± 0.335 a | 47.686 ± 1.361 a | 310.787 ± 7.953 c |
| C1 | 0.370 ± 0.106 b | 1.825 ± 0.104 ab | 44.488 ± 2.183 b | 357.079 ± 11.582 a |
| C2 | 0.322 ± 0.041 b | 1.482 ± 0.144 b | 44.488 ± 2.206 b | 357.079 ± 9.367 a |
| C3 | 0.425 ± 0.113 ab | 1.825 ± 0.180 ab | 47.686 ± 1.859 a | 357.079 ± 8.116 a |
| C4 | 0.370 ± 0.083 b | 1.589 ± 0.226 ab | 44.488 ± 2.667 b | 357.079 ± 5.219 a |
| C5 | 0.561 ± 0.094 a | 1.659 ± 0.083 ab | 44.488 ± 1.407 b | 333.129 ± 13.823 b |
| Sample | Surimi (g) | TGase (w/w, %) | Egg-White Protein (w/w, %) | Curdlan (w/w, %) | Lys (w/w, %) | NaCl (w/w, %) | Cold Water (%) |
|---|---|---|---|---|---|---|---|
| CK | 300 | 0.4 | 7.0 | – | 0.2 | 0.5 | 3 |
| C1 | 300 | 0.4 | 0 | 0.2 | 0.2 | 0.5 | 3 |
| C2 | 300 | 0.4 | 0 | 0.4 | 0.2 | 0.5 | 3 |
| C3 | 300 | 0.4 | 0 | 0.6 | 0.2 | 0.5 | 3 |
| C4 | 300 | 0.4 | 0 | 0.8 | 0.2 | 0.5 | 3 |
| C5 | 300 | 0.4 | 0 | 1.0 | 0.2 | 0.5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Liang, G.; Huang, Q.; Ling, F.; Pan, W.; Cao, Y.; Chen, M. Curdlan-Induced Significant Enhancement of Lipid Oxidation Control and Gelling Properties of Low-Salt Marine Surimi Gel Containing Transglutaminase and Lysine. Gels 2025, 11, 535. https://doi.org/10.3390/gels11070535
Ma W, Liang G, Huang Q, Ling F, Pan W, Cao Y, Chen M. Curdlan-Induced Significant Enhancement of Lipid Oxidation Control and Gelling Properties of Low-Salt Marine Surimi Gel Containing Transglutaminase and Lysine. Gels. 2025; 11(7):535. https://doi.org/10.3390/gels11070535
Chicago/Turabian StyleMa, Wenhui, Guangcan Liang, Qiliang Huang, Feng Ling, Weilin Pan, Yungang Cao, and Miao Chen. 2025. "Curdlan-Induced Significant Enhancement of Lipid Oxidation Control and Gelling Properties of Low-Salt Marine Surimi Gel Containing Transglutaminase and Lysine" Gels 11, no. 7: 535. https://doi.org/10.3390/gels11070535
APA StyleMa, W., Liang, G., Huang, Q., Ling, F., Pan, W., Cao, Y., & Chen, M. (2025). Curdlan-Induced Significant Enhancement of Lipid Oxidation Control and Gelling Properties of Low-Salt Marine Surimi Gel Containing Transglutaminase and Lysine. Gels, 11(7), 535. https://doi.org/10.3390/gels11070535







