Fabrication and Performance Study of 3D-Printed Ceramic-in-Gel Polymer Electrolytes
Abstract
1. Introduction
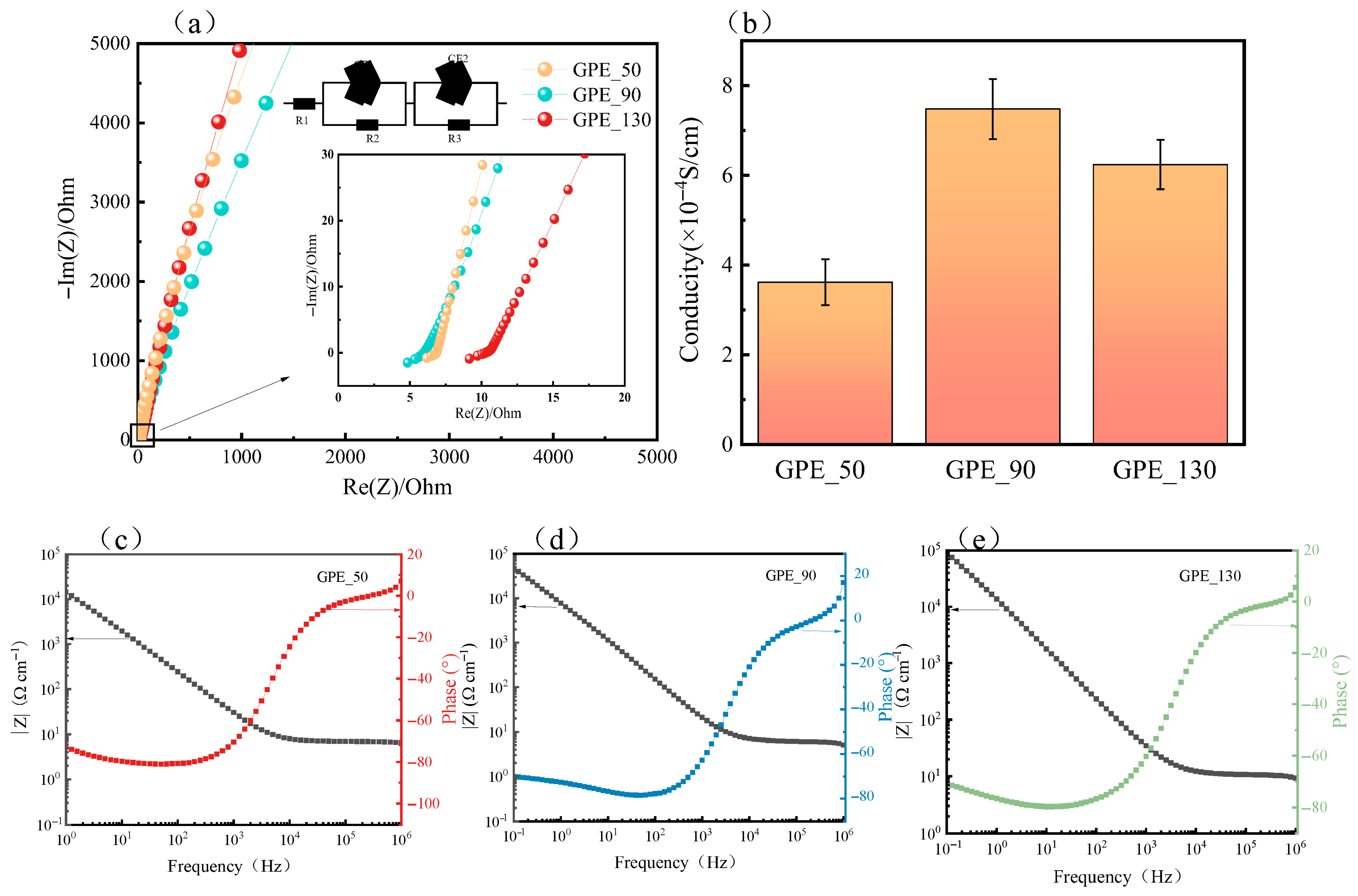
2. Results and Discussion
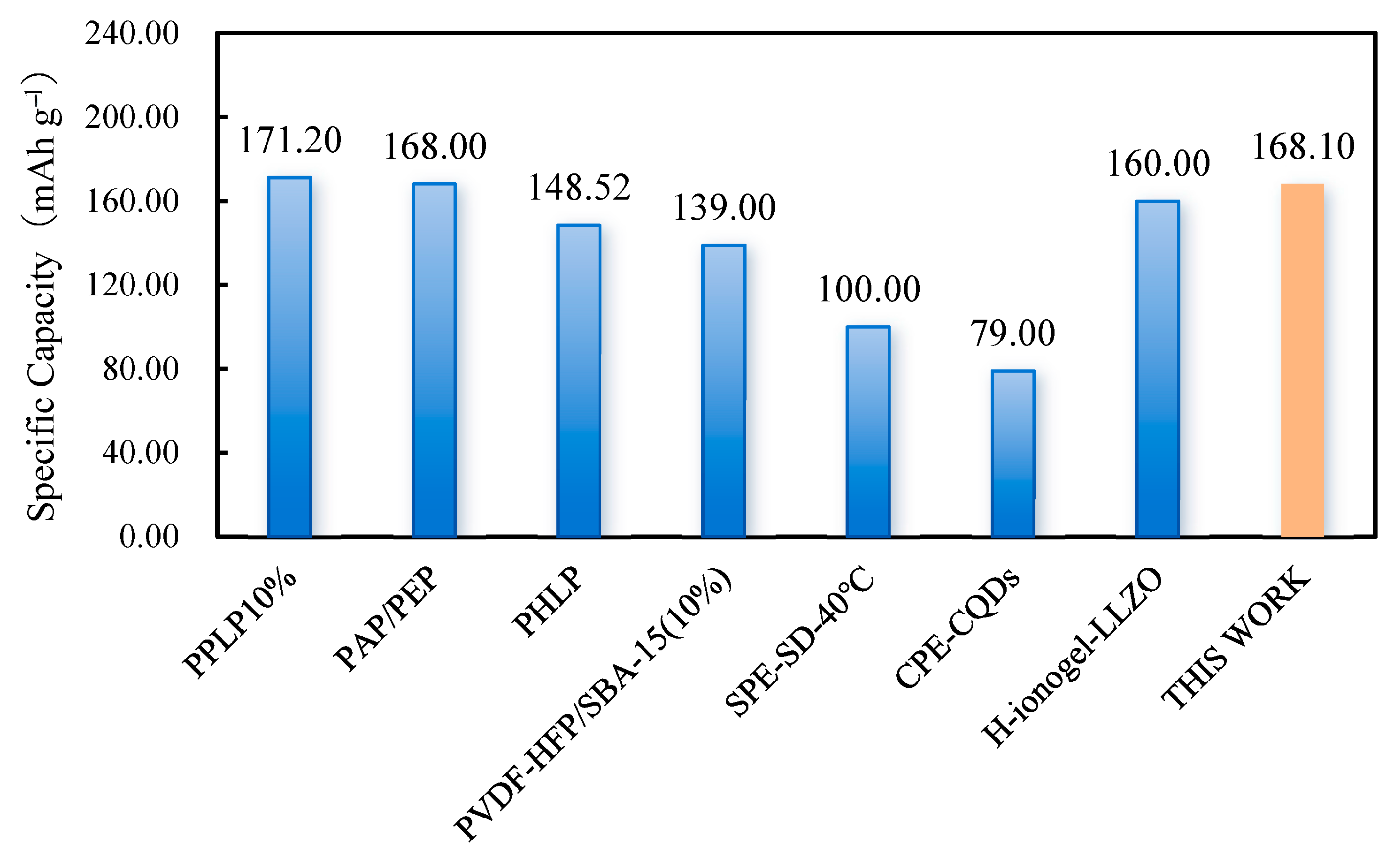
| SSEs | Method | 0.1 C (mAh g−1) | 0.2 C (mAh g−1) | 0.5 C (mAh g−1) | After Cycles (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|
| PPLP10% | Solution casting | 171.20 (25 °C) | \ | 167.20 (25 °C) | 157.30, 97.8% (1 C, 100) | [40] |
| PAP/PEP | Solution casting | 168.00 (25 °C) | 150.00 (25 °C) | 120.40 (25 °C) | 140.00, 93.3% (0.2 C, 100) | [29] |
| PHLP | Solution casting | 148.52 (25 °C) | 141.74 (25 °C) | 118.68 (25 °C) | 123.16, 88.29% (0.33 C, 200) | [41] |
| LLZO/LIC LISE | In situ sintering | 167.20 (60 °C) | 165.50 (60 °C) | \ | 144.20, 89.0% (0.5 C, 200) | [42] |
| PVDF-HFP/SBA-15 (10%) | 3D printing | 139.00 (25 °C) | \ | \ | \ | [43] |
| LiPF6 Gel10 | 3D printing | \ | 145.00 (25 °C) | 144 (25 °C) | 117.00, 96% (1 C, 150) | [44] |
| SPE-SD-40 °C | 3D printing | 100.00 (40 °C) | 40.00 (40 °C) | \ | \ | [45] |
| GPE-CQDs | 3D printing | 79.00 (25 °C) | 78.00 (25 °C) | 74.00 (25 °C) | 74.00, 92% (0.2 C, 200) | [46] |
| H-ionogel-LLZO | 3D printing | 160.00 (50 °C) | 153.00 (50 °C) | 148.00 (50 °C) | 125.00, 88.1% (0.5 C, 200) | [47] |
| This work (GPE_90) | 3D printing | 168.10 (25 °C) | 166.90 (25 °C) | 147.00 (25 °C) | 152.80, 92.8% (0.2 C, 100) | This Work |
3. Conclusions
4. Materials and Methods
4.1. Materials
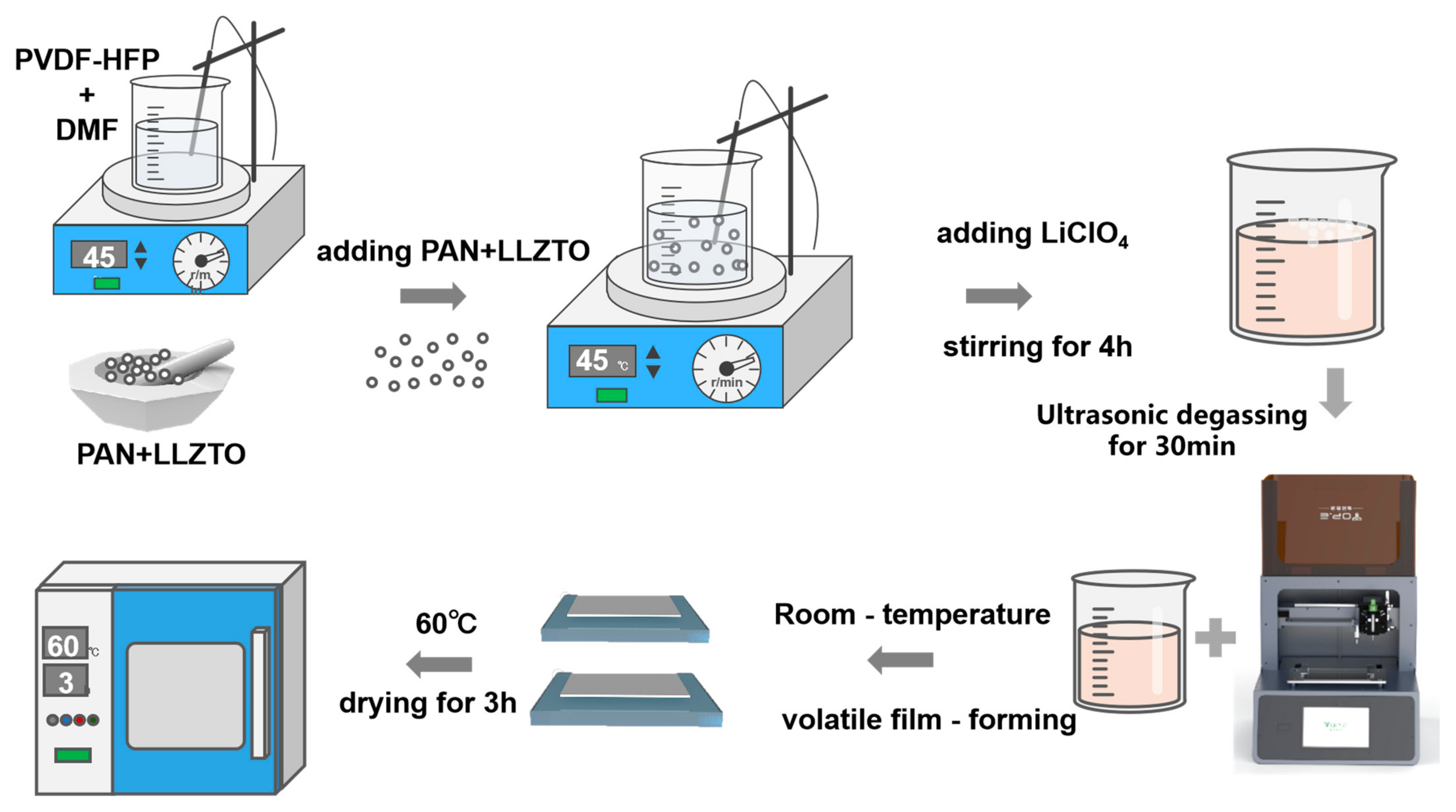
4.2. Preparation of Gel Polymer Electrolytes (GPEs)
4.3. Assembling the Battery
4.4. Material Characterization
4.5. Electrochemical Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Luo, M.; Yang, R.; Hu, X.; Lu, C. Achieve high dielectric and energy-storage density properties by employing cyanoethyl cellulose as fillers in PVDF-based polymer composites. Materials 2023, 16, 4201. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.-H.; Kim, J.-M.; Yoon, C.S.; Sun, Y.-K. High-energy-density, long-life li-metal batteries via application of external pressure. ACS Energy Lett. 2023, 8, 2970–2978. [Google Scholar] [CrossRef]
- Tan, D.H.; Banerjee, A.; Chen, Z.; Meng, Y.S. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; He, M.; Gao, S.; Hou, W.; Ma, Y.; Huo, H.; Du, C.; Yin, G.; Zuo, P. Molecular Regulation and Intermolecular Chemistry in Gel Polymer Electrolytes for High-Voltage Lithium Batteries. Adv. Sci. 2025, 12, 2417169. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, Z.; Xu, J.; Sun, Z.; Wu, X.; Han, J.; Wang, Y.H.; Wang, M.S. Zwitterionic cellulose-based polymer electrolyte enabled by aqueous solution casting for high-performance solid-state batteries. Angew. Chem. 2024, 136, e202400477. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, Y.; Sakai, T.; Quartarone, E.; Mustarelli, P. Conduction Mechanisms of PVDF-Type Gel Polymer Electrolytes of Lithium Prepared by a Phase Inversion Process. J. Phys. Chem. B 2000, 104, 11460–11464. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Wang, J.; Huang, D.; Chen, L.; Yang, G. Hybrid gel polymer electrolyte fabricated by electrospinning technology for polymer lithium-ion battery. Eur. Polym. J. 2015, 67, 365–372. [Google Scholar] [CrossRef]
- Yan, J.; Huang, S.; Von Lim, Y.; Xu, T.; Kong, D.; Li, X.; Yang, H.Y.; Wang, Y. Direct-ink writing 3D printed energy storage devices: From material selectivity, design and optimization strategies to diverse applications. Mater. Today 2022, 54, 110–152. [Google Scholar] [CrossRef]
- Nguyen, H.B.T.; Ding, L.; Pohle, B.; Schmeida, T.; Nguyen, H.B.A.; Mikhailova, D. Ternary PEO/PVDF-HFP-Based Polymer Electrolytes for Li-Ion Batteries. Batteries 2025, 11, 45. [Google Scholar] [CrossRef]
- Halder, B.; Mohamed, M.G.; Kuo, S.-W.; Elumalai, P. Review on composite polymer electrolyte using PVDF-HFP for solid-state lithium-ion battery. Mater. Today Chem. 2024, 36, 101926. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- He, Z.; Rault, F.; Lewandowski, M.; Mohsenzadeh, E.; Salaün, F. Electrospun PVDF nanofibers for piezoelectric applications: A review of the influence of electrospinning parameters on the β phase and crystallinity enhancement. Polymers 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, J.; Sun, H.; Yang, W.; Wei, L.; Zhao, X. Induced Electron Traps via the PCBM in P (VDF-HFP) Composites to Enhance Dielectric and Energy Storage Performance. Polymers 2024, 16, 3030. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, P.; Abhilash, K.; Nalini, B.; Perumal, P.; Selvin, P.C. Free-standing, high Li-ion conducting hybrid PAN/PVdF/LiClO4/Li0.5La0.5TiO3 nanocomposite solid polymer electrolytes for all-solid-state batteries. J. Solid State Electrochem. 2021, 25, 905–917. [Google Scholar] [CrossRef]
- Hu, L.; Gao, X.; Wang, H.; Song, Y.; Zhu, Y.; Tao, Z.; Yuan, B.; Hu, R. Progress of Polymer Electrolytes Worked in Solid-State Lithium Batteries for Wide-Temperature Application. Small 2024, 20, 2312251. [Google Scholar] [CrossRef]
- Farooqui, U.; Ahmad, A.; Hamid, N. Effect of polyaniline (PANI) on Poly (vinylidene fluoride-co-hexaflouro propylene)(PVDF-co-HFP) polymer electrolyte membrane prepared by breath figure method. Polym. Test. 2017, 60, 124–131. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Bao, C.; You, Z.; Lu, Y.; Wen, Z. Interfacial engineering for high-performance garnet-based solid-state lithium batteries. SusMat 2024, 4, 72–105. [Google Scholar] [CrossRef]
- Blake, A.J.; Kohlmeyer, R.R.; Hardin, J.O.; Carmona, E.A.; Maruyama, B.; Berrigan, J.D.; Huang, H.; Durstock, M.F. 3D printable ceramic–polymer electrolytes for flexible high-performance Li-ion batteries with enhanced thermal stability. Adv. Energy Mater. 2017, 7, 1602920. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, Y.; Yao, W.; Yuan, Y.; Deivanayagam, R.; Foroozan, T.; Huang, Z.; Song, B.; Rojaee, R.; Shokuhfar, T. Elevated-temperature 3D printing of hybrid solid-state electrolyte for Li-ion batteries. Adv. Mater. 2018, 30, 1800615. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, X.; Liu, M.; Duan, S.; Ren, Y.; Ma, H.; Tang, K.; Shi, J.; Hou, S.; Jin, H. Direct ink writing of Li1. 3Al0. 3Ti1. 7 (PO4) 3-based solid-state electrolytes with customized shapes and remarkable electrochemical behaviors. Small 2021, 17, 2002866. [Google Scholar] [CrossRef]
- Chen, B.; Willenbacher, N. High-precision direct ink writing of Li6.4La3Zr1.4Ta0.6O12. J. Eur. Ceram. Soc. 2022, 42, 7491–7500. [Google Scholar] [CrossRef]
- Li, C.; Deng, S.; Feng, W.; Cao, Y.; Bai, J.; Dou, Q.; Tian, X.; Dong, Y.; Xia, F. Lithium salt regulation enabling 3D printed solid-state electrolytes to achieve ultra-long cycle performance of LiNi0.8Mn0.1Co0.1O2. Chem. Eng. J. 2024, 494, 152770. [Google Scholar] [CrossRef]
- Singer, C.; Schmalzbauer, S.; Daub, R. Influence of the slurry composition on thin-film components for the wet coating process of sulfide-based all-solid-state batteries. J. Energy Storage 2023, 68, 107703. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Huang, X.; Chen, S.; Wang, G. Honeycomb-like porous gel polymer electrolyte membrane for lithium ion batteries with enhanced safety. Sci. Rep. 2014, 4, 6007. [Google Scholar] [CrossRef]
- Wang, D.; Cai, D.; Zhong, Y.; Jiang, Z.; Zhang, S.; Xia, X.; Wang, X.; Tu, J. A three-dimensional electrospun Li6. 4La3Zr1. 4Ta0. 6O12–poly (vinylidene fluoride-hexafluoropropylene) gel polymer electrolyte for rechargeable solid-state lithium ion batteries. Front. Chem. 2021, 9, 751476. [Google Scholar]
- Park, J.-H.; Kim, M.; Kim, M.-Y.; Jeong, J.; Jung, H.-G.; Yoon, W.Y.; Chung, K.Y. Correlation between the particle size of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte and lithium-ion transport in composite cathodes for all-solid-state lithium-ion batteries. Chem. Eng. J. 2024, 481, 148436. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Han, T.Y.-J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors based on three-dimensional hierarchical graphene aerogels with periodic macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef]
- Beshahwured, S.L.; Wu, Y.-S.; Wu, S.-h.; Chien, W.-C.; Jose, R.; Lue, S.J.; Yang, C.-C. Flexible hybrid solid electrolyte incorporating ligament-shaped Li6. 25Al0. 25La3Zr2O12 filler for all-solid-state lithium-metal batteries. Electrochim. Acta 2021, 366, 137348. [Google Scholar] [CrossRef]
- Hun, Q.; Lan, L.; Lu, X.; Hu, Q.; Liang, X.; Guo, Y.; Wang, Y. Bilayer Heterostructure Electrolytes Were Prepared by a UV-Curing Process for High Temperature Lithium-Ion Batteries. Polymers 2024, 16, 2972. [Google Scholar] [CrossRef]
- Satapathy, S.; Pawar, S.; Gupta, P.; Varma, K. Effect of annealing on phase transition in poly (vinylidene fluoride) films prepared using polar solvent. Bull. Mater. Sci. 2011, 34, 727–733. [Google Scholar] [CrossRef]
- Janakiraman, S.; Surendran, A.; Ghosh, S.; Anandhan, S.; Venimadhav, A. Electroactive poly (vinylidene fluoride) fluoride separator for sodium ion battery with high coulombic efficiency. Solid State Ion. 2016, 292, 130–135. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, K.; An, Y.; Liu, W.; Li, C.; Zheng, S.; Zhang, X.; Wang, L.; Sun, X.; Ma, Y. Rapid ion transport induced by the enhanced interaction in composite polymer electrolyte for all-solid-state lithium-metal batteries. J. Phys. Chem. Lett. 2021, 12, 10603–10609. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, X.; Wang, W.; Wang, Q.; Zhou, X.; Liu, Y.; Guo, B. Dense PVDF-type polymer-in-ceramic electrolytes for solid state lithium batteries. RSC Adv. 2020, 10, 22417–22421. [Google Scholar] [CrossRef] [PubMed]
- Mouraliraman, D.; Shaji, N.; Praveen, S.; Nanthagopal, M.; Ho, C.W.; Varun Karthik, M.; Kim, T.; Lee, C.W. Thermally Stable PVDF-HFP-Based Gel Polymer Electrolytes for High-Performance Lithium-Ion Batteries. Nanomaterials 2022, 12, 1056. [Google Scholar] [CrossRef]
- Yi, L.; Chen, X.; Zou, C.; Liu, J.; Cao, X.; Tao, X.; Zang, Z.; Zheng, L.; Liu, L.; Wang, X. Construction and performance of PVDF-HFP-matrix comb-like gel copolymer electrolyte for high-performance quasi-solid-state lithium-metal batteries. J. Energy Storage 2023, 68, 107810. [Google Scholar] [CrossRef]
- Duan, T.; Cheng, H.; Sun, Q.; Liu, Y.; Nie, W.; Chu, Y.; Xu, Q.; Lu, X. Reinforcing interfacial compatibility of LLZTO/PVDF-HFP composite electrolytes by chemical interaction for solid-state lithium metal batteries. J. Power Sources 2024, 589, 233789. [Google Scholar] [CrossRef]
- Nguyen, M.C.; Nguyen, H.L.; Duong, T.P.M.; Kim, S.H.; Kim, J.Y.; Bae, J.H.; Kim, H.K.; Lim, S.N.; Ahn, W. Highly Safe, Ultra-Thin MOF-Based Solid Polymer Electrolytes for Superior All-Solid-State Lithium-Metal Battery Performance. Adv. Funct. Mater. 2024, 34, 2406987. [Google Scholar] [CrossRef]
- Lu, Q.; He, Y.-B.; Yu, Q.; Li, B.; Kaneti, Y.V.; Yao, Y.; Kang, F.; Yang, Q.-H. Dendrite-Free, High-Rate, Long-Life Lithium Metal Batteries with a 3D Cross-Linked Network Polymer Electrolyte. Adv. Mater. 2017, 29, 1604460. [Google Scholar] [CrossRef]
- Fan, W.; Li, N.W.; Zhang, X.; Zhao, S.; Cao, R.; Yin, Y.; Xing, Y.; Wang, J.; Guo, Y.G.; Li, C. A dual-salt gel polymer electrolyte with 3D cross-linked polymer network for dendrite-free lithium metal batteries. Adv. Sci. 2018, 5, 1800559. [Google Scholar] [CrossRef]
- Xu, K.; Xu, C.; Jiang, Y.; Cai, J.; Ni, J.; Lai, C. Sandwich structured PVDF-HFP-based composite solid electrolytes for solid-state lithium metal batteries. Ionics 2022, 28, 3243–3253. [Google Scholar] [CrossRef]
- Lee, H.; Song, Y.-W.; Kim, M.-Y.; Lee, J.; Ryu, J.; Noh, Y.; Kim, S.-J.; Kim, J.; Lim, J. Composite solid electrolyte with improved ionic conductivity and high lithium transference number through reduced PVDF-HFP crystallinity. Solid State Ion. 2024, 411, 116571. [Google Scholar] [CrossRef]
- Kou, W.; Guo, Z.; Li, W.; Liu, S.; Zhang, J.; Zhang, X.; Wu, W.; Wang, J. Highly conductive thin lamellar Li7La3Zr2O12/Li3InCl6 composite inorganic solid electrolyte for high-performance all-solid-state lithium battery. J. Membr. Sci. 2023, 687, 122080. [Google Scholar] [CrossRef]
- Robert, V.; Loganathan, S.; Sivashanmugam, A.; Thomas, S.; Valapa, R.B. 3D Printed P (VdF-HFP)/SBA-15 composite-based gel polymer electrolyte versus commercial celgard: A comparative investigation on thermal stability and electrochemical performance for lithium-ion battery application. J. Am. Ceram. Soc. 2024, 107, 5903–5922. [Google Scholar] [CrossRef]
- Bae, J.; Oh, S.; Lee, B.; Lee, C.H.; Chung, J.; Kim, J.; Jo, S.; Seo, S.; Lim, J.; Chung, S. High-performance, printable quasi-solid-state electrolytes toward all 3D direct ink writing of shape-versatile Li-ion batteries. Energy Storage Mater. 2023, 57, 277–288. [Google Scholar] [CrossRef]
- Bourseau, F.; Grugeon, S.; Lafont, U.; Dupont, L. 3D Printing of solvent-free PEO-Polyolefin solid polymer electrolyte by Fused Filament Fabrication. Virtual Phys. Prototyp. 2024, 19, e2409975. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, H.; Ding, H.; Jabbar, K.A.; Gao, L.; Zhao, G. Ceria Quantum Dot Filler-Modified Polymer Electrolytes for Three-Dimensional-Printed Sodium Solid-State Batteries. Polymers 2024, 16, 1707. [Google Scholar] [CrossRef]
- Wei, L.; Feng, Y.; Ge, S.; Liu, S.; Ma, Y.; Yan, J. Three-Dimensionally Printed Ionogel-Coated Ceramic Electrolytes for Solid-State Lithium Batteries. ACS Nano 2025, 19, 5789–5800. [Google Scholar] [CrossRef]

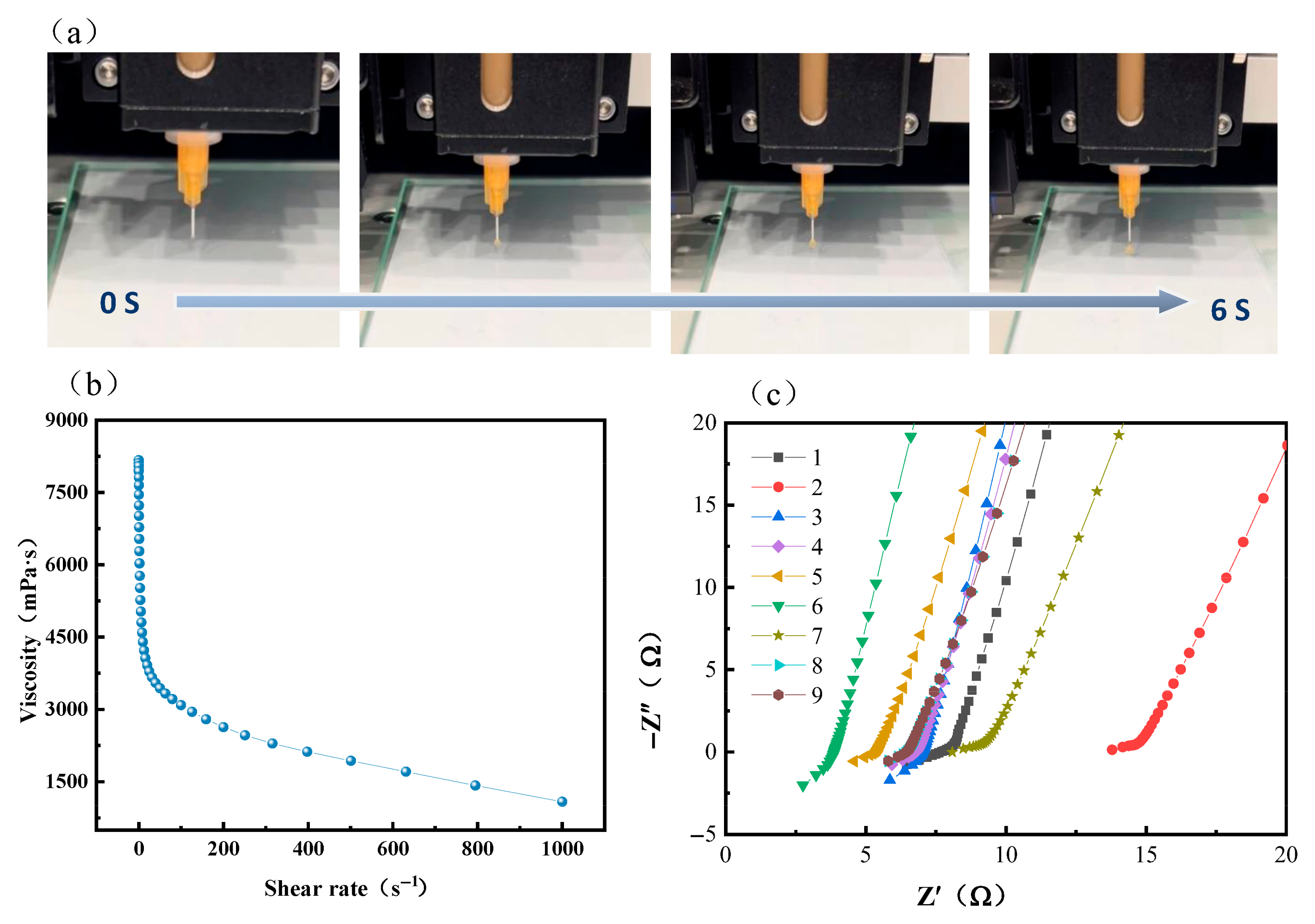
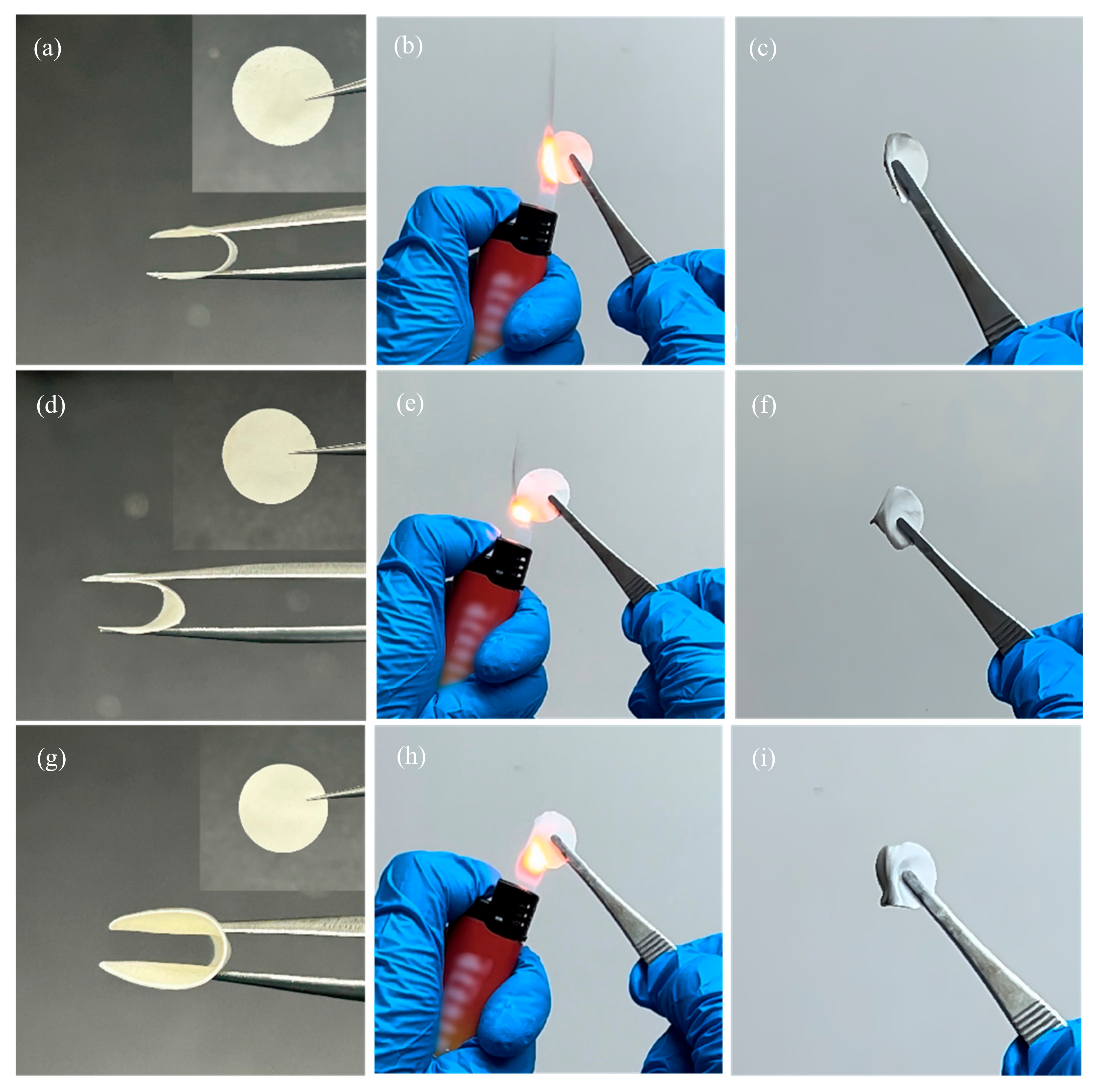
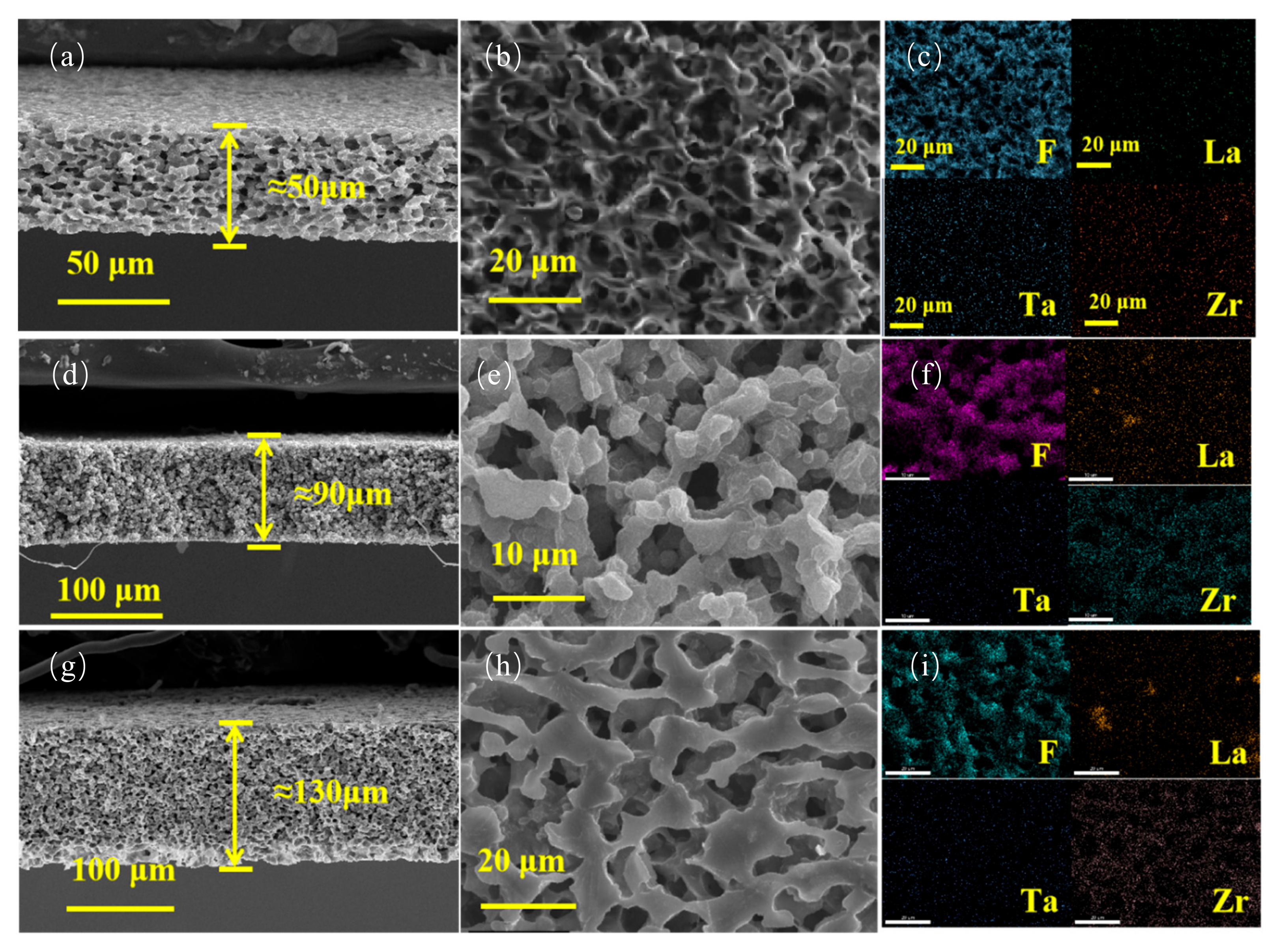
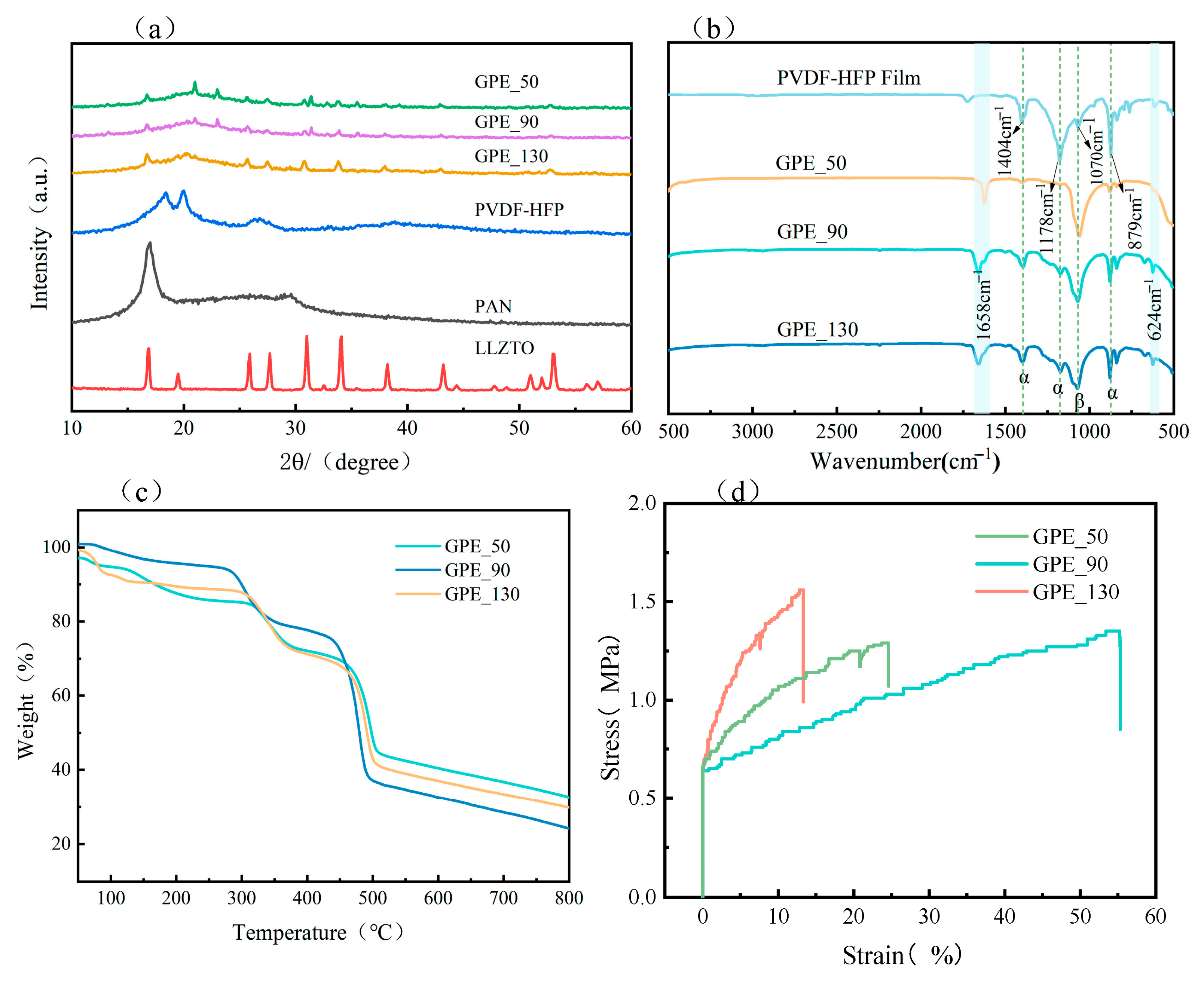

| Number | Printing Speed (mm/s) | Filling Density (%) | Ionic Conductivity (S·cm−1) |
|---|---|---|---|
| 1 | 20 | 50 | 2.9 × 10−4 |
| 2 | 20 | 70 | 1.98 × 10−4 |
| 3 | 20 | 90 | 3.18 × 10−4 |
| 4 | 40 | 50 | 2.8 × 10−4 |
| 5 | 40 | 70 | 5.77 × 10−4 |
| 6 | 40 | 90 | 2.93 × 10−4 |
| 7 | 60 | 50 | 1.09 × 10−4 |
| 8 | 60 | 70 | 9.41 × 10−5 |
| 9 | 60 | 90 | 1.47 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Qin, W.; Hun, Q.; Mao, N.; Li, J.; Liang, X.; Long, Y.; Guo, Y. Fabrication and Performance Study of 3D-Printed Ceramic-in-Gel Polymer Electrolytes. Gels 2025, 11, 534. https://doi.org/10.3390/gels11070534
Yao X, Qin W, Hun Q, Mao N, Li J, Liang X, Long Y, Guo Y. Fabrication and Performance Study of 3D-Printed Ceramic-in-Gel Polymer Electrolytes. Gels. 2025; 11(7):534. https://doi.org/10.3390/gels11070534
Chicago/Turabian StyleYao, Xiubing, Wendong Qin, Qiankun Hun, Naiyao Mao, Junming Li, Xinghua Liang, Ying Long, and Yifeng Guo. 2025. "Fabrication and Performance Study of 3D-Printed Ceramic-in-Gel Polymer Electrolytes" Gels 11, no. 7: 534. https://doi.org/10.3390/gels11070534
APA StyleYao, X., Qin, W., Hun, Q., Mao, N., Li, J., Liang, X., Long, Y., & Guo, Y. (2025). Fabrication and Performance Study of 3D-Printed Ceramic-in-Gel Polymer Electrolytes. Gels, 11(7), 534. https://doi.org/10.3390/gels11070534






