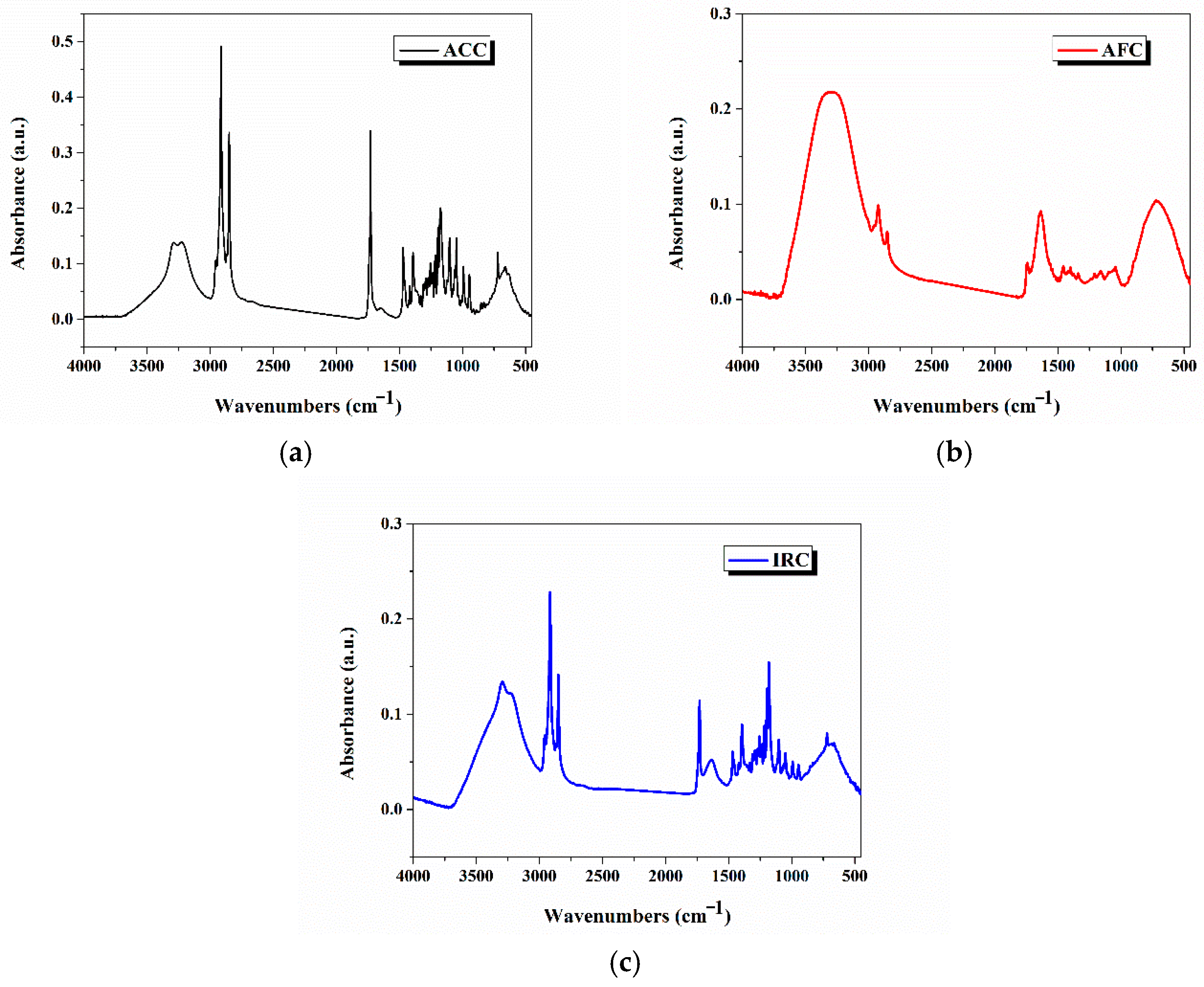
2.9.1. pH on the Skin Surface
The human skin not only serves as a barrier against external infections, but also harbors a diverse microbiome that includes viruses, fungi, bacteria, and archaea. Disorders of the skin microbiome can impair immune function and lead to autoimmune and inflammatory diseases. The pH value is a key characteristic of the skin for the microbiome. Cosmetic skincare products are essential for maintaining the microbial balance as they interact with the skin’s pH and microbiome. Products with unphysiological pH levels can disrupt the skin microbiome. A combination electrode, consisting of an H
+ ion-sensitive electrode and an additional reference electrode, is the basis for the Skin-pH-Meter PH 905 measurements. The results of the skin pH measurements are represented in
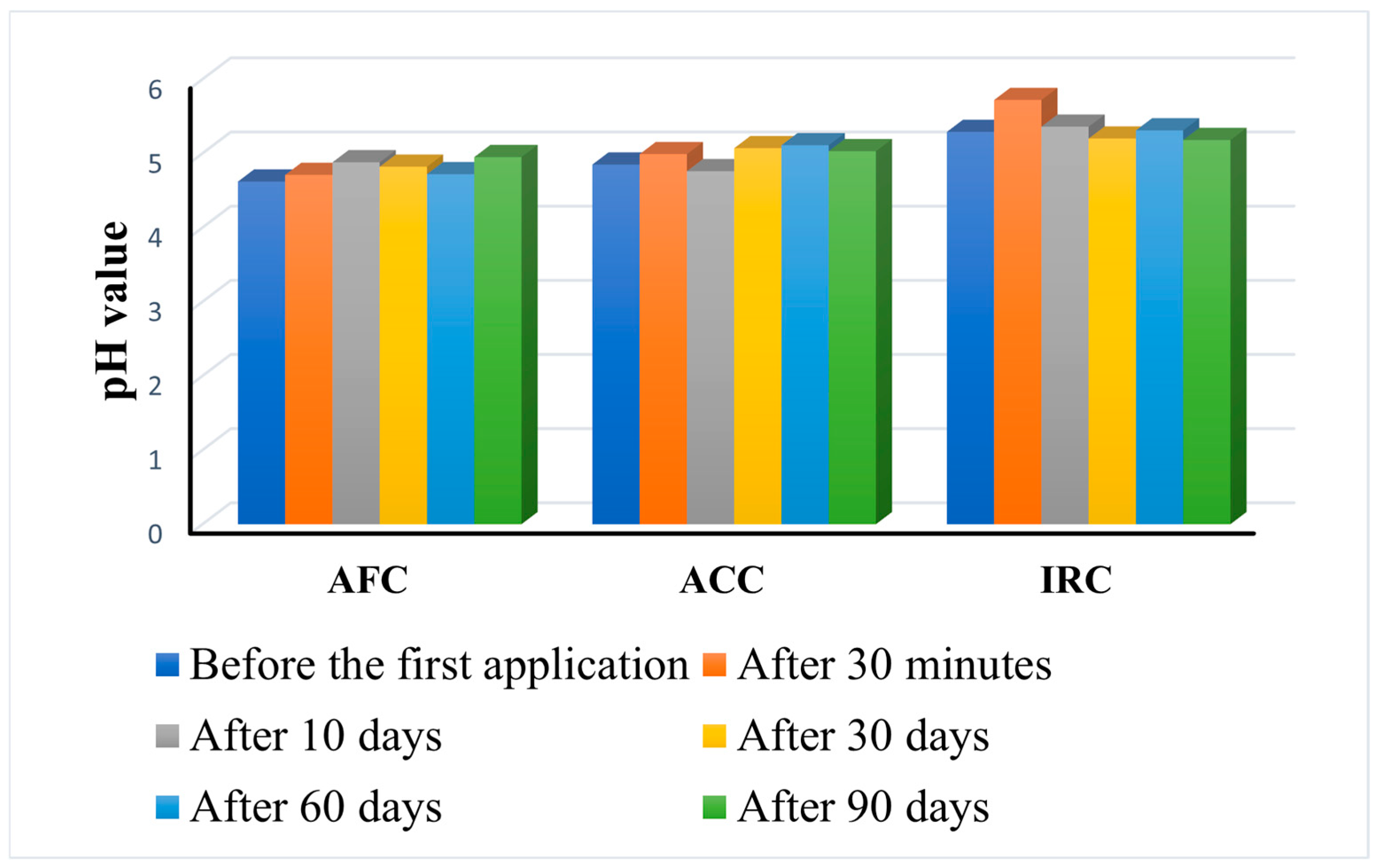
Figure 9.
As an obvious conclusion, none of the products alter the pH of the skin, which means that they are well tolerated even with long-term use.
However, the behavior of the products is different for the three formulations. AFC gradually increases the skin pH to the highest recorded value after 3 months of daily use (4.95 ± 0.31 compared to 4.62 ± 0.53 before use). ACC also increases the skin pH, but the highest value (5.11 ± 0.54) was measured after 60 days of constant use, while it decreases slightly after 90 days (5.03 ± 0.60). IRC manifests differently, leading to a remarkable increase in pH 30 min after the first application (5.72 ± 0.67 compared to 5.29 ± 0.84 before the first application), but the value slowly decrease over time inducing, after 90 days of application, a final pH value lower (5.18 ± 0.71) than before application.
Although there were some differences in skin pH between volunteers, with subject no. 17 (age 29) having the lowest pH prior to application (4.4), and subject no. 3 (age 47), having the highest pH value at the beginning of the study (5.4), the trend was similar during the study period. Considering that IRC has the lowest pH (5.05) of all three products, its manifestation after application on the skin is not surprising. Nevertheless, the change in skin pH is not linear for any of the products. There are variations between different time intervals, but it is significant that there are no differences in responses between subjects.
In summary, an increased variety of the natural skin pH was observed during the course of the investigation. According to the Shannon diversity index [
54], none of the test products significantly alter the diversity of the skin microbiome. Janssens-Böcker et al. [
55] proved that using low-pH skincare products does not alter the diversity of the skin microbiota and may even improve skin microbiome diversity and health by lowering the numbers of some harmful bacteria. The acidic pH of the stratum corneum appears to play an important role both in the antimicrobial defense of the skin and in the formation of the permeability barrier [
56]. The pH of the skin changes greatly throughout the stratum corneum, which presumably plays a role in regulating enzymatic activity and skin renewal [
57]. Numerous endogenous factors, including skin moisture, perspiration, sebum, anatomic location, genetic predisposition, and age, influence the pH of the skin [
58]. Variations in pH have been linked to the etiology of skin conditions such as irritant contact dermatitis, atopic dermatitis, ichthyosis, acne vulgaris, and Candida albicans infections [
59]. According to Schmid-Wendtner et al. [
60] findings, the prevention and treatment of certain skin conditions may benefit from the use of skin products with a pH of roughly 6.
2.9.2. Hydration Level
Improving skin hydration can lower sebum production, enhance the function of the skin barrier, and lower the risk of hyperkeratinization and colonization by microorganisms. Thus, topical anti-acne products with moisturizing properties may be more useful for the treatment of acne, particularly in individuals with dry, irritated skin. In addition, a more moisturizing formulation leads to better adherence to the treatment [
61]. Corneometry is currently one of the most widely used methods in the field of dermocosmetics. The experts of the EEMCO (European group on efficacy measurement and evaluation of cosmetics and other products) expressly recommend the Corneometer
® (Cologne, Germany) for testing the moisture content of the skin [
62].
The stratum corneum, the uppermost layer of the skin, is used as a dielectric medium in the analysis based on capacitance measurements. Its dielectric properties change with increasing water content. Water has a higher dielectric constant (81) than most other substances (usually <7), being the basis for the measurement [
63]. When Hua et al. compared Courage + Khazaka corneometry with other devices, they found that while the results for certain skin attributes were related to the measurements of other devices, the results for skin hydration and TEWL showed minimal variability [
64]. According to Holm et al. [
65], corneometry is a useful non-invasive tool for measuring the severity of atopic eczema in patients.
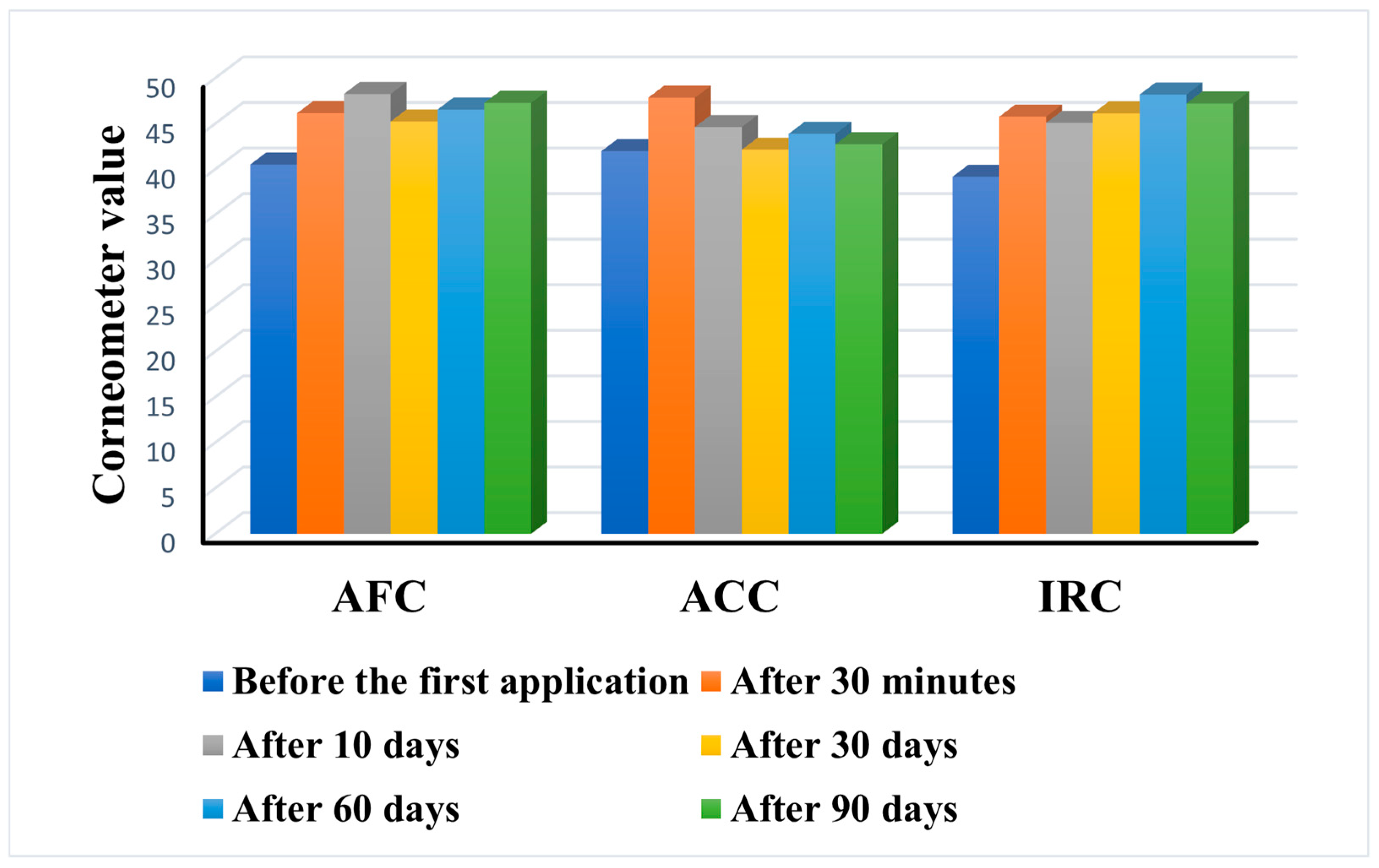
The results of the skin hydration levels are presented in
Figure 10.
All three products were found to notably increase the skin’s moisture content after a single application. Even after three months of daily use, all three formulations were found to be moisturizing, but the performance of each product was different. The highest increase in skin hydration was achieved by IRC with a value of 47.22 ± 2.02 after 90 days, compared to 39.17 ± 4.18 before the first application. A similar behavior was also observed for AFC, with a final value of 47.28 ± 1.7 compared to 40.52 ± 2.47 at the beginning. ACC showed the weakest effect with a slight increase from 41.98 ± 3.25 to 42.74 ± 2.59 after 90 days. While AFC and IRC led to a constant increase in hydration over time, AFC showed a fluctuating behavior post-application.
In addition, the performance of the products in six of the subjects differed from the mean behavior registered in the other participants. For subject no. 5 (age 38), only AFC led to an increase in skin hydration, while IRC and ACC had no effect on the parameter. Subject no. 17 (age 29) showed a significant decrease in hydration level after 90 days of ACC application (from 48.06 to 42.13). Subject no. 20 (age 50) showed no change in hydration level at any time following ACC and IRC application. Subject no. 22 (age 27) and subject no. 34 (age 25) showed a significant increase in hydration after the application of ACC. The 90-day application of IRC in subject no. 36 (age 27) resulted in a slight decrease in skin hydration.
The moisturizing ability of the three products can be explained by their components. In AFC, the extract of
Chlorella vulgaris and the phytosterol esters from
Crambe abyssinica in particular help to retain moisture in the skin due to the content of amino acids, vitamins, and fatty acids, which leads to increased hydration [
66]. IRC also contains
Crambe abyssinica oil and betaine, which are known for their moisturizing effects [
67]. Meanwhile, ACC also contains a large amount of
Crambe abyssinica oil.
Skin damage caused by excessive dryness or oiliness can be prevented by moisturizing the skin, which also helps to prevent common skin conditions such as acne. In addition to protecting the skin barrier and reducing the formation of excess oil that can clog pores and lead to further acne breakouts, a moisturizing agent also helps maintain adequate moisture levels [
68]. The use of moisturizers as supportive therapy has been shown to improve the treatment of some dermatological conditions [
69].
2.9.3. Melanin Content
An important factor in the development of acne is inflammation, which causes capillary hyperplasia, prolonged vasodilation, and aberrant melanin synthesis, which in turn leads to postinflammatory erythema (PIE) and postinflammatory hyperpigmentation (PIH). While PIH is more common in people with darker complexions, PIE mainly affects people with lighter skin tones [
70]. A significant percentage of acne patients experience PIH over an extended period of time, which emphasises the need for effective treatment methods. Therefore, anti-acne products should have effective properties to reduce pigmentation alterations. The results of the melanin content are presented in
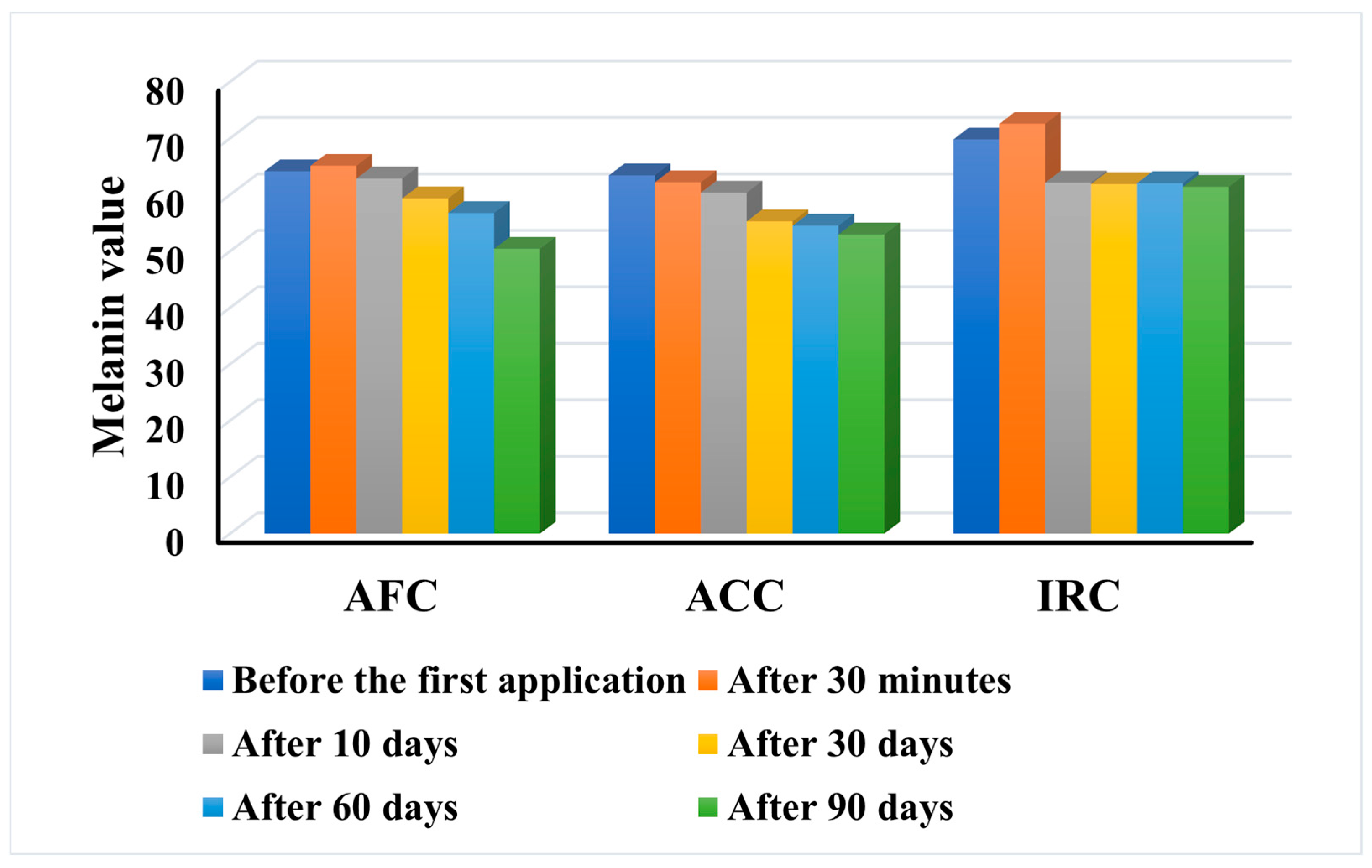
Figure 11.
The results indicate that all three tested products lead to a reduction in the melanin content of the skin after prolonged use. However, the behaviour of the products is different. While AFC and IRC show a slight increase in the melanin value 30 min after the first application, only ACC leads to a decrease in the parameter after the first application and maintains this decrease constant over time, from 63.44 to 52.96. Nevertheless, the greatest decrease in melanin was recorded after the application of AFC, with a decrease in the median value from 64.15 before the test to 50.42 after three months of daily application. The same pattern as with the AFC was observed with the IRC, but the decrease was much smaller in this case, from 69.8 to 61.39. However, in two subjects (no. 22—age 27 and no. 17—age 29), the melanin content was not affected by IRC, with final values similar to the initial values, but they responded with a reduction after the application of ACC and AFC. Also, one subject (no. 38—age 46) showed an increase in melanin content after the application of ACC (from 59.84 to 62.16), but a decrease after the application of AFC and IRC. It is important to note that in all subjects, the melanin content decreased after the continuous application of ACC and AFC, and only in 9 out of 40 (22.5%), the parameter also decreased after the first application of AFC. In the remaining 31 subjects (77.5%), the melanin content increased slightly but decreased again after long-term use.
The results demonstrate the positive effect of all three products on reducing hyperpigmentation of the skin, with AFC showing the highest efficacy, but ACC showing a constant behavior.
Chlorella vulgaris extracts have been found to suppress melanogenesis by inhibiting tyrosinase, a key enzyme in melanin synthesis [
71]. In addition, Cho et al. demonstrated that betaine reduces the amount of melanin in the cells by directly inhibiting the activity of the tyrosinase enzyme and downregulating MITF at both the transcriptional and post-translational levels. Three signaling pathways seem to be involved simultaneously: activation of the ERK signaling pathway, AKT-GSK3β activation, and PKA-CREB repression [
72]. Moreover, the study conducted by Kamei et al. [
73] proved that vitamin E suppresses the activity of tyrosinase, a crucial cascade enzyme involved in melanin synthesis in melanoma cells, by 34%, apart from the 28% inhibition of melanin synthesis in B16 cells. According to the results of the present study, the combination of these ingredients in the AFC formulation appears to lead to increased efficacy in reducing the melanin content of the skin. According to the study by Kim H-K [
74], melanin production in HRM-2 mice was significantly and strikingly reduced by the radish extract. Additionally, the radish extract cream showed superior skin-whitening and anti-wrinkle properties in humans, demonstrating its strong whitening effects. Its presence in the ACC formulation probably gave rise to its constant and pronounced anti-melanogenic activity. On the other hand, IRC contains niacinamide that can both enhance PIH and decrease melanosome transfer from melanocytes to adjacent keratinocytes [
75].
2.9.5. Transepidermal Water Loss (TEWL)
As a vital component of the body’s metabolism, water is continuously evaporating from the skin. The unit of measurement for water content (Transepidermal Water Loss, or TEWL) is g/m
2/h. However, water loss will increase as soon as the skin’s barrier function is compromised, even if the damage is minimal and imperceptible to the naked eye. Its measurement is a fundamental measurement in all types of topical product applications and an essential parameter for assessing the skin’s water barrier performance. Skin’s barrier capability and resistance are directly connected, and both are dependent on the stratum corneum’s level of moisture [
81]. The results of transepidermal water loss (TEWL) are presented in
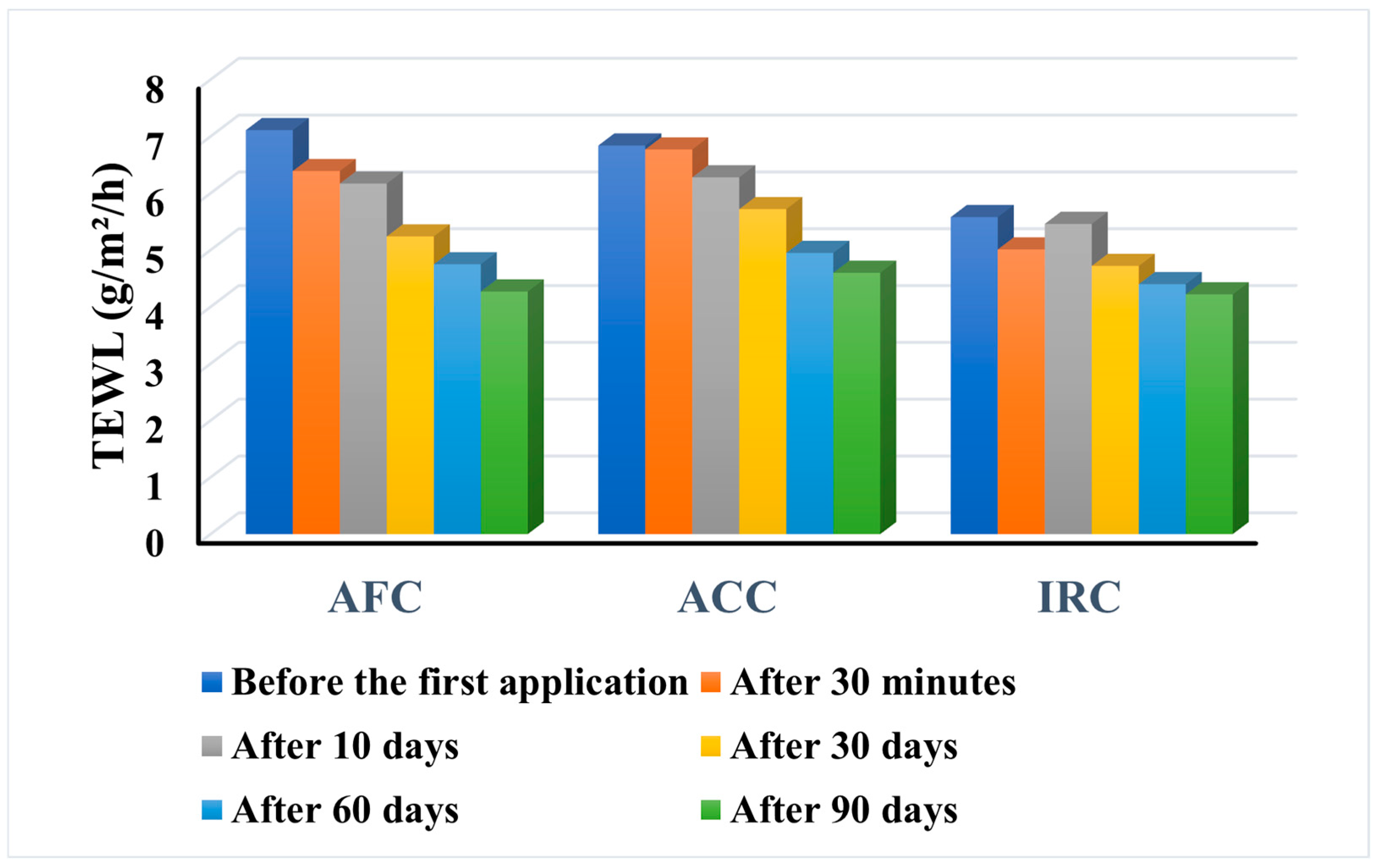
Figure 13.
The three dermatocosmetic products reduced the TEWL from the initial value to a constant application over 90 days. For all three samples, TEWL decreased even immediately after the first application, and for ACC and AFC, this trend persists, gradually decreasing over time. In the case of IRC, the value recorded after 10 days is higher than the value recorded 30 min after the first application, but from this point onwards it decreases steadily over time. However, some different behaviors were observed. In the case of AFC, subjects no. 1 (age 38) and no. 15 (age 50) showed an increase in TEWL in the first 30 days, and the parameter only began to decrease after 60 days of use. In contrast, subject no. 31 (age 25) showed only a slight change in the indicator, from 4.43 at the beginning of use to 4.37 after 90 days. For ACC, seven of the subjects (175.5%) showed a consistent decrease in TEWL from the first application to the end of the study, while subject no. 37 (age 48) was the only subject to show an increase in the parameter after 90 days of use (from 6.55 to 7.06). For IRC, subjects no. 10 (age 33) and no. 29 (age 45) showed a significant decrease in TEWL from 5.78 and 5.42 at baseline to 3.05 and 3.20, respectively, after three months of daily use.
The results prove a drastic reduction in TEWL after the application of all tested products, which helps to retain hydration and a healthy skin state by forming a protective layer on top of the skin. The skin’s capacity to retain moisture or the integrity of the skin barrier function can be determined by measuring TEWL [
82]. Clinical data indicates a strong correlation between acne and skin barrier dysfunction. The skin’s capacity to hold onto moisture is improved by a decrease in TEWL or an enhancement in skin barrier function [
83]. Using moisturizers concurrently as an adjuvant acne treatment has therapeutic effects and improves skin condition by reducing inflammation and irritation [
84]. As a result, acne and skin conditions can recover rather quickly. The study by Gala M. proved that regular application of moisturizers greatly enhanced skin hydration; repaired or strengthened the skin barrier; decreased skin oiliness; lessened the visibility of acne and prevented the emergence of new acne; decreased skin redness; lessened the visibility of blemishes or lightening them; and lowered the quantity, intensity and visibility of pores.
The registered results are in line with the results of the study by Morin et al., which proved that skin hydration is based on two processes and can thus be explained. The first hydration process takes place in the first few minutes after application of the product and follows first-order kinetics. Their results confirm that the process is primarily related to the filling of cavities and not to hydration-induced changes in the molecular components of the stratum corneum, which would require longer equilibration times. The second hydration process occurs at least one hour after application and has a slower rate with a linear progression. This process is thought to be caused by hydration-related changes in the stratum corneum, including changes in the kinetics and molecular structure of the proteins and lipids of the stratum corneum, the enlargement of corneocytes, and/or the development of water inclusions [
85].
2.9.6. Skin Topography
The skin’s surface topography is made up of scales, wrinkles, and lines. A network-like structure made up of primary and secondary lines can be recognized as a polygon. Measurements of skin surface roughness are often used in dermatological treatment and research [
86]. The complex structure of human skin, its mechanical behavior, and its microscale topography all play a role in its ability to tolerate topical products. Some dermatological products can damage the skin and impair its functionality [
87]. Skin topography analysis is an important test that provides important information about the behavior of the product on the skin during its continuous use.
SELS parameters, surface, texture, and ageing parameters were determined. The SELS parameters (Surface Evaluation of the Living Skin): SEr, SEsc, SEsm, and SEw represent the roughness, desquamation dryness, smoothness, and wrinkles of the skin. A higher SEr value indicates greater roughness, while a lower SEsc value signifies less desquamation/dryness. Conversely, a lower SEsm value indicates smoother skin, and a higher SEw value suggests more wrinkles. The surface parameters are represented by the surface area (if it is high, the skin quality is poor) and the volume (if it is high, the skin is hydrated). The texture parameters are represented by contrast (a low value indicates a good skin condition), entropy (a high value means a well hydrated skin), variance (a high value indicates a rough skin), energy (a high value reveals a young, hydrated, elastic skin) and homogeneity (a high value shows a hydrated skin). The ageing parameters are made up of the anisotropy index, which indicates the directionality of the lines (high values are characteristic of old skin), and the total number of cells—polygons between the lines (a high value is an indicator of young skin).
Black and white images of the skin show wrinkles and black lines, skin peeling represented by very large pixels, and light-coloured areas representing plateaus of the skin’s microrelief. 3D color images show differences in the height and roughness of the skin surface. The results of skin topography parameters for AFC are presented in
Table 5.
The results of the study show that AFC has a positive effect on the properties of the skin after the first application, which increases with long-term use, resulting in a significant reduction in roughness and dryness and a marked improvement in skin hydration, quality, and suppleness. In addition, it showed a significant anti-ageing effect, which is reflected in the reduction in the SEw value and the anisotropy index, as well as in the increase in the total number of polygons.
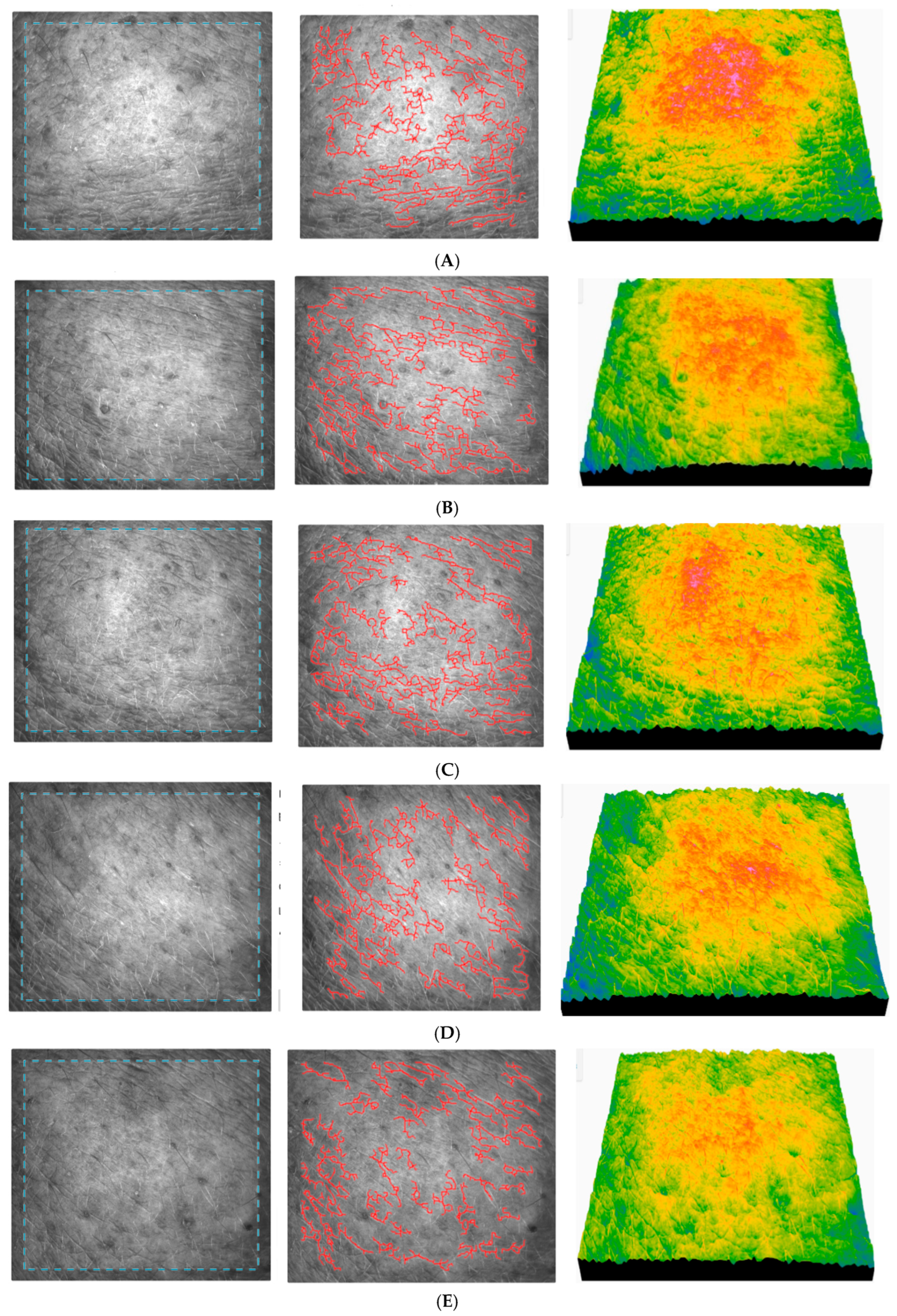
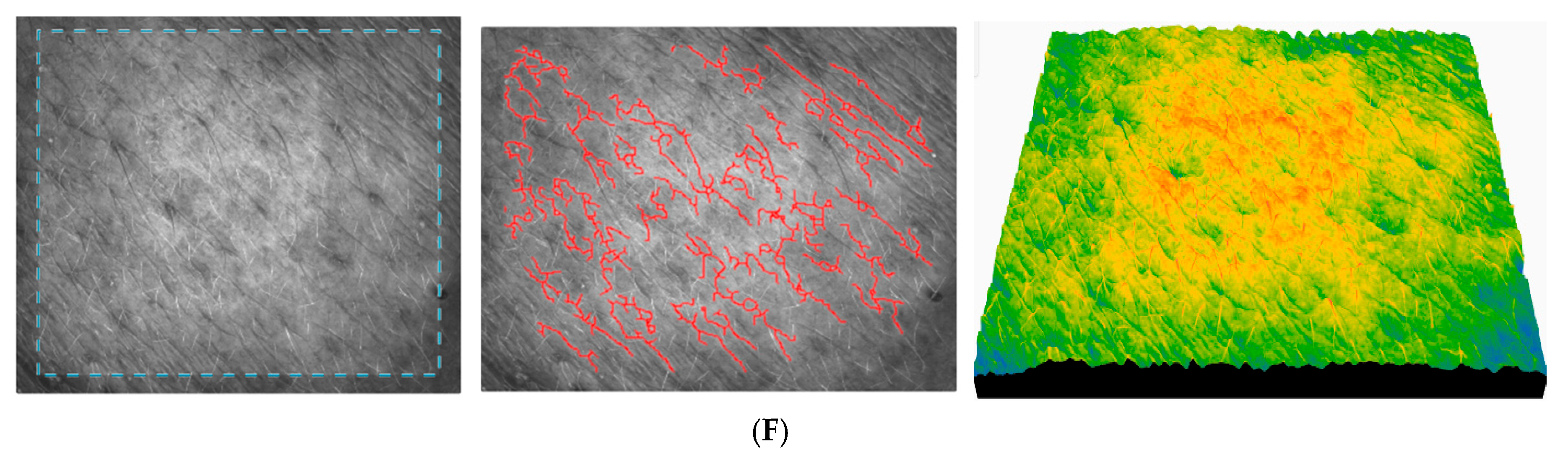
Figure 14 registered for subject no. 2 (age 25) shows the images of skin topography registered for some subjects during the application of AFC.
The skin before the application of AFC registered for subject no. 2 (age 25) (
Figure 14A) shows a moderate roughness, a slightly irregular relief, a slight peeling, a moderately hydrated and smooth skin, and a clear presence of fine wrinkles. It can be seen that skin roughness decreases in the long term, which is a clear sign of improved skin texture. The SEsc parameter gradually decreases and reaches its minimum after 90 days (
Figure 14F), which indicates a clear smoothing of the skin. The SEw parameter decreases significantly up to 90 days, indicating a reduction in fine lines. The skin becomes smoother after application of the cream, especially between 30 and 90 days. The texture parameters improve significantly up to 30–90 days, indicating a smoother, more even, and younger texture. The relatively constant number of cells indicates stable epidermal activity, with no signs of inflammation or excessive regeneration.
The 50-year-old subject exhibited skin with mild roughness, indicating moderate skin relief and smoothness, with a moderate to high SEw score indicating the onset of structural changes in the skin (
Figure S1 from the Supplementary Materials). The beginnings of photo-ageing are evident, but no significant peeling is observed. After application of the cream, a decrease in skin roughness is observed, and peeling is significantly reduced, indicating good skin hydration with surface smoothing and active cell regeneration. A homogenization of the skin texture and a reduction in the depth of fine lines are also observed, indicating improved hydration, probably also due to the stimulation of collagen synthesis, given the increase in volume.
At baseline, the facial skin of subject 18 (
Figure S2 from the Supplementary Materials) has moderate SEr and SEw values, and the skin appears to be fairly smooth and slightly wrinkled. SEsc is very low, which is a good indicator that the skin is well hydrated (no visible scales). The texture appears quite even (moderate contrast and entropy). After applying the cream, the skin condition changes for the better. The surface parameters indicate a smoothing of the macrorelief of the skin and a stabilization of the skin. The texture becomes more homogeneous, smoother, and even, with active skin regeneration and subsequent stabilization. As a conclusion, the skin of all subjects notably improved its microrelief and mechanical properties after the continuous application of AFC during 90 days.
The mechanical properties change in parallel with the biological changes that take place in the epidermis during cell development. The uppermost skin layer and the stratum corneum are commonly identified with different mechanical responses [
88]. Compared to the initial physiological state [
89], the apparent stiffness of the stratum corneum decreases by orders of magnitude when the corneocytes are hydrated and swell due to water uptake [
90]. Due to the presence of essential amino acids (including proline, lysine, and glycine) and peptides in the composition of
Chlorella vulgaris extract, it has been shown to enhance the production of collagen, increase skin suppleness, and reduce the visibility of wrinkles and fine lines [
91].
Chlorella vulgaris extract also contains large amounts of polysaccharides that bind moisture, improve the skin barrier, and increase hydration [
92]. The vitamin B complex (B1, B2, B3, B6, B12) it contains improves the overall tone and texture of the skin, stimulates cell metabolism, and promotes skin regeneration. Alpha-linolenic acid (ALA), an essential fatty acid, helps to maintain the lipid barrier of the skin, reducing irritation and dryness [
93].
The results of skin topography parameters for ACC are presented in
Table 6.
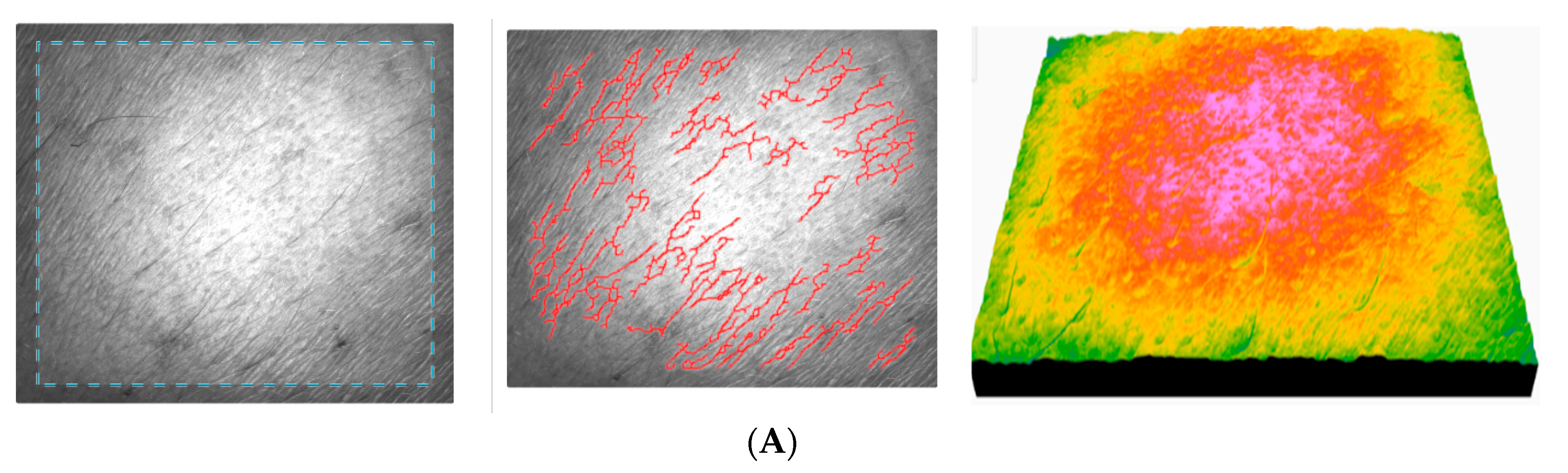
Similar to AFC performance, ACC also improves the skin’s microrelief and mechanical properties when applied over a longer period of time, but its efficiency is lower. However, evaluation of the skin by light scattering shows a significant decrease in roughness and an increase in smoothness, indicating a more uniform appearance in depth, and the fine lines become less pronounced. The reduction in surface area indicates that the skin becomes more uniform on the surface. The texture parameters indicate that the skin is more moisturized, and the homogeneity of the skin is improved. The change in the total cell count indicates stable cell activity, with a clear improvement, but no greater enhancement than for AFC. Some representative images were selected for the presentation (
Figure 15).
With test subject no. 7, both roughness and dryness decrease, the skin becomes smoother, and wrinkles are reduced. The uniformity of the skin also improves, and the relief becomes less pronounced. The texture parameters indicate a natural, more even, and less rigid texture. The total number of cells has increased after a continuous 90-day application of ACC.
In subject no. 26 (
Figure S3 from the Supplementary Materials), the behavior is different, and after the application of ACC, an increase in roughness, a strong deterioration of the superficial skin layer towards the end, a possible excessive peeling (dryness), and a decrease in skin smoothness were observed. This indicates a more uneven appearance in depth, and the fine lines become more pronounced, which is a sign of dehydration. The increase in surface area indicates that the skin is becoming more irregular on the surface, and the volume variations emphasize an increase in skin relief. In the texture parameters, it can be observed that the skin becomes more contrasted and “textured”, which is a sign of irregularity, and that the uniformity of texture and homogeneity of the skin decrease. The fluctuations in the total cell count indicate unstable cell activity, with no clear improvement, but no clear regression either. ACC appears to cause irritation, dryness, or aggression of the skin in subject no. 26, aged 49 years. Compared to the other test subjects, however, this appears to be an isolated case, and the behavior could be triggered by a sensitivity to one of the product’s ingredients.
For subject no. 37 (
Figure S4 from the Supplementary Materials), the roughness decreases after the constant application of ACC, which means a regeneration of the epidermis and an improvement of the surface. The skin becomes smoother, finer, and the anti-wrinkle effect appears. A temporary inflammatory reaction or structural expansion is observed, but the increase in volume is a sign of hydration and replenishment of the skin. The skin texture parameters indicate a possible unevenness of the texture in certain phases, a temporary unevenness, and a slightly disorganized structure in the long term. The increase in the number of cells is a sign of increased cellular activity and regeneration of the epidermis. The high anisotropy at the end (90 days) could indicate an uneven reorganization of the dermal fibers. Overall, ACC seems to have more positive effects on young people than on older people.
The results of the study by Zhang et al. [
94] indicate that rice protein hydrolysate is essential for skin protection due to its high antioxidant capacity, its effective protection of skin tissue, and its inhibition of water loss. Furthermore, the rice protein hydrolysate has been shown to diminish human face wrinkles by 11.8% [
95], block the development of aging symptoms in mice [
96], and lower the melanin content of human epidermal melanocyte cells [
97].
The results of skin topography parameters for IRC are presented in
Table 7.
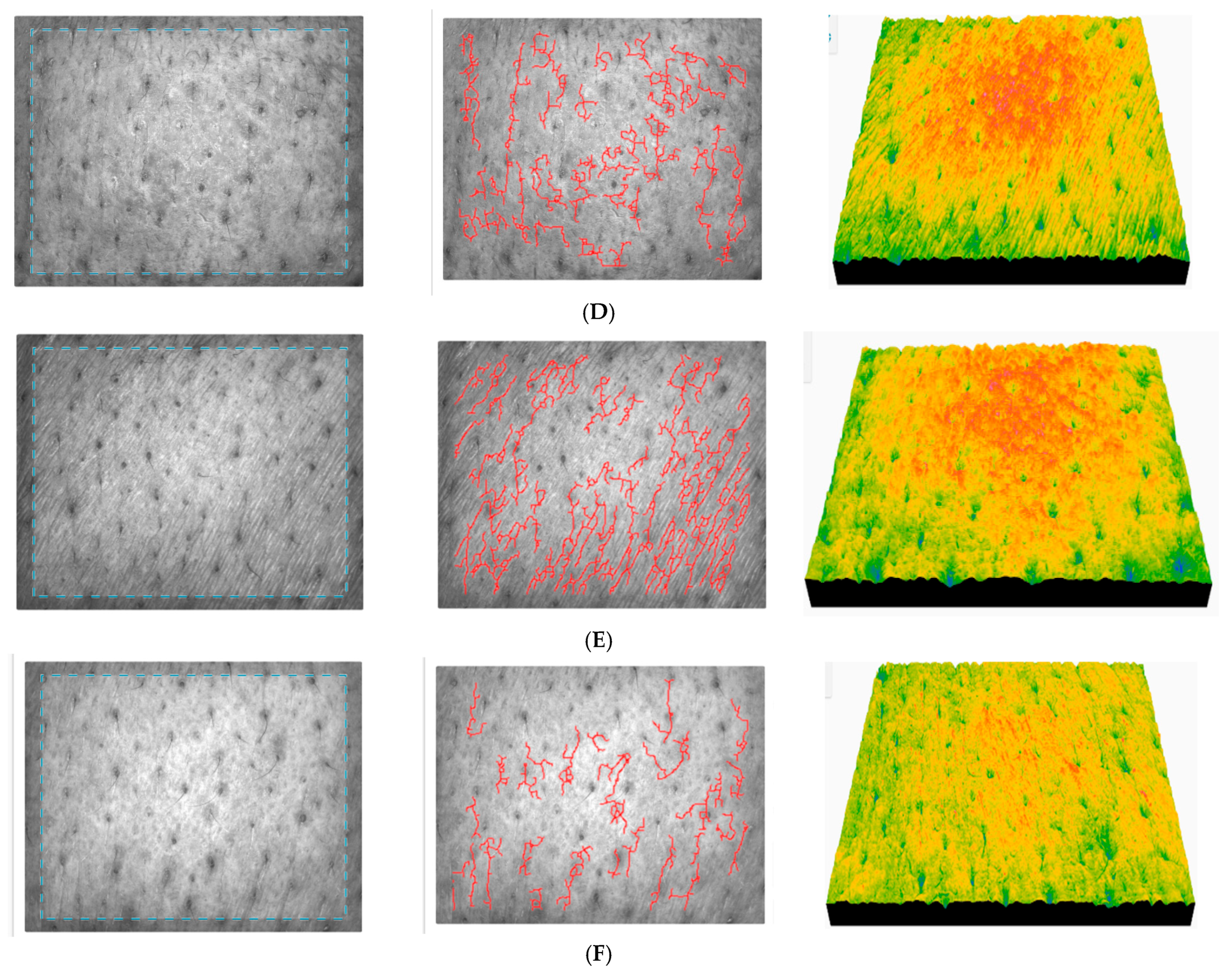
It can be seen that IRC produces the greatest improvement in skin topography of all three products analyzed. The positive effect was observed from the first application and significantly improved the biophysical properties of the skin during continuous application, with the highest point being reached after 90 days of use. It appears to be suitable for all skin types, both young and mature, with a marked improvement in the overall quality of the skin, especially texture, internal organization, and cellular activity. Roughness slowly diminished and smoothness improved significantly, with fine lines softened in the long term. The internal organization of the skin has improved according to the anisotropy index, and cell activity has considerably improved. The images registered for subject 9 (
Figure 16) highlight a positive course with an important reduction in skin roughness. The smoothness of the skin is improved in the long-term application, accompanied by a clear reduction in fine lines and wrinkles. The surface parameters signal a clear improvement in skin uniformity in the long term, and a significant cellular recovery was detected.
The data for subject no. 14 (age 47) (
Figure S5 from the Supplementary Materials) indicate that the roughness of the skin has decreased, accompanied by strong hydration, followed by excellent soothing and restoration of smoothness. The fine lines were initially accentuated (signs of dehydration/contracture), but after daily use, they are significantly reduced. Texture is more uniform, balanced, and normalized compared to baseline. The anisotropy index indicates that the internal organization of the skin is largely restored, and the cellular regeneration is active.
The results recorded for subject no. 15 (
Figure S6 from the Supplementary Materials) shows a constant improvement in skin quality. The skin is less rough and becomes smoother, while the fine wrinkles are significantly improved. The uniformity of the skin is partially restored, as is hydration, which is also confirmed by the texture parameters. The change in the total cell count attests to clearly activated cell regeneration, a sign of a positive adaptive reaction of the skin.
To summarize, IRC proved to be the most effective of the three products in terms of improving the skin’s microrelief. It was followed by the AFC and then the ACC. The IRC behavior can be explained by the high content of rice protein hydrolysate and vitamin E. Mango butter has also been proven to reduce wrinkles and roughness due to its bioactive components such as phytosterols, tocopherols, and triterpenes [
98]. The study conducted by Mandawgade et al. [
99] showed that mango butter results in exceptional softness, actively restoring moisture for improved skin protection and rebuilding a naturally occlusive, protective skin barrier, leaving skin feeling smooth, moisturized, and silky. However, Camargo et al. [
100] demonstrated that panthenol in skin care formulations has an enhanced protective impact in maintaining skin integrity after 30 days of treatment and leads to a remarkable reduction in TEWL. Also, niacinamide, included in the IRC formulation, was revealed to considerably lower pigmentation, inflammation, and oxidative stress in the skin [
101]. Marques et al. stated that niacinamide may be able to partially prevent and/or reverse a number of biophysical alterations linked to skin aging through a variety of multimodal methods [
102]. In conclusion, IRC has a very balanced formulation that contributes to strong skin activity by restoring skin functionality and preventing premature ageing.