A Review of High-Temperature Resistant Silica Aerogels: Structural Evolution and Thermal Stability Optimization
Abstract
1. Introduction
2. Structural Evolution and Sintering Mechanism at High Temperatures
2.1. High-Temperature Structural Evolution
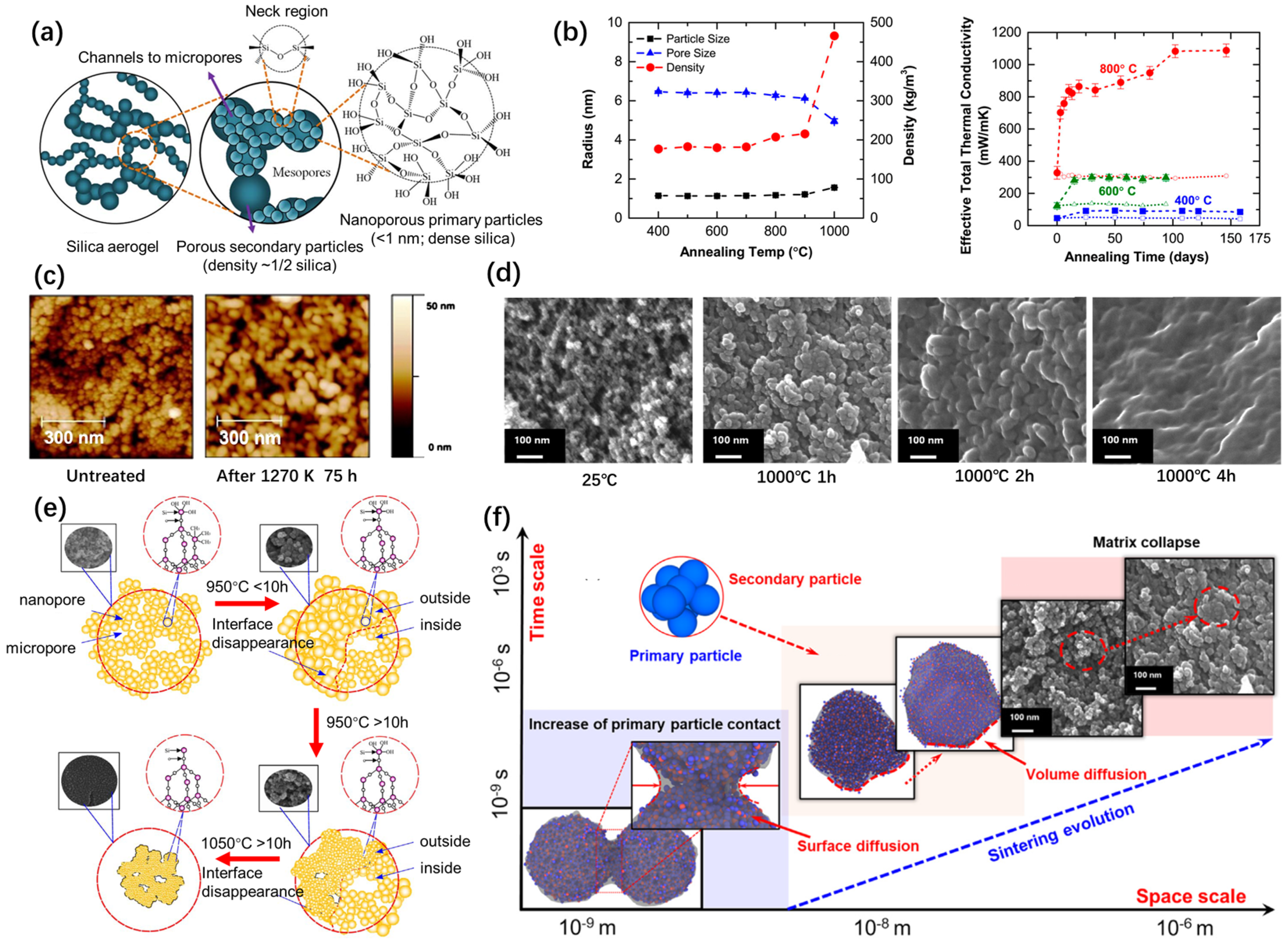
2.2. Sintering Driving Forces and Models
3. Methods for Improving the Thermal Stability of Silica Aerogels
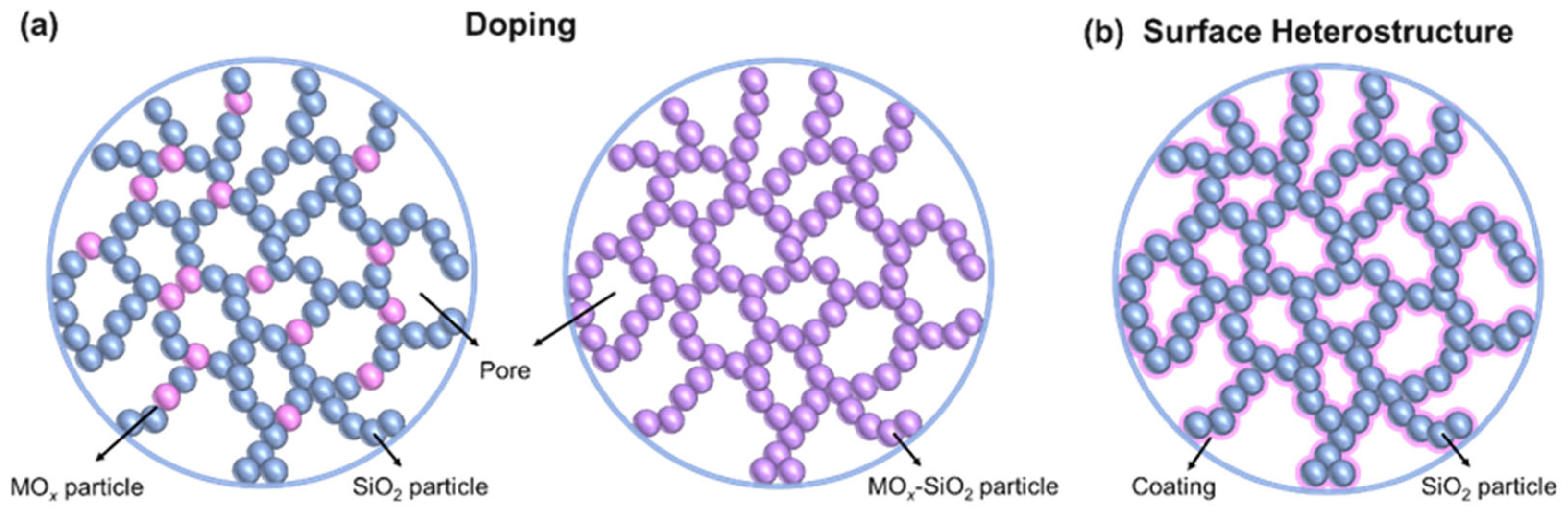
3.1. Heteroatom Doping
3.1.1. Alumina-Doped Silica Aerogels
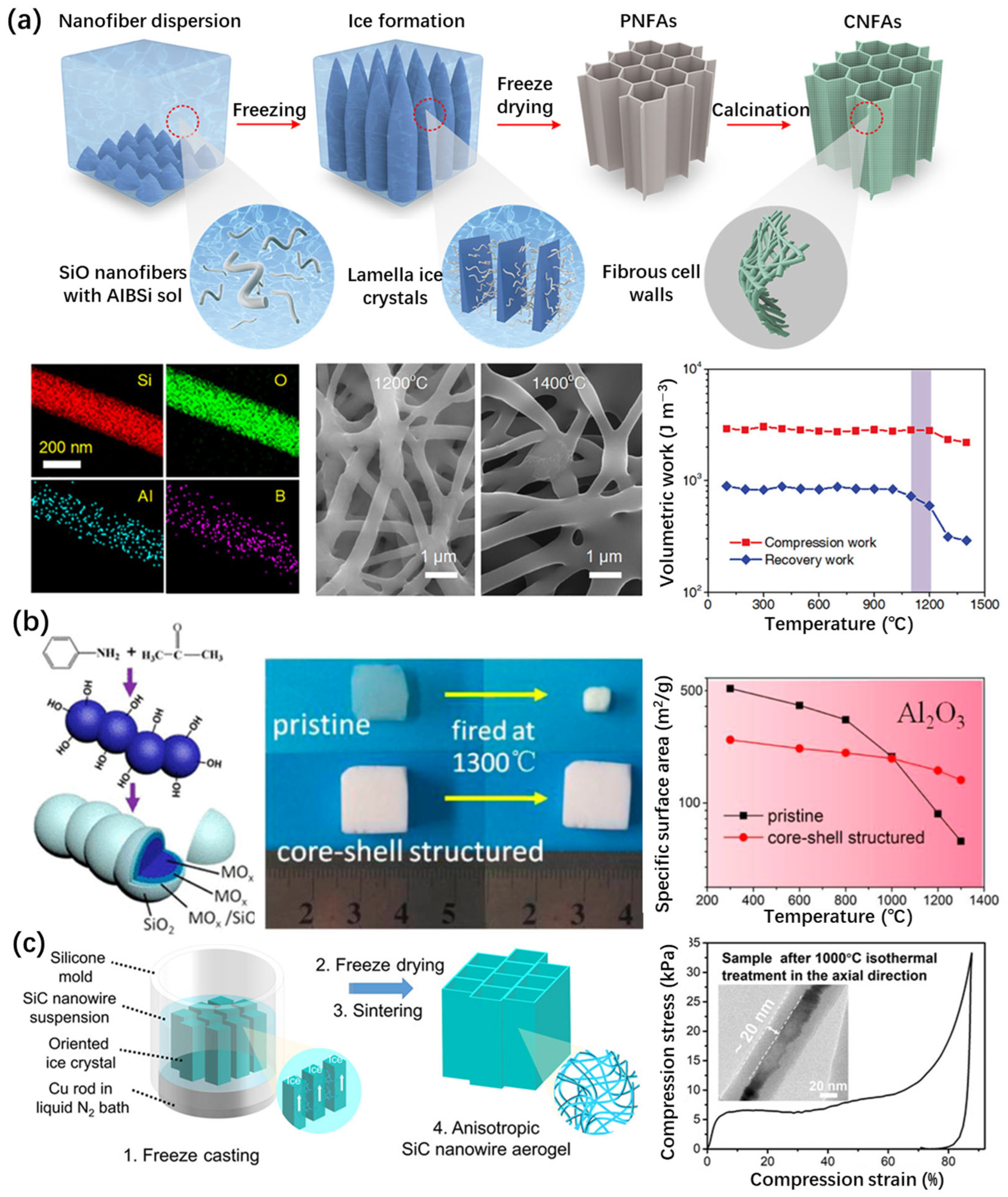
- The organic aluminum precursor-based route. The precursors employed in this route are primarily organosilicons (TEOS and TMOS) and organic aluminum compounds (aluminum isopropoxide and aluminum tri-sec-butoxide), while the sol–gel method (Figure 5a) combined with the supercritical drying technique was adopted [64,65,66]. During the preparation of Al2O3–SiO2 aerogels, the hydrolysis of organic aluminum compounds occurs significantly faster than that of organosilicons, resulting in a non-uniform solution due to sedimentation that occurs before gelation. It becomes necessary to introduce chelating agents to inhibit the hydrolysis of organic aluminum compounds [65,66,67] or to promote the hydrolysis process of organosilicons [68], thereby achieving a synchronized reaction process between these two precursors. As early as 1993, Komarneni et al. [59] carried out a study on the Al2O3–SiO2 system aerogel, using Al doping amounts of 1% and 10%. Tetramethoxysilane (TMOS) and boehmite were used as the starting materials. The presence of alumina as a refractory phase hindered their densification, resulting in surface areas ranging from 500 to 600 m2/g, with mesopore diameters of approximately 6 nm, after being heated at 1000 °C;
- The inorganic aluminum precursor-based route. In this route, inorganic aluminum salts, mainly including aluminum chloride and aluminum nitrate, are used as precursors, along with organosilicon [69,70,71,72]. In order to reduce the cost, natural or industrial waste containing silicon and aluminum can be adopted as precursors. Rutiser et al. [60] prepared aerogels from silica combined with 1 to 10 mol% of minerals containing aluminum, such as kaolinite, montmorillonite, boehmite, and mullite. When annealed at 1000 °C, alumina-doped silica aerogels still retained a very high surface area compared to sintered aerogels prepared from silica only. Specific surface areas of up to 425 m2/g were achieved by the 1% kaolinite aerogels after 8 h of heat treatment.

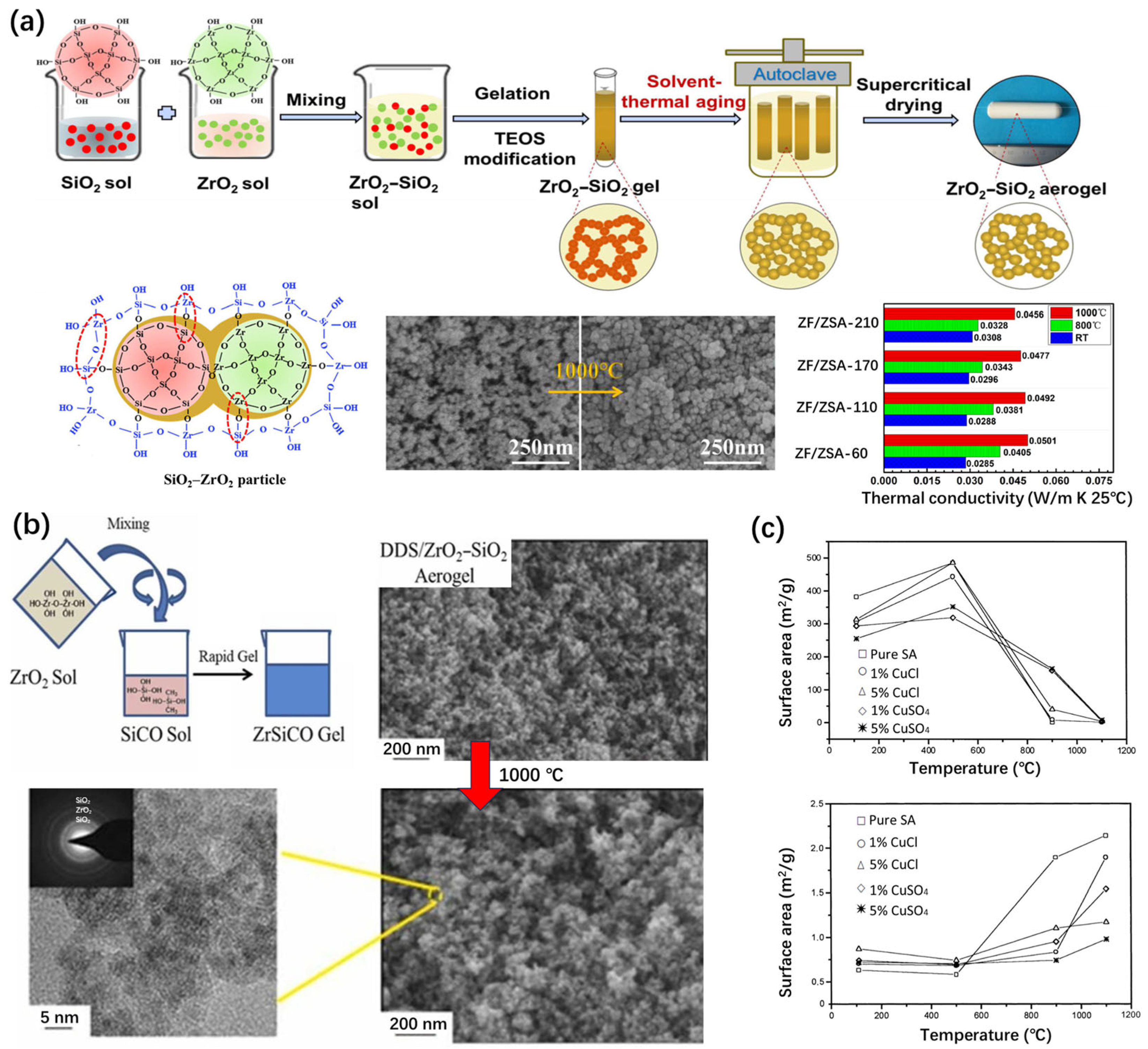
3.1.2. Zirconia-Doped Silica Aerogels
3.1.3. Silica Aerogels Doped with Other Elements
3.2. Construction of the Surface Heterostructure
3.2.1. The Sol–Gel Method
3.2.2. The Thin-Film Deposition Technique
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fricke, J.; Emmerling, A. Aerogels—Preparation, Properties, Applications. In Chemistry, Spectroscopy and Applications of Sol-Gel Glasses; Reisfeld, R., JJørgensen, C.K., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 37–87. ISBN 978-3-540-47588-0. [Google Scholar]
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Akimov, Y.K. Fields of application of aerogels (review). Instrum. Exp. Tech. 2003, 46, 287–299. [Google Scholar] [CrossRef]
- Jones, S.M. Aerogel: Space exploration applications. J. Sol-Gel Sci. Technol. 2006, 40, 351–357. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel: Synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Hegde, N.D.; Hirashima, H. Absorption and desorption of organic liquids in elastic superhydrophobic silica aerogels. J. Colloid Interface Sci. 2007, 305, 124–132. [Google Scholar] [CrossRef]
- Hamann, T.W.; Martinson, A.B.F.; Elam, J.W.; Pellin, M.J.; Hupp, J.T. Atomic layer deposition of TiO2 on aerogel templates: New photoanodes for dye-sensitized solar cells. J. Phys. Chem. C 2008, 112, 10303–10307. [Google Scholar] [CrossRef]
- Jung, S.-B.; Park, S.-W.; Yang, J.-K.; Park, H.-H.; Kim, H. Application of SiO2 aerogel film for interlayer dielectric on GaAs with a barrier of Si3N4. Thin Solid Film. 2004, 447–448, 580–585. [Google Scholar] [CrossRef]
- Li, C.; Chen, Z.; Dong, W.; Lin, L.; Zhu, X.; Liu, Q.; Zhang, Y.; Zhai, N.; Zhou, Z.; Wang, Y.; et al. A review of silicon-based aerogel thermal insulation materials: Performance optimization through composition and microstructure. J. Non-Cryst. Solids 2021, 553, 120517. [Google Scholar] [CrossRef]
- Caps, R.; Fricke, J. Infrared radiative heat transfer in highly transparent silica aerogel. Sol. Energy 1986, 36, 361–364. [Google Scholar] [CrossRef]
- Riffat, S.B.; Qiu, G. A Review of state-of-the-art aerogel applications in buildings. Int. J. Low-Carbon Technol. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Buratti, C.; Moretti, E.; Zinzi, M. High energy-efficient windows with silica aerogel for building refurbishment: Experimental characterization and preliminary simulations in different climate conditions. Buildings 2017, 7, 8. [Google Scholar] [CrossRef]
- Strobach, E.; Bhatia, B.; Yang, S.; Zhao, L.; Wang, E.N. High temperature stability of transparent silica aerogels for solar thermal applications. APL Mater. 2019, 7, 081104. [Google Scholar] [CrossRef]
- Bheekhun, N.; Abu Talib, A.R.; Hassan, M.R. Aerogels in aerospace: An overview. Adv. Mater. Sci. Eng. 2013, 2013, 406065. [Google Scholar] [CrossRef]
- Fricke, J.; Emmerling, A. Aerogels. J. Am. Ceram. Soc. 1992, 75, 2027–2035. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W.; Roth, E.P. Sol→gel→glass: II. Physical and structural evolution during constant feating rate experiments. J. Non-Cryst. Solids 1985, 72, 345–368. [Google Scholar] [CrossRef]
- Wang, C.; Bai, L.; Xu, H.; Qin, S.; Li, Y.; Zhang, G. A Review of high-temperature aerogels: Composition, mechanisms, and properties. Gels 2024, 10, 286. [Google Scholar] [CrossRef]
- Emmerling, A.; Lenhard, W.; Fricke, J.; Van De Vorst, G.A.L. Densification behaviour of silica aerogels upon isothermal sintering. J. Sol-Gel Sci. Technol. 1997, 8, 837–842. [Google Scholar] [CrossRef]
- Kuchta, L.; Fajnor, V.Š. About the synthesis and thermal stability of SiO2-aerogel. J. Therm. Anal. 1996, 46, 515–520. [Google Scholar] [CrossRef]
- Bouaziz, J.; Bourret, D.; Sivade, A.; Grill, C. Phase separation during isothermal sintering of (1 − x)SiO2-xLn2O3 aerogels. J. Non-Cryst. Solids 1992, 145, 71–74. [Google Scholar] [CrossRef]
- Wagh, P.B.; Pajonk, G.M.; Haranath, D.; Rao, A.V. Influence of temperature on the physical properties of citric acid catalyze TEOS silica aerogels. Mater. Chem. Phys. 1997, 50, 76–81. [Google Scholar] [CrossRef]
- Buscarino, G.; Ardizzone, V.; Vaccaro, G.; Gelardi, F.M. Sintering process of amorphous SiO2 nanoparticles investigated by AFM, IR and Raman techniques. J. Non-Cryst. Solids 2011, 357, 1866–1870. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Feng, J.; Chen, Q.; Zhang, S.; Li, L.; Feng, J. Nanostructure evolution of silica aerogels under rapid heating from 600 °C to 1300 °C via in-situ TEM observation. Ceram. Int. 2020, 46, 12489–12498. [Google Scholar] [CrossRef]
- Huang, D.; Guo, C.; Zhang, M.; Shi, L. Characteristics of nanoporous silica aerogel under high temperature from 950 °C to 1200 °C. Mater. Des. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Yang, M.Y.; Tang, G.H.; Sheng, Q.; Guo, L.; Zhang, H. Atomic-level sintering mechanism of silica aerogels at high temperatures: Structure evolution and solid thermal conductivity. Int. J. Heat Mass Transf. 2022, 199, 123456. [Google Scholar] [CrossRef]
- Marlière, C.; Despetis, F.; Etienne, P.; Woignier, T.; Dieudonné, P.; Phalippou, J. Very large-scale structures in sintered silica aerogels as evidenced by atomic force microscopy and ultra-small angle X-Ray scattering experiments. J. Non-Cryst. Solids 2001, 285, 148–153. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Chen, Q.; Zhang, S.; Li, L.; Feng, J.; Feng, J. Sintering behavior of SiO2 aerogel composites reinforced by mullite fibers via in-situ rapid heating TEM observations. J. Eur. Ceram. Soc. 2020, 40, 127–135. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Chen, G.; Feng, M.; Dai, H.; Yuan, B.; Chen, X. Effect of heat treatment on hydrophobic silica aerogel. J. Hazard. Mater. 2019, 362, 294–302. [Google Scholar] [CrossRef]
- Shuttleworth, R. The surface tension of solids. Proc. Phys. Soc. Sect. A 1950, 63, 444. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Wang, H. Densification and grain growth during sintering of nanosized particles. Int. Mater. Rev. 2008, 53, 326–352. [Google Scholar] [CrossRef]
- Lu, H.M.; Jiang, Q. Size-dependent surface energies of nanocrystals. J. Phys. Chem. B 2004, 108, 5617–5619. [Google Scholar] [CrossRef]
- Mazlan, M.R.; Jamadon, N.H.; Rajabi, A.; Sulong, A.B.; Mohamed, I.F.; Yusof, F.; Jamal, N.A. Necking mechanism under various sintering process parameters–A review. J. Mater. Res. Technol. 2023, 23, 2189–2201. [Google Scholar] [CrossRef]
- Scherer, G.W. Cell models for viscous sintering. J. Am. Ceram. Soc. 1991, 74, 1523–1531. [Google Scholar] [CrossRef]
- Sempéré, R.; Bourret, D.; Woignier, T.; Phalippou, J.; Jullien, R. Scaling approach to sintering of fractal matter. Phys. Rev. Lett. 1993, 71, 3307–3310. [Google Scholar] [CrossRef]
- Olivi-Tran, N.; Jullien, R. Numerical simulations of sintering, application to partially densified aerogels. J. Sol-Gel Sci. Technol. 1997, 8, 813–817. [Google Scholar] [CrossRef]
- Phalippou, J.; Despetis, F.; Calas, S.; Faivre, A.; Dieudonné, P.; Sempéré, R.; Woignier, T. Comparison between sintered and compressed aerogels. Opt. Mater. 2004, 26, 167–172. [Google Scholar] [CrossRef]
- Ristic, M.; Milosevic, S.D. Frenkel’s theory of sintering. Sci. Sinter. 2006, 38, 7–11. [Google Scholar] [CrossRef]
- Olevsky, E.A. Theory of sintering: From discrete to continuum. Mater. Sci. Eng. R Rep. 1998, 23, 41–100. [Google Scholar] [CrossRef]
- Saliger, R.; Heinrich, T.; Gleissner, T.; Fricke, J. Sintering behaviour of alumina-modified silica aerogels. J. Non-Cryst. Solids 1995, 186, 113–117. [Google Scholar] [CrossRef]
- Scherer, G.W. Sintering of low-density glasses: I, Theory. J. Am. Ceram. Soc. 1977, 60, 236–239. [Google Scholar] [CrossRef]
- Scherer, G.W. Viscous sintering with a pore-size distribution and rigid inclusions. J. Am. Ceram. Soc. 1988, 71, C447–C448. [Google Scholar] [CrossRef]
- Scherer, G.W.; Calas, S.; Sempéré, R. Densification kinetics and structural evolution during sintering of silica aerogel. J. Non-Cryst. Solids 1998, 240, 118–130. [Google Scholar] [CrossRef]
- Scherer, G.W.; Calas, S.; Sempéré, R. Sintering aerogels. J. Sol-Gel Sci. Technol. 1998, 13, 937–943. [Google Scholar] [CrossRef]
- de la Rosa-Fox, N.; Gago-Duport, L.; Esquivias, L. Aggregation process in silica aerogels on sintering. J. Non-Cryst. Solids 1995, 192–193, 534–538. [Google Scholar] [CrossRef]
- Jullien, R.; Olivi-Train, N.; Hasmy, A.; Woignier, T.; Phalippou, J.; Bourret, D.; Sempéré, R. Scaling theory and numerical simulations of aerogel sintering. J. Non-Cryst. Solids 1995, 188, 1–10. [Google Scholar] [CrossRef]
- Olivi-Tran, N.; Jullien, R. Numerical simulations of aerogel sintering. Phys. Rev. B 1995, 52, 258–267. [Google Scholar] [CrossRef]
- Tandon, P.; Rosner, D.E. Sintering kinetics and transport property evolution of large multi-particle aggregates. Chem. Eng. Commun. 1996, 151, 147–168. [Google Scholar] [CrossRef]
- Hawa, T.; Zachariah, M.R. Development of a phenomenological scaling law for fractal aggregate sintering from molecular dynamics simulation. J. Aerosol Sci. 2007, 38, 793–806. [Google Scholar] [CrossRef]
- Eggersdorfer, M.L.; Kadau, D.; Herrmann, H.J.; Pratsinis, S.E. Multiparticle sintering dynamics: From fractal-like aggregates to compact structures. Langmuir 2011, 27, 6358–6367. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Luo, T.; Liu, K. Evolution process of fault silica aerogel under high temperatures: A molecular dynamics approach. Gels 2024, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Ni, X.; Chen, J.; Zheng, H. Synthesis of Cu/SiO2 composite films via gamma-irradiation route and their optical absorption properties. Mater. Res. Bull. 2008, 43, 2421–2426. [Google Scholar] [CrossRef]
- Piao, L.; Li, Y.; Chen, J.; Chang, L.; Lin, J.Y.S. Methane decomposition to carbon nanotubes and hydrogen on an alumina supported nickel aerogel catalyst. Catal. Today 2002, 74, 145–155. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Saha, S.K.; Chakravorty, D. Silver nanowires grown in the pores of a silica gel. Appl. Phys. Lett. 2000, 77, 3770–3772. [Google Scholar] [CrossRef]
- Weiping, C.; Lide, Z. Synthesis and structural and optical properties of mesoporous silica containing silver nanoparticles. J. Phys. Condens. Matter 1997, 9, 7257. [Google Scholar] [CrossRef]
- Motojima, S.; Kawaguchi, M.; Nozaki, K.; Iwanaga, H. Preparation of coiled carbon fibers by catalytic pyrolysis of acetylene, and its morphology and extension characteristics. Carbon 1991, 29, 379–385. [Google Scholar] [CrossRef]
- Komarneni, S.; Roy, R.; Selvaraj, U.; Malla, P.B.; Breval, E. Nanocomposite aerogels: The SiO2–Al2O3 system. J. Mater. Res. 1993, 8, 3163–3167. [Google Scholar] [CrossRef]
- Rutiser, C.; Komarneni, S.; Roy, R. Composite aerogels of silica and minerals of different morphologies. Mater. Lett. 1994, 19, 221–224. [Google Scholar] [CrossRef]
- Aravind, P.R.; Mukundan, P.; Krishna Pillai, P.; Warrier, K.G.K. Mesoporous silica–alumina aerogels with high thermal pore stability through hybrid sol–gel route followed by subcritical drying. Microporous Mesoporous Mater. 2006, 96, 14–20. [Google Scholar] [CrossRef]
- Ling, X.; Li, B.; Li, M.; Hu, W.; Chen, W. Thermal stability of Al-modified silica aerogels through epoxide-assisted sol–gel route followed by ambient pressure drying. J. Sol-Gel Sci. Technol. 2018, 87, 83–94. [Google Scholar] [CrossRef]
- Yao, J.; Gao, X.; Wu, Y.; Zhao, X.; Li, X. High-temperature resistant ambient pressure-dried aluminum doped silica aerogel from inorganic silicon and aluminum sources. Ceram. Int. 2022, 48, 15006–15016. [Google Scholar] [CrossRef]
- Shalygin, A.S.; Kozhevnikov, I.V.; Gerasimov, E.Y.; Andreev, A.S.; Lapina, O.B.; Martyanov, O.N. The impact of Si/Al ratio on properties of aluminosilicate aerogels. Microporous Mesoporous Mater. 2017, 251, 105–113. [Google Scholar] [CrossRef]
- Hernandez, C.; Pierre, A.C. Evolution of the texture and structure of SiO2–Al2O3 xerogels and aerogels as a function of the Si to Al molar ratio. J. Sol-Gel Sci. Technol. 2001, 20, 227–243. [Google Scholar] [CrossRef]
- Tamon, H.; Sone, T.; Mikami, M.; Okazaki, M. Preparation and characterization of silica–titania and silica–alumina aerogels. J. Colloid Interface Sci. 1997, 188, 493–500. [Google Scholar] [CrossRef]
- Liu, R.; Dong, X.; Xie, S.; Jia, T.; Xue, Y.; Liu, J.; Jing, W.; Guo, A. Ultralight, thermal insulating, and high-temperature-resistant mullite-based nanofibrous aerogels. Chem. Eng. J. 2019, 360, 464–472. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Shalygin, A.S.; Pakharukova, V.P.; Glazneva, T.S.; Martyanov, O.N. Mesoporous aerogel-like Al-Si oxides obtained via supercritical antisolvent precipitation of alumina and silica sols. J. Supercrit. Fluids 2019, 149, 110–119. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Cui, S.; Wang, L.; Shen, X. Synthesis of a novel Al2O3–SiO2 composite aerogel with high specific surface area at elevated temperatures using inexpensive inorganic salt of aluminum. Ceram. Int. 2016, 42, 874–882. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Shen, X.; Cui, S.; Wang, L. Novel Al2O3–SiO2 composite aerogels with high specific surface area at elevated temperatures with different alumina/silica molar ratios prepared by a non-alkoxide sol–gel method. RSC Adv. 2016, 6, 5611–5620. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Wang, P.; Xu, B.; Ma, Y.; Wen, W.; Yang, Y.; Fang, D. Porous carbon-bonded carbon fiber composites impregnated with SiO2-Al2O3 aerogel with enhanced thermal insulation and mechanical properties. Ceram. Int. 2018, 44, 3484–3487. [Google Scholar] [CrossRef]
- Wu, X.; Ding, J.; Kong, Y.; Sun, Z.; Shao, G.; Li, B.; Wu, J.; Zhong, Y.; Shen, X.; Cui, S. Synthesis of a novel three-dimensional Na2SO4@SiO2@Al2O3-SiO2 phase change material doped aerogel composite with high thermal resistance and latent heat. Ceram. Int. 2018, 44, 21855–21865. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Ghica, M.E.; Durães, L. An overview on alumina-silica-based aerogels. Adv. Colloid Interface Sci. 2020, 282, 102189. [Google Scholar] [CrossRef] [PubMed]
- Garvie, R.C.; Chan, S.-K. Stability limits in the monoclinic-tetragonal transformations of zirconia. Phys. B+C 1988, 150, 203–211. [Google Scholar] [CrossRef]
- Hu, Z.; He, J.; Li, X.; Ji, H.; Su, D.; Qiao, Y. Improvement of thermal stability of ZrO2–SiO2 aerogels by an inorganic–organic synergetic surface modification. J. Porous Mater. 2017, 24, 657–665. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Zhi, M.; Hong, Z. Synthesis of high temperature resistant ZrO2-SiO2 composite aerogels via “Thiol-Ene” click reaction. J. Sol-Gel Sci. Technol. 2018, 87, 734–742. [Google Scholar] [CrossRef]
- Liu, B.; Gao, M.; Liu, X.; Zhao, X.; Zhang, J.; Yi, X. Thermally stable nanoporous ZrO2/SiO2 hybrid aerogels for thermal insulation. ACS Appl. Nano Mater. 2019, 2, 7299–7310. [Google Scholar] [CrossRef]
- Yu, H.; Tong, Z.; Qiao, Y.; Yang, Z.; Yue, S.; Li, X.; Su, D.; Ji, H. High thermal stability of SiO2–ZrO2 aerogels using solvent-thermal aging. J. Solid State Chem. 2020, 291, 121624. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Zhang, H.; Huang, S.; Wu, S.; Liang, Z. A three-dimensional network modifier (dimethyldiethoxysilane) makes ZrO2-SiO2 aerogel with excellent thermal insulation performance and high-temperature stability. Colloids Surf. Physicochem. Eng. Asp. 2023, 671, 131716. [Google Scholar] [CrossRef]
- de Sousa, E.M.B.; Porto, A.O.; Schilling, P.J.; Alves, M.C.M.; Mohallem, N.D.S. Study of the structural evolution of copper-doped porous silica gels. J. Phys. Chem. Solids 2000, 61, 853–861. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Huang, S.; Wang, Y.; Guan, X.; Liang, Z.; Ye, X. Rapid ambient pressure drying preparation of bulk ZrO2-SiO2 aerogels with high thermal stability. Ceram. Int. 2024, 50, 48011–48020. [Google Scholar] [CrossRef]
- Li, G.; Zhu, T.; Deng, Z.; Zhang, Y.; Jiao, F.; Zheng, H. Preparation of Cu-SiO2 composite aerogel by ambient drying and the influence of synthesizing conditions on the structure of the aerogel. Chin. Sci. Bull. 2011, 56, 685–690. [Google Scholar] [CrossRef]
- Kong, Y.; Zhong, Y.; Shen, X.; Hu, D.; Cui, S.; Teng, K.; Zhang, J. Preparation of monolithic C/SiO2 composite aerogels with low density. J. Nanjing Univ. Technol. Sci. Ed. 2012, 34, 6–10. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Y.; Shen, X.; Cui, S.; Kong, Y.; Ji, L.; Li, B. Properties and characterization of SiO2 monolithic aerogels doped with yttrium. Chin. J. Inorg. Chem. 2014, 30, 793–799. Available online: http://www.ccspublishing.org.cn/article/doi/10.11862/CJIC.2014.136?pageType=en (accessed on 24 January 2025).
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Surface chemical modification of silica aerogels using various alkyl-alkoxy/chloro silanes. Appl. Surf. Sci. 2003, 206, 262–270. [Google Scholar] [CrossRef]
- Parvathy Rao, A.; Venkateswara Rao, A. Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents. J. Mater. Sci. 2010, 45, 51–63. [Google Scholar] [CrossRef]
- Wu, G.; Yu, Y.; Cheng, X.; Zhang, Y. Preparation and surface modification mechanism of silica aerogels via ambient pressure drying. Mater. Chem. Phys. 2011, 129, 308–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Li, X.; Wang, L.; Nie, C. Facile preparation of a phenyl-reinforced flexible silica aerogel with excellent thermal stability and fire resistance. Mater. Chem. Front. 2021, 5, 4214–4224. [Google Scholar] [CrossRef]
- Si, Y.; Wang, X.; Dou, L.; Yu, J.; Ding, B. Ultralight and fire-resistant ceramic nanofibrous aerogels with temperature-invariant superelasticity. Sci. Adv. 2018, 4, eaas8925. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z.; Liu, B.; Zhang, F. Robust, highly thermally stable, core–shell nanostructured metal oxide aerogels as high-temperature thermal superinsulators, adsorbents, and catalysts. Chem. Mater. 2014, 26, 5761–5772. [Google Scholar] [CrossRef]
- Su, L.; Wang, H.; Niu, M.; Dai, S.; Cai, Z.; Yang, B.; Huyan, H.; Pan, X. Anisotropic and hierarchical SiC@SiO2 nanowire aerogel with exceptional stiffness and stability for thermal superinsulation. Sci. Adv. 2020, 6, eaay6689. [Google Scholar] [CrossRef]
- Tai, Y.; Tajiri, K. Preparation, thermal stability, and CO oxidation activity of highly loaded Au/titania-coated silica aerogel catalysts. Appl. Catal. A Gen. 2008, 342, 113–118. [Google Scholar] [CrossRef]
- Tai, Y.; Murakami, J.; Tajiri, K.; Ohashi, F.; Daté, M.; Tsubota, S. Oxidation of carbon monoxide on Au nanoparticles in titania and titania-coated silica aerogels. Appl. Catal. A Gen. 2004, 268, 183–187. [Google Scholar] [CrossRef]
- Oke, J.A.; Jen, T.-C. Atomic layer deposition and other thin film deposition techniques: From principles to film properties. J. Mater. Res. Technol. 2022, 21, 2481–2514. [Google Scholar] [CrossRef]
- Parsons, G.N.; George, S.M.; Knez, M. Progress and future directions for atomic layer deposition and ALD-based chemistry. MRS Bull. 2011, 36, 865–871. [Google Scholar] [CrossRef]
- Adomaitis, R.A. A ballistic transport and surface reaction model for simulating atomic layer deposition processes in high-aspect-ratio nanopores. Chem. Vap. Depos. 2011, 17, 353–365. [Google Scholar] [CrossRef]
- Elam, J.W. Coatings on high aspect ratio structures. In Atomic Layer Deposition of Nanostructured Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 227–249. ISBN 978-3-527-63991-5. [Google Scholar]
- Ylilammi, M.; Ylivaara, O.M.E.; Puurunen, R.L. Modeling growth kinetics of thin films made by atomic layer deposition in lateral high-aspect-ratio structures. J. Appl. Phys. 2018, 123, 205301. [Google Scholar] [CrossRef]
- Cremers, V.; Puurunen, R.L.; Dendooven, J. Conformality in atomic layer deposition: Current status overview of analysis and modelling. Appl. Phys. Rev. 2019, 6, 021302. [Google Scholar] [CrossRef]
- Yim, J.; Verkama, E.; Velasco, J.A.; Arts, K.; Puurunen, R.L. Conformality of atomic layer deposition in microchannels: Impact of process parameters on the simulated thickness profile. Phys. Chem. Chem. Phys. 2022, 24, 8645–8660. [Google Scholar] [CrossRef]
- Reiter, T.; Aguinsky, L.F.; Rodrigues, F.; Weinbub, J.; Hössinger, A.; Filipovic, L. Modeling the impact of incomplete conformality during atomic layer processing. Solid-State Electron. 2024, 211, 108816. [Google Scholar] [CrossRef]
- Peng, Q.; Sun, X.-Y.; Spagnola, J.C.; Hyde, G.K.; Spontak, R.J.; Parsons, G.N. Atomic layer deposition on electrospun polymer fibers as a direct route to Al2O3 microtubes with precise wall thickness control. Nano Lett. 2007, 7, 719–722. [Google Scholar] [CrossRef]
- Elam, J.W.; Libera, J.A.; Huynh, T.H.; Feng, H.; Pellin, M.J. Atomic layer deposition of aluminum oxide in mesoporous silica gel. J. Phys. Chem. C 2010, 114, 17286–17292. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Biener, J.; Wang, Y.M.; Baumann, T.F.; Wu, K.J.; van Buuren, T.; Hamza, A.V.; Satcher, J.H., Jr.; Elam, J.W.; Pellin, M.J. Atomic layer deposition of ZnO on ultralow-density nanoporous silica aerogel monoliths. Appl. Phys. Lett. 2005, 86, 083108. [Google Scholar] [CrossRef]
- Elam, J.W.; Xiong, G.; Han, C.Y.; Wang, H.H.; Birrell, J.P.; Welp, U.; Hryn, J.N.; Pellin, M.J.; Baumann, T.F.; Poco, J.F.; et al. Atomic layer deposition for the conformal coating of nanoporous materials. J. Nanomater. 2006, 2006, 064501. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Biener, J.; Baumann, T.F.; Wang, Y.M.; Hamza, A.V.; Li, Z.; Lee, D.K.; Gordon, R.G. Mechanisms of atomic layer deposition on substrates with ultrahigh aspect ratios. Langmuir 2008, 24, 943–948. [Google Scholar] [CrossRef]
- Mane, A.U.; Greene, J.P.; Nolen, J.A.; Sampathkumaran, U.; Owen, T.W.; Winter, R.; Elam, J.W. Refractory nanoporous materials fabricated using tungsten atomic layer deposition on silica aerogels. Appl. Surf. Sci. 2012, 258, 6472–6478. [Google Scholar] [CrossRef]
- Gayle, A.J.; Berquist, Z.J.; Chen, Y.; Hill, A.J.; Hoffman, J.Y.; Bielinski, A.R.; Lenert, A.; Dasgupta, N.P. Tunable atomic layer deposition into ultra-high-aspect-ratio (>60000:1) aerogel monoliths enabled by transport modeling. Chem. Mater. 2021, 33, 5572–5583. [Google Scholar] [CrossRef]
- Berquist, Z.J.; Gayle, A.J.; Dasgupta, N.P.; Lenert, A. Transparent refractory aerogels for efficient spectral control in high-temperature solar power generation. Adv. Funct. Mater. 2022, 32, 2108774. [Google Scholar] [CrossRef]
- Yang, S.; Strobach, E.; Bierman, D.; Zhao, L.; Bhatia, B.; Wang, E.N. Effect of Al2O3 ALD coating on thermal stability of silica aerogel. J. Porous Mater. 2022, 29, 193–200. [Google Scholar] [CrossRef]

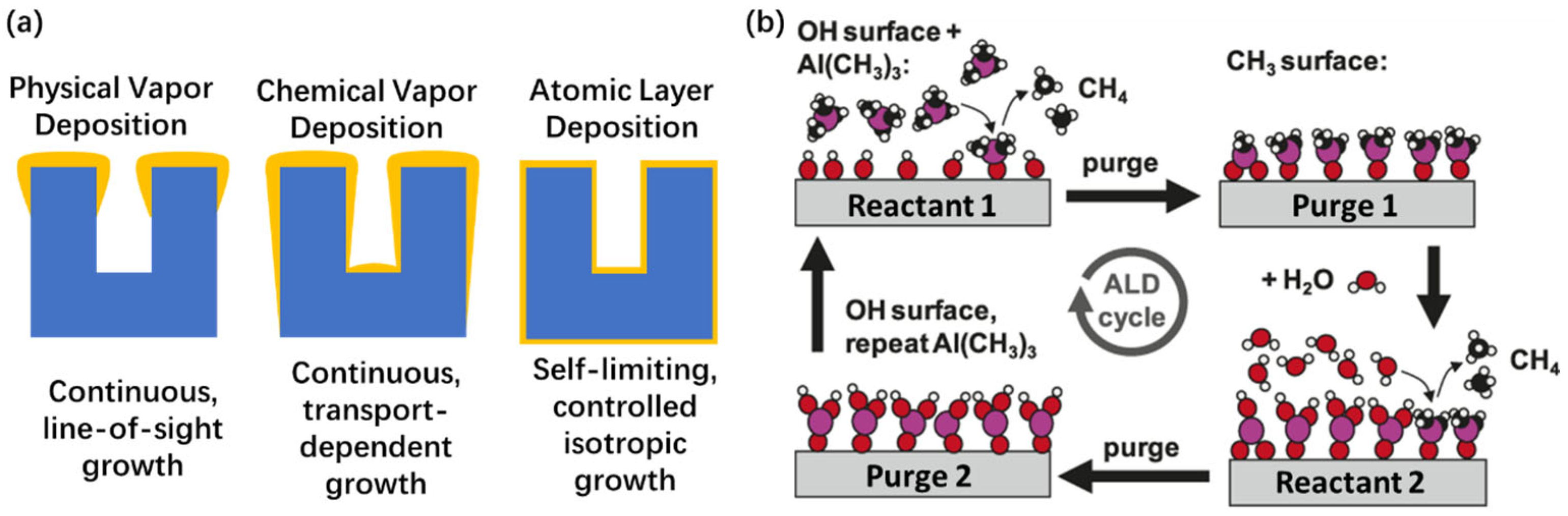

| Technique | PVD | CVD | ALD |
|---|---|---|---|
| Uniformity | ∼80 range | ∼10 range | ∼80 range |
| Conformity | <50% | <70% | <100% |
| Cleanliness | Particle | Particle | No particle |
| Deposition rate | Fast | Fast | Poor |
| Vacuum | High | High/Medium | Medium |
| Temperature range | Low | Low | Wide |
| Technology | ∼100 nm | ∼90–65 nm | No limit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhang, W.; Huang, H.; Li, W.; Ling, H.; Zhang, H. A Review of High-Temperature Resistant Silica Aerogels: Structural Evolution and Thermal Stability Optimization. Gels 2025, 11, 357. https://doi.org/10.3390/gels11050357
Zhu Z, Zhang W, Huang H, Li W, Ling H, Zhang H. A Review of High-Temperature Resistant Silica Aerogels: Structural Evolution and Thermal Stability Optimization. Gels. 2025; 11(5):357. https://doi.org/10.3390/gels11050357
Chicago/Turabian StyleZhu, Zhenyu, Wanlin Zhang, Hongyan Huang, Wenjing Li, Hao Ling, and Hao Zhang. 2025. "A Review of High-Temperature Resistant Silica Aerogels: Structural Evolution and Thermal Stability Optimization" Gels 11, no. 5: 357. https://doi.org/10.3390/gels11050357
APA StyleZhu, Z., Zhang, W., Huang, H., Li, W., Ling, H., & Zhang, H. (2025). A Review of High-Temperature Resistant Silica Aerogels: Structural Evolution and Thermal Stability Optimization. Gels, 11(5), 357. https://doi.org/10.3390/gels11050357





