High-Quality Application of Crayfish Muscle in Surimi Gels: Fortification of Blended Gels by Transglutaminase
Abstract
1. Introduction
2. Results and Discussion
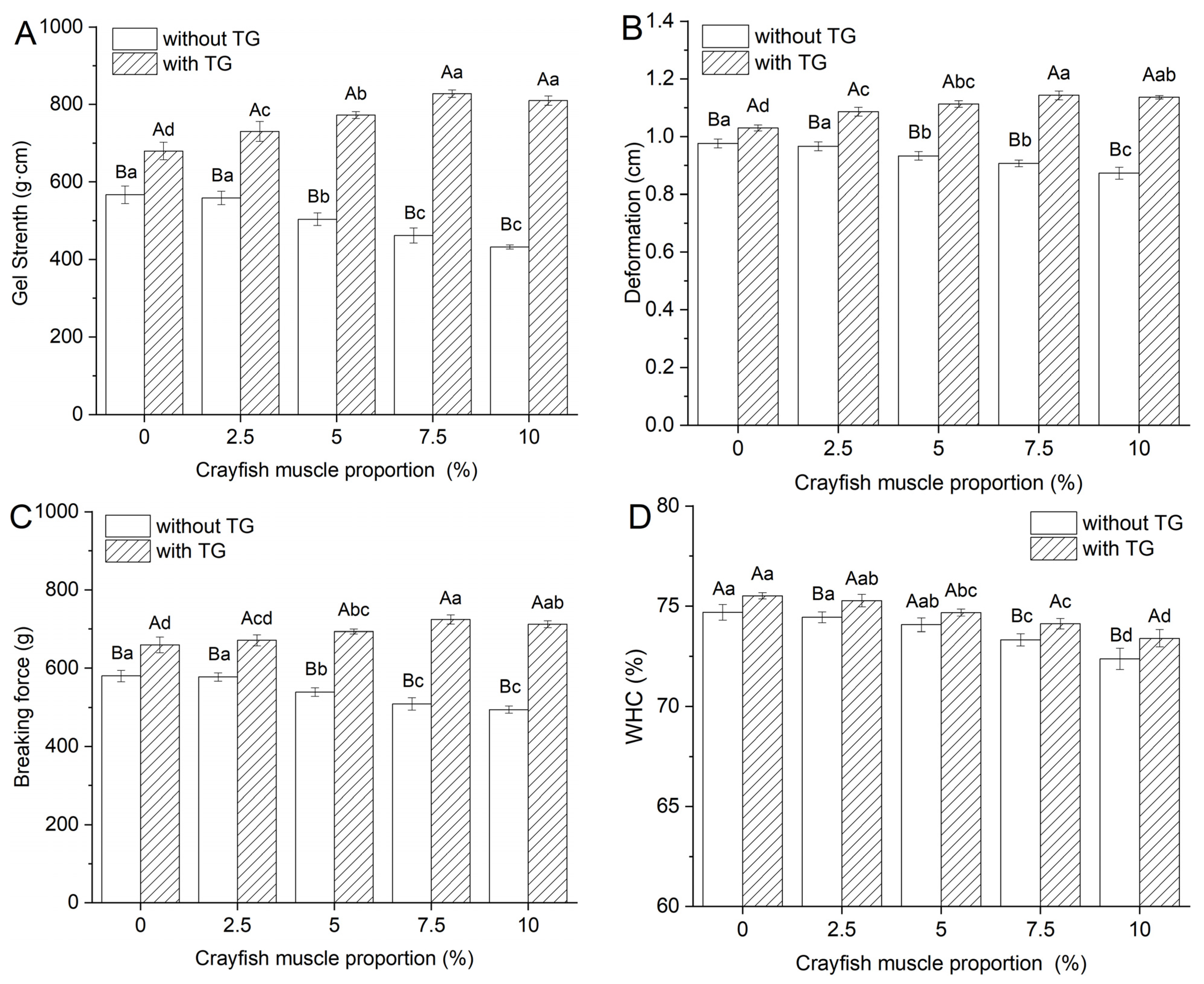
2.1. Effect of TGase and Crayfish Meat on the Texture Properties of Blended Gels
2.1.1. Texture Properties
2.1.2. Effect of TGase and Crayfish Muscle on the Water Holding Capacity of Blended Gels
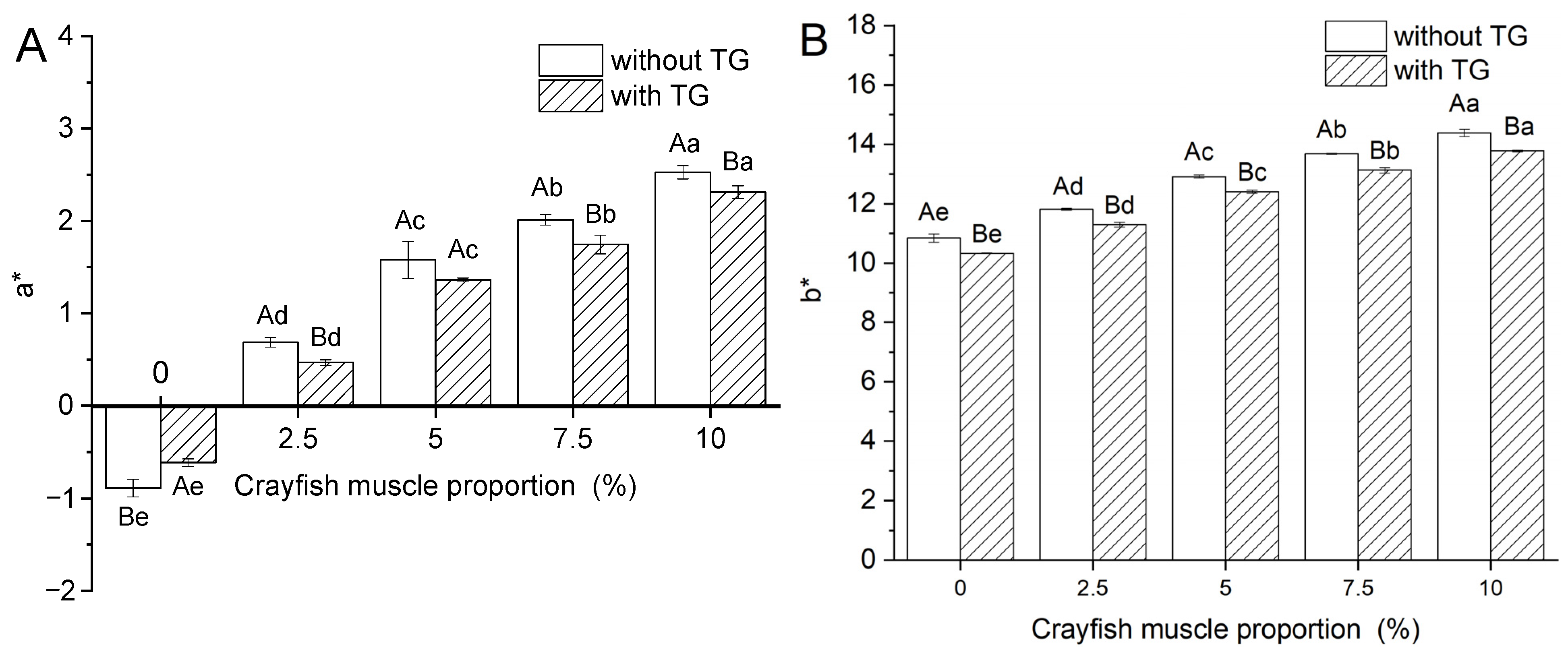
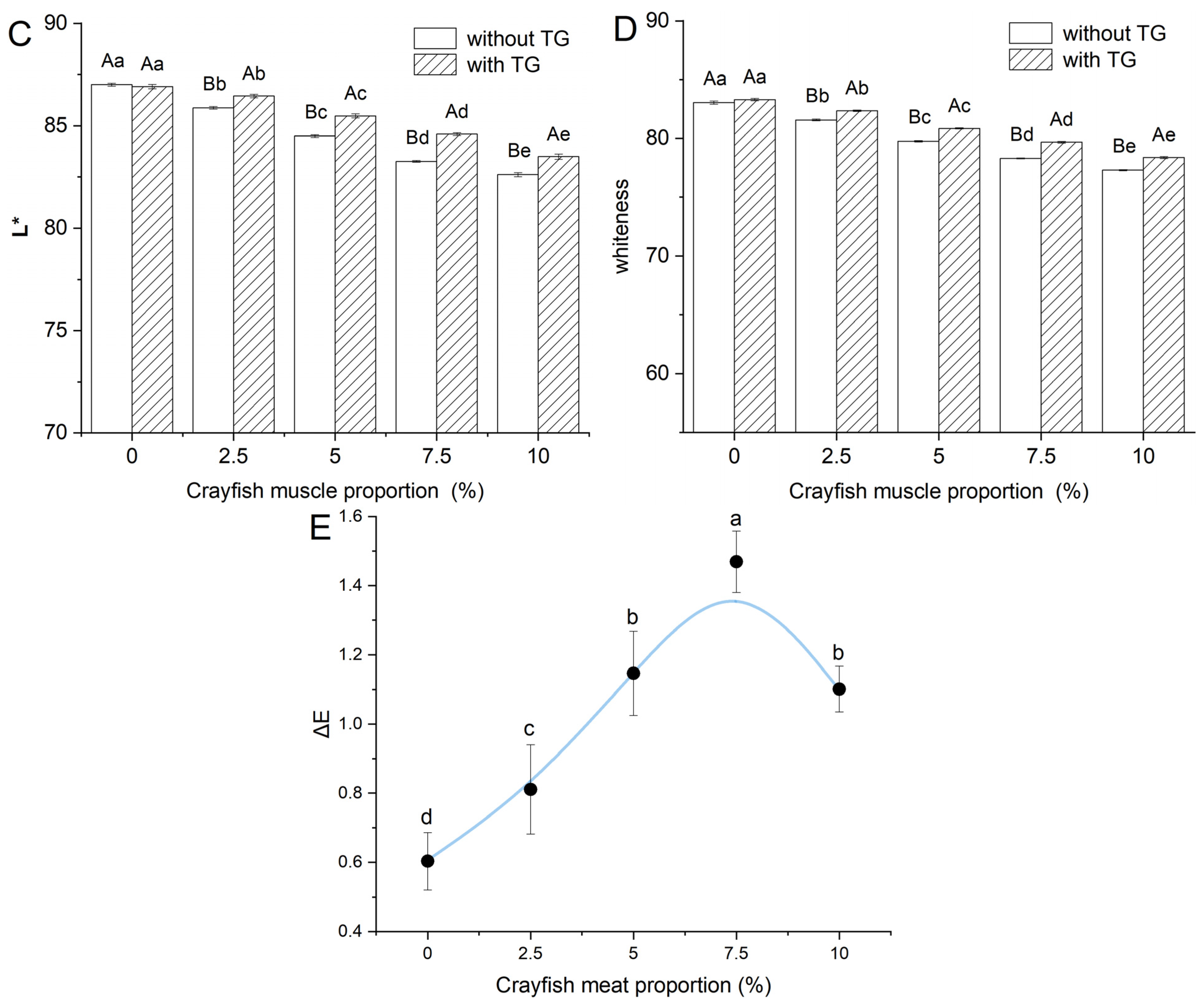
2.2. Effect of TGase and Crayfish Muscle on the Color Properties of Blended Gels
2.3. Effect of TGase and Crayfish Muscle on the Water Distribution of Blended Gels
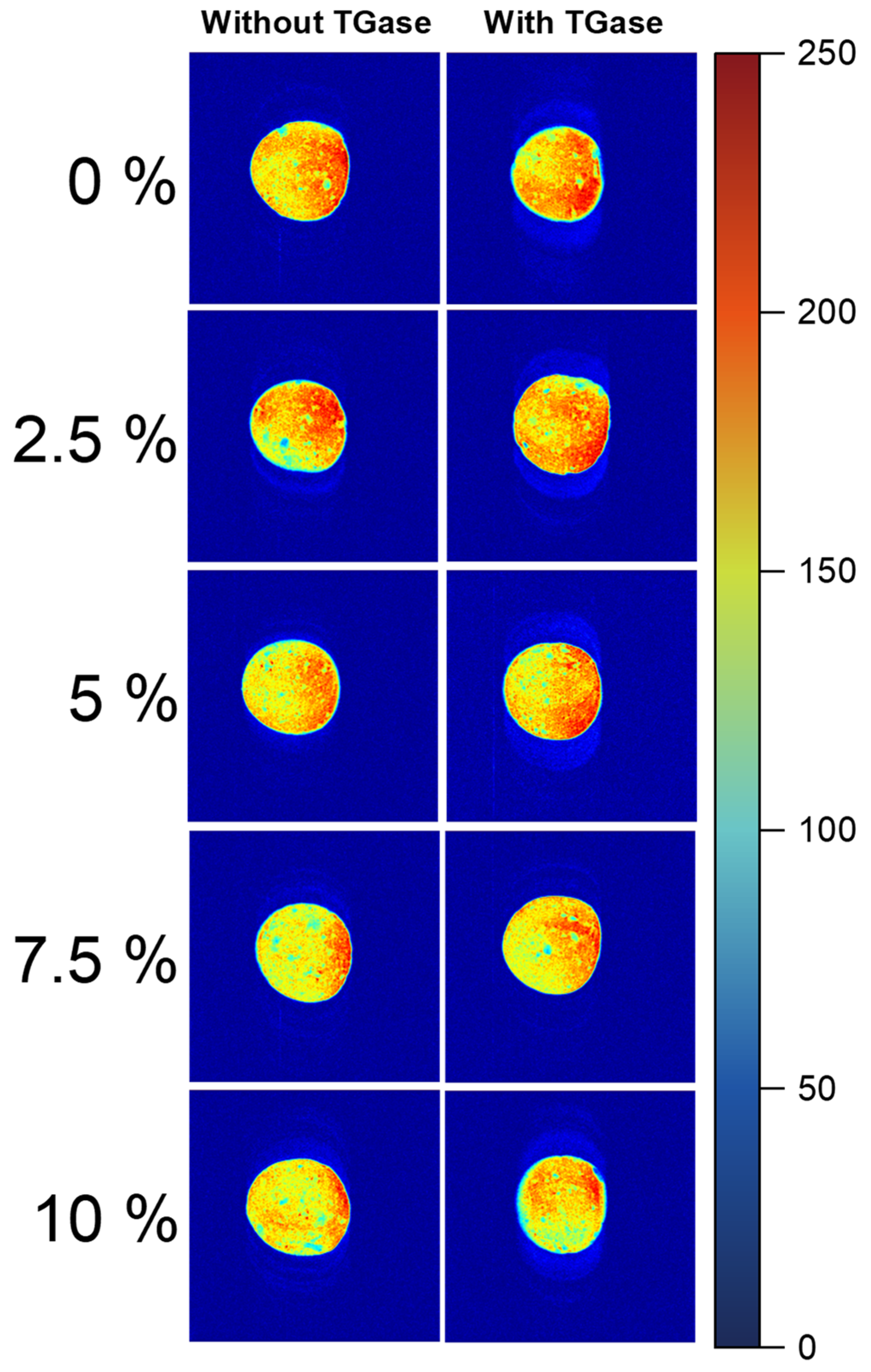
2.4. Effect of TGase and Crayfish Muscle on the Magnetic Resonance Imaging of Blended Gels
2.5. Effect of TGase and Crayfish Muscle on the Microstructural of Blended Gels
2.6. Effect of TGase and Crayfish Muscle on the Chemical Interactions of Blended Gels
2.7. Effect of TGase and Crayfish Muscle on the Total Sulfhydryl Content of Blended Gels
2.8. Effect of TGase and Crayfish Muscle on the Fourier Transform Infrared Spectroscopy of Blended Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Surimi and Crayfish Muscle Blended Gels
4.3. Gel Strength
4.4. WHC
4.5. Whiteness
4.6. Low-Field Nuclear Magnetic Resonance
4.7. Magnetic Resonance Imaging
4.8. SEM
4.9. Chemical Interactions
4.10. Total Sulfhydryl Content
4.11. FTIR
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harlioğlu, M.M.; Harlioğlu, A.G. Threat of Non-Native Crayfish Introductions into Turkey: Global Lessons. Rev. Fish Biol. Fish. 2006, 16, 171–181. [Google Scholar] [CrossRef]
- Bureau of Fisheries Management, Chinese Ministry of Agriculture and Rural Affairs. China Fishery Statistics Yearbook 2024; China Agriculture Press: Beijing, China, 2024; pp. 24, 89. ISBN 978-7-109-32126-7.
- Adegbusi, H.S.; Ismail, A.; Mohd. Esa, N.; Daud, Z.A.M. Effects of Formulated Nigerian Yellow Maize, Soybean, and Crayfish Blends on Some Growth Performance and Physiological Status. Food Prod. Process. Nutr. 2023, 5, 14. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, K.; Chen, J.; Wei, G.; Li, J.; Zheng, B.; Song, Y.; Gao, P.; Zhou, R. Enhancement of Myofibrillar Protein Gelation by Plant Proteins for Improved Surimi Gel Characteristics: Mechanisms and Performance. LWT 2024, 198, 116045. [Google Scholar] [CrossRef]
- Lee, Y.E.J.; Goh, H.M.; Huang, D.-G. Pea Protein Soften Surimi Gels and Improve In Vitro Digestibility: Potential High Protein Foods for Elderly. Food Hydrocoll. 2024, 159, 110664. [Google Scholar] [CrossRef]
- Liang, F.; Lin, L.; He, T.; Zhou, X.; Jiang, S.; Lu, J. Effect of Transglutaminase on Gel Properties of Surimi and Precocious Chinese Mitten Crab (Eriocheir sinensis) Meat. Food Hydrocoll. 2020, 98, 105261. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Lu, J.; Yang, M.; Zhang, M.; Lin, L.; Wang, C. Microwave-Mediated Fibrous Blended Gels of Crab Meat and Surimi: Fibrous Protein Production Method in the Perspective of Structural Remodeling. Innov. Food Sci. Emerg. Technol. 2024, 100, 103910. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, L.; Gao, P.; Yu, P.; Yang, F.; Yu, D.; Chen, H.; Xia, W. Study on the Effect and Mechanism of Chicken Breast on the Gel Properties of Silver Carp (Hypophthalmichtys molitrix) Surimi. J. Sci. Food Agric. 2023, 104, 1132–1142. [Google Scholar] [CrossRef]
- Niu, F.; Li, X.; Lin, C.; Hu, X.; Zhang, B.; Pan, W. The Mechanism of Egg White Protein to Enhance the Thermal Gel Properties of Giant Squid (Dosidicus gigas) Surimi. Food Chem. 2024, 469, 142601. [Google Scholar] [CrossRef] [PubMed]
- Rawdkuen, S.; Benjakul, S.; Visessanguan, W.; Lanier, T.C. Chicken Plasma Protein Affects Gelation of Surimi from Bigeye Snapper (Priacanthus tayenus). Food Hydrocoll. 2004, 18, 259–270. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Wang, X. Effects of Hydrolyzed Wheat Gluten on the Properties of High-Temperature (≥100 °C) Treated Surimi Gels. Food Hydrocoll. 2015, 45, 196–202. [Google Scholar] [CrossRef]
- Shafiee, S.; Goli, M.; Khoshkhoo, Z.; Hosseini, S.E. Optimization of Hydrolysis Conditions (Temperature, Time, and Concentration of Alkalase) of Rainbow Trout Viscera Using the Response Surface Methodology. J. Food Process. Preserv. 2021, 45, e15456. [Google Scholar] [CrossRef]
- Li, Q.; Ye, T.; Zhu, Y.; Xia, L.; Lin, L.; Lu, J. Sustainable Development of Fishery Resources: Precipitation of Protein from Surimi Rinsing Wastewater by Low-Temperature Plasma. Food Chem. 2024, 463 Pt 2, 141286. [Google Scholar] [CrossRef]
- Luan, D.; Wang, C.; Li, S.; Xue, Q.; Wang, X.; Xue, C.; Wang, Y. Improving Gel Properties of Low-Salt Silver Carp Surimi through Single-Mode Microwave-Assisted Processing. Innov. Food Sci. Emerg. Technol. 2024, 97, 103841. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, M.; Ai, C.; Cao, H.; Xiao, J.; Imran, M.; Chen, L.; Teng, H. Ultrasonic Treatment Combined with Curdlan Improves the Gelation Properties of Low-Salt Nemipterus Virgatus Surimi. Int. J. Biol. Macromol. 2023, 248, 125899. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Ma, J.; Yang, K.; Feng, X.; You, X.; Wang, S.; Zhang, Y.; Xiong, G.; Wang, L.; et al. Effects of Radio Frequency Heating on Water Distribution and Structural Properties of Grass Carp Myofibrillar Protein Gel. Food Chem. 2020, 343, 128557. [Google Scholar] [CrossRef]
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation Characteristics of Tropical Surimi under Water Bath and Ohmic Heating. LWT 2012, 46, 97–103. [Google Scholar] [CrossRef]
- Piao, X.; Li, J.; Zhao, Y.; Guo, L.; Zheng, B.; Zhou, R.; Ostrikov, K. Oxidized Cellulose Nanofibrils-Based Surimi Gel Enhancing Additives: Interactions, Performance and Mechanisms. Food Hydrocoll. 2022, 133, 107893. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Teng, H. Phase Behavior of the Gelation Process of Myofibrillar Protein-Curdlan Blended System: Discussion Based on Rheology and Gel Properties. Food Chem. 2023, 437, 137839. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Ding, Y.; Xiang, X.; Yang, Q.; Wei, Z.; Song, H.; Liu, S.; Zhou, X. Improved Texture Properties and Toughening Mechanisms of Surimi Gels by Double Network Strategies. Food Hydrocoll. 2024, 152, 109900. [Google Scholar] [CrossRef]
- Shi, T.; Yuan, L.; Kong, Y.; Bao, Y.; Zhang, H.; Lu, C.; Jin, W.; Monto, A.R.; Gao, R. Towards Higher-Quality Low Salt Surimi Gels: Significance of the Combinatorial Effects of Chickpea Protein with Transglutaminase on Their Micro-Structures. LWT 2024, 199, 116103. [Google Scholar] [CrossRef]
- Yu, N.; Yang, F.; Gong, H.; Zhou, J.; Jie, C.; Wang, W.; Chen, X.; Sun, L. Gel & Three-Dimensional Printing Properties of Sheep Plasma Protein-Surimi Induced by Transglutaminase. J. Food Eng. 2022, 323, 111006. [Google Scholar] [CrossRef]
- Li, Q.; Yi, S.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Different Thermal Treatment Methods and TGase Addition Affect Gel Quality and Flavour Characteristics of Decapterus maruadsi Surimi Products. Foods 2021, 11, 66. [Google Scholar] [CrossRef]
- Martínez, M.A.; Robledo, V.; Velazquez, G.; Ramírez, J.A.; Vázquez, M.; Uresti, R.M. Effect of Precooking Temperature and Microbial Transglutaminase on the Gelling Properties of Blue Crab (Callinectes sapidus) Proteins. Food Hydrocoll. 2014, 35, 264–269. [Google Scholar] [CrossRef]
- Huang, P.-H.; Cheng, Y.-T.; Hsieh, H.-C.; Ko, W.-C.; Lu, W.-C.; Li, P.-H. Effects of Transglutaminase on the Physicochemical Properties of Surimi and Kamaboko Prepared by Thermal and Low Level-Pressure Treatments. LWT 2023, 183, 114863. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Y.; Wu, C.; Yuan, C.; Ishimura, G.; Liu, W.; Chen, S. γ-PGA and MTGase Improve the Formation of ε-(γ-Glutamyl) Lysine Cross-Links within Hairtail (Trichiurus haumela) Surimi Protein. Food Chem. 2018, 242, 330–337. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Lu, M.; Ai, C.; Cao, H.; Xiao, J.; Zhong, S.; Teng, H. Effect of Cellulose on Gel Properties of Heat-Induced Low-Salt Surimi Gels: Physicochemical Characteristics, Water Distribution and Microstructure. Food Chem. X 2023, 19, 100820. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, N.; Wei, W.; Hu, X.; Tan, Y.; Yu, Y.; Deng, Y.; Bi, C.; Zhang, L.; Zhang, H. Assessing the Dynamic Extrusion-Based 3D Printing Process for Power-Law Fluid Using Numerical Simulation. J. Food Eng. 2020, 275, 109861. [Google Scholar] [CrossRef]
- Terwilliger, N.B.; Ryan, M.C.; Towle, D. Evolution of Novel Functions: Cryptocyanin Helps Build New Exoskeleton in Cancer magister. J. Exp. Biol. 2005, 208, 2467–2474. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Y.; Yuan, X.; Zhao, Y.; Kang, Z.; Zhu, M.; Ma, H. Effect of Low-Frequency Alternating Magnetic Field on the Rheological Properties, Water Distribution and Microstructure of Low-Salt Pork Batters. LWT 2022, 159, 113164. [Google Scholar] [CrossRef]
- Li, J.; Munir, S.; Yu, X.; Yin, T.; You, J.; Liu, R.; Xiong, S.; Hu, Y. Double-Crosslinked Effect of TGase and EGCG on Myofibrillar Proteins Gel Based on Physicochemical Properties and Molecular Docking. Food Chem. 2021, 345, 128655. [Google Scholar] [CrossRef]
- Mi, H.; Zhao, Y.; Li, Y.; Chen, J.; Liu, H.; Yi, S.; Li, X.; Li, J. Combining Effect of Soybean Protein Isolate and Transglutaminase on the Gel Properties of Zhikong Scallop (Chlamys farreri) Adductor Muscle. LWT 2021, 138, 110727. [Google Scholar] [CrossRef]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined Use of Rheology, LF-NMR, and MRI for Characterizing the Gel Properties of Hairtail Surimi with Potato Starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Feng, J.; Liu, Y.; Suo, R.; Jin, J.; Wang, W. Ultrasonic Pretreatment Improves the Gelation Properties of Low-Salt Penaeus Vannamei (Litopenaeus vannamei) Surimi. Ultrason. Sonochem. 2022, 86, 106031. [Google Scholar] [CrossRef]
- Du, J.; Zhou, C.; Xia, Q.; Wang, Y.; Geng, F.; He, J.; Sun, Y.; Pan, D.; Cao, J. The Effect of Fibrin on Rheological Behavior, Gelling Properties and Microstructure of Myofibrillar Proteins. LWT 2022, 153, 112457. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, N.; Gao, P.; Yu, D.; Yang, F.; Xu, Y.; Xia, W. Influence of L-Arginine Addition on the Gel Properties of Reduced-Salt White Leg Shrimp (Litopenaeus vannamei) Surimi Gel Treated with Microbial Transglutaminase. LWT 2023, 173, 114310. [Google Scholar] [CrossRef]
- Jia, R.; Jiang, Q.; Kanda, M.; Tokiwa, J.; Nakazawa, N.; Osako, K.; Okazaki, E. Effects of Heating Processes on Changes in Ice Crystal Formation, Water Holding Capacity, and Physical Properties of Surimi Gels during Frozen Storage. Food Hydrocoll. 2019, 90, 254–265. [Google Scholar] [CrossRef]
- Kang, S.; Shao, Y.; Li, Z.; Chang, W.; Song, J.; Hu, Y.; Li, S.; Luan, G. Mechanism of Transglutaminase on Film-Forming and Structural Properties of Soybean Protein and Its Fractions: A Study in Different pH Environments. Food Hydrocoll. 2024, 157, 110394. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in Chemical Interactions and Gel Properties of Heat-induced Surimi Gels from Silver Carp (Hypophthalmichthys molitrix) Fillets During Setting and Heating: Effects of Different Washing Solutions. Food Hydrocoll. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Guan, Z.; Jia, Z.; Zhang, Z.; Yang, C.; Wang, J. Transglutaminase-Crosslinked Cold-Set Pea Protein Isolate Gels Modified by pH Shifting: Properties, Structure and Formation Mechanisms. Food Hydrocoll. 2024, 154, 110158. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, X.; Hu, N.; Zhao, C.; Wu, Y.; Liu, J. Study on Properties of TGase-Induced Pea Protein–Zein Complex Gels. J. Food Eng. 2023, 354, 111578. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, X.; Guo, K.; Li, S.; Li, T. Effects of TGase on the Rheological Behaviors, Structural Properties and Molecular Forces of Cowpea Protein Isolate and Cowpea Albumin Gels. Int. J. Biol. Macromol. 2024, 291, 139154. [Google Scholar] [CrossRef]
- Shuai, X.; Gao, L.; Geng, Q.; Li, T.; He, X.; Chen, J.; Liu, C.; Dai, T. Effects of Moderate Enzymatic Hydrolysis on Structure and Functional Properties of Pea Protein. Foods 2022, 11, 2368. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Tang, S.; Mi, S.; Lu, L.; Zeng, Q.-Y.; Xia, M.; Cai, Z. Improved Effect of Ultrasound-Assisted Enzymolysis on Egg Yolk Powder: Structural Properties, Hydration Properties and Stability Characteristics. Food Chem. 2022, 382, 132549. [Google Scholar] [CrossRef]
- Li, J.; Munir, S.; Yu, X.; Yin, T.; You, J.; Liu, R.; Xiong, S.; Hu, Y. Interaction of Myofibrillar Proteins and Epigallocatechin Gallate in the Presence of Transglutaminase in Solutions. Food Funct. 2020, 11, 9560–9572. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Xavier, K.A.M.; Nayak, B.B.; Dhanapal, K.; Balange, A.K. Instrumental Quality Attributes of Single Washed Surimi Gels of Tilapia: Effect of Different Washing Media. LWT 2017, 86, 385–392. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Weng, W.; Yang, H.; Li, B. Characteristics and Application of Fish Oil-in-Water Pickering Emulsions Structured with Tea Water-Insoluble Proteins/κ-Carrageenan Complexes. Food Hydrocoll. 2021, 114, 106562. [Google Scholar] [CrossRef]
- Wei, W.; Hu, W.; Zhang, X.-Y.; Zhang, F.-P.; Sun, S.-Q.; Liu, Y.; Xu, C.-H. Analysis of Protein Structure Changes and Quality Regulation of Surimi during Gelation Based on Infrared Spectroscopy and Microscopic Imaging. Sci. Rep. 2018, 8, 5566. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Effects of Emulsified Lard and TGase on Gel Properties of Threadfin Bream (Nemipterus virgatus) Surimi. LWT 2021, 146, 111513. [Google Scholar] [CrossRef]
- Oujifard, A.; Benjakul, S.; Prodpran, T.; Seyfabadi, J. Properties of Red Tilapia (Oreochromis niloticus) Protein Based Film as Affected by Cryoprotectants. Food Hydrocoll. 2013, 32, 245–251. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, Y.; Ye, T.; Nie, Y.; Jiang, S.; Lin, L.; Lu, J. Physicochemical Properties and Microstructure of Composite Surimi Gels: The Effects of Ultrasonic Treatment and Olive Oil Concentration. Ultrason. Sonochem. 2022, 88, 106065. [Google Scholar] [CrossRef]
- Dong, M.; Sun, Y.; Xiong, D.-M.; Song, Q.; Jia, J.; Liu, X.; Sheng, L.; Duan, X. Comparison of the Effects of pH-Shifting, Acetic Acid Modification, and TGase Treatment on the Physicochemical and Functional Properties of Wheat Gluten Protein. Food Bioprocess Technol. 2023, 17, 245–256. [Google Scholar] [CrossRef]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar Protein Conformation Enhance Gel Properties of Mixed Surimi Gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Fan, D.; Huang, L.; Li, B.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Acoustic Intensity in Ultrasound Field and Ultrasound-Assisted Gelling of Surimi. LWT 2017, 75, 497–504. [Google Scholar] [CrossRef]
- Herrero, A.M.; Cambero, M.I.; Ordóñez, J.A.; De La Hoz, L.; Carmona, P. Raman Spectroscopy Study of the Structural Effect of Microbial Transglutaminase on Meat Systems and Its Relationship with Textural Characteristics. Food Chem. 2008, 109, 25–32. [Google Scholar] [CrossRef]
- Gao, X.; Xie, Y.; Yin, T.; Hu, Y.; You, J.; Xiong, S.; Liu, R. Effect of High Intensity Ultrasound on Gelation Properties of Silver Carp Surimi with Different Salt Contents. Ultrason. Sonochem. 2021, 70, 105326. [Google Scholar] [CrossRef]
- Ma, Y.; Shan, A.; Wang, R.; Zhao, Y.; Chi, Y. Characterization of Egg White Powder Gel Structure and Its Relationship with Gel Properties Influenced by Pretreatment with Dry Heat. Food Hydrocoll. 2021, 110, 106149. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical Interactions Involved In Microwave Heat-induced Surimi Gel Fortified with Fish Oil and Its Formation Mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]


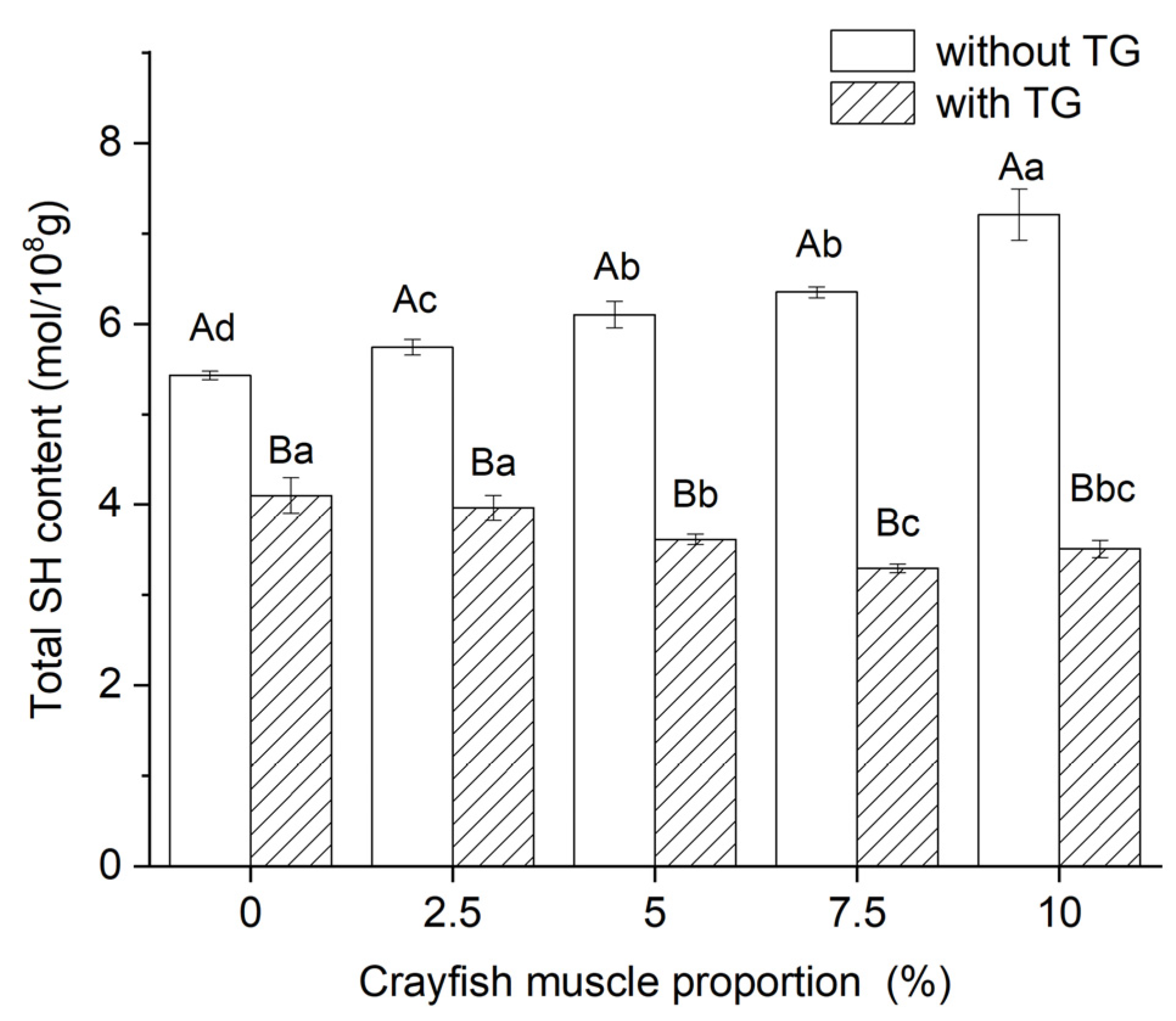
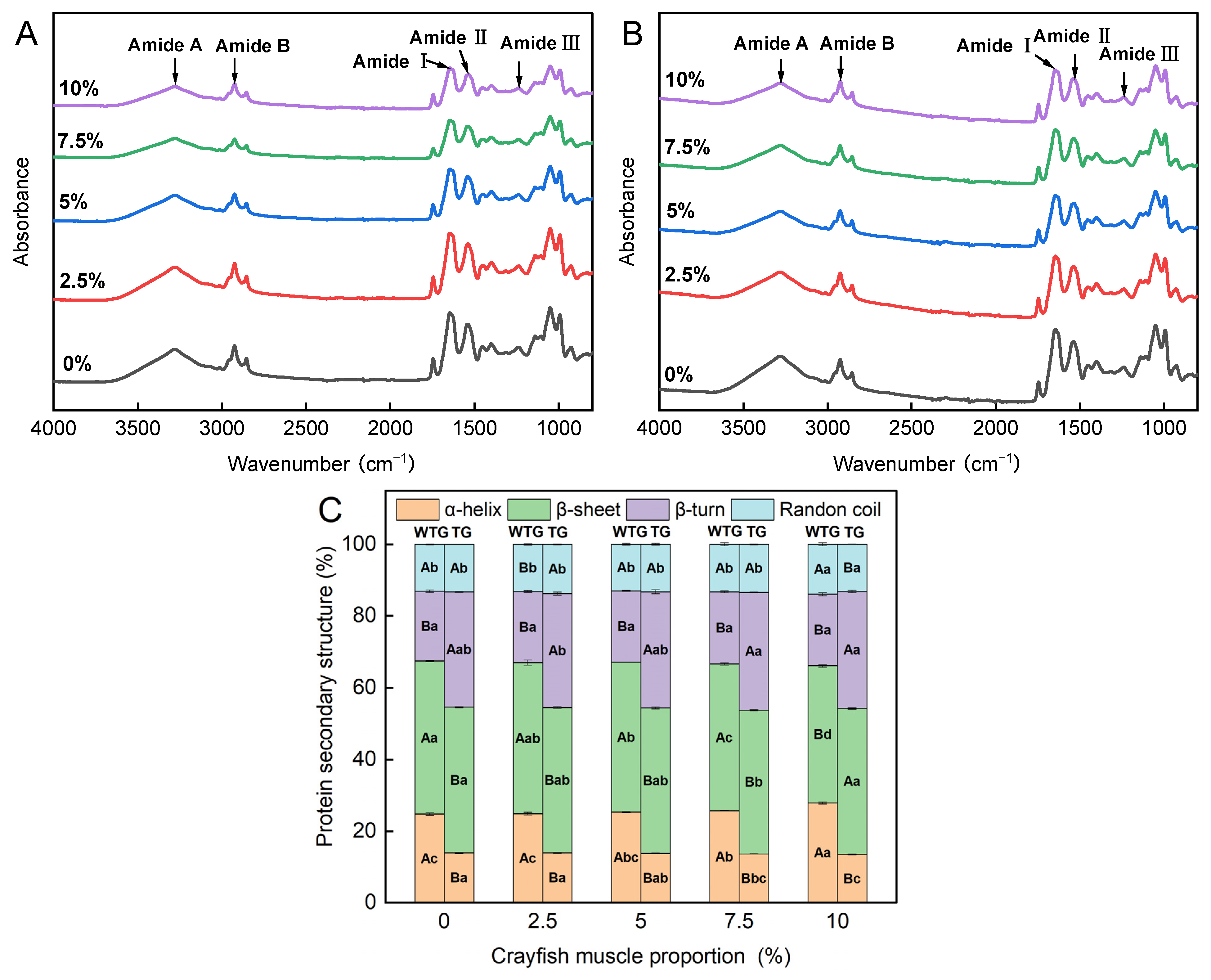
| Crayfish Muscle Content (%) | Hydrogen Bonds | Ionic Bonds | Hydrophobic Interactions | |||
|---|---|---|---|---|---|---|
| Without TGase | With TGase | Without TGase | With TGase | Without TGase | With TGase | |
| 0 | 1.66 ± 0.04 Ba | 2.31 ± 0.12 Aa | 0.88 ± 0.03 Ae | 0.35 ± 0.04 Be | 21.99 ± 0.32 Aa | 20.1 ± 0.33 Bd |
| 2.5 | 1.27 ± 0.10 Bb | 2.09 ± 0.11 Ab | 1.25 ± 0.08 Ad | 0.66 ± 0.02 Bd | 20.21 ± 0.23 Bb | 21.1 ± 1.04 Ac |
| 5 | 0.97 ± 0.13 Bc | 1.74 ± 0.06 Ac | 1.60 ± 0.10 Ac | 0.95 ± 0.08 Bc | 18.1 ± 0.33 Bc | 22.82 ± 1.54 Ab |
| 7.5 | 0.47 ± 0.16 Bd | 1.37 ± 0.03 Ad | 2.04 ± 0.15 Ab | 1.27 ± 0.05 Bb | 16.55 ± 0.28 Bd | 25.6 ± 0.74 Aa |
| 10 | 0.21 ± 0.10 Be | 0.85 ± 0.05 Ae | 2.34 ± 0.12 Aa | 1.69 ± 0.03 Ba | 14.16 ± 0.9 Be | 25.62 ± 0.36 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Q.; Yang, M.; Wang, H.; Wang, M.; Lin, L.; Lu, J. High-Quality Application of Crayfish Muscle in Surimi Gels: Fortification of Blended Gels by Transglutaminase. Gels 2025, 11, 204. https://doi.org/10.3390/gels11030204
Wang H, Li Q, Yang M, Wang H, Wang M, Lin L, Lu J. High-Quality Application of Crayfish Muscle in Surimi Gels: Fortification of Blended Gels by Transglutaminase. Gels. 2025; 11(3):204. https://doi.org/10.3390/gels11030204
Chicago/Turabian StyleWang, Hongyi, Qiang Li, Mengru Yang, Hong Wang, Mengtao Wang, Lin Lin, and Jianfeng Lu. 2025. "High-Quality Application of Crayfish Muscle in Surimi Gels: Fortification of Blended Gels by Transglutaminase" Gels 11, no. 3: 204. https://doi.org/10.3390/gels11030204
APA StyleWang, H., Li, Q., Yang, M., Wang, H., Wang, M., Lin, L., & Lu, J. (2025). High-Quality Application of Crayfish Muscle in Surimi Gels: Fortification of Blended Gels by Transglutaminase. Gels, 11(3), 204. https://doi.org/10.3390/gels11030204







