Revealing the Impact of Gel Electrolytes on the Performance of Organic Electrochemical Transistors
Abstract
1. Introduction
2. Results and Discussion
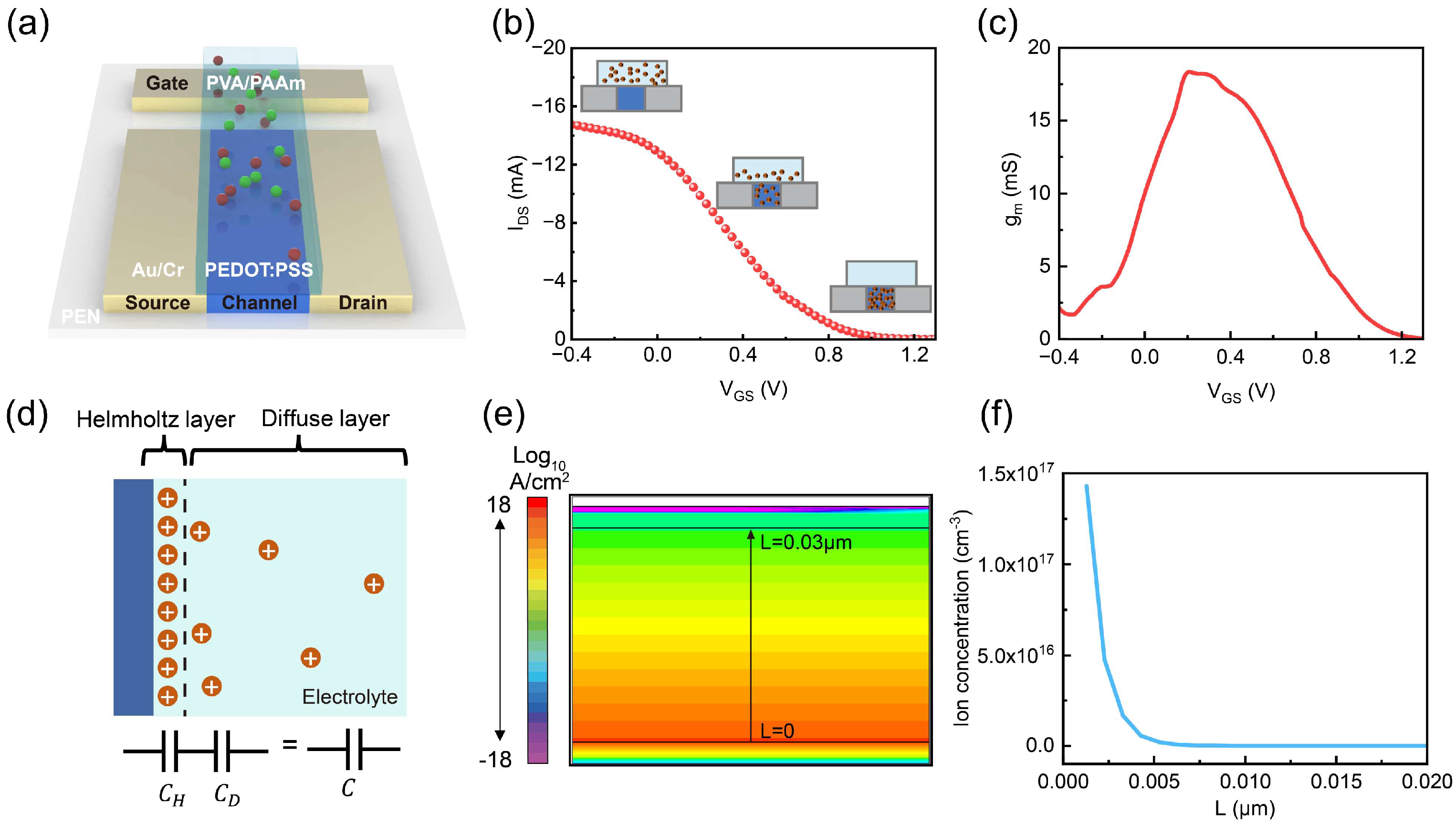
2.1. Analysis of the Operating Mechanism of Gel-Based OECTs
2.2. Simulation of Ion Distribution and Capacitance Under Different Conditions
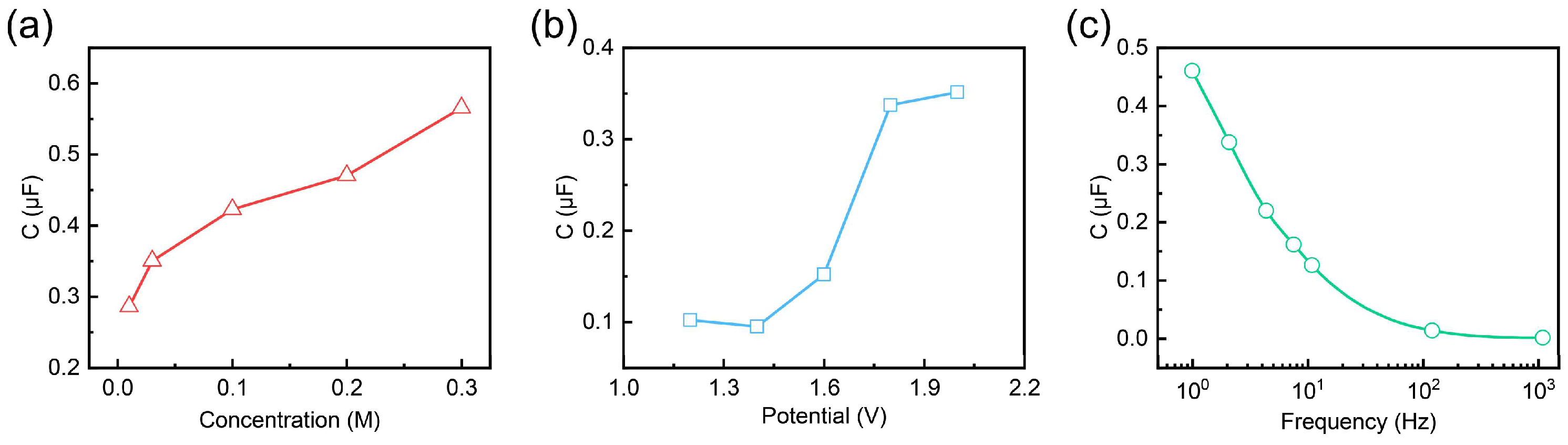
2.3. Experimental Analysis of Factors Influencing the Capacitance of Gel Electrolytes
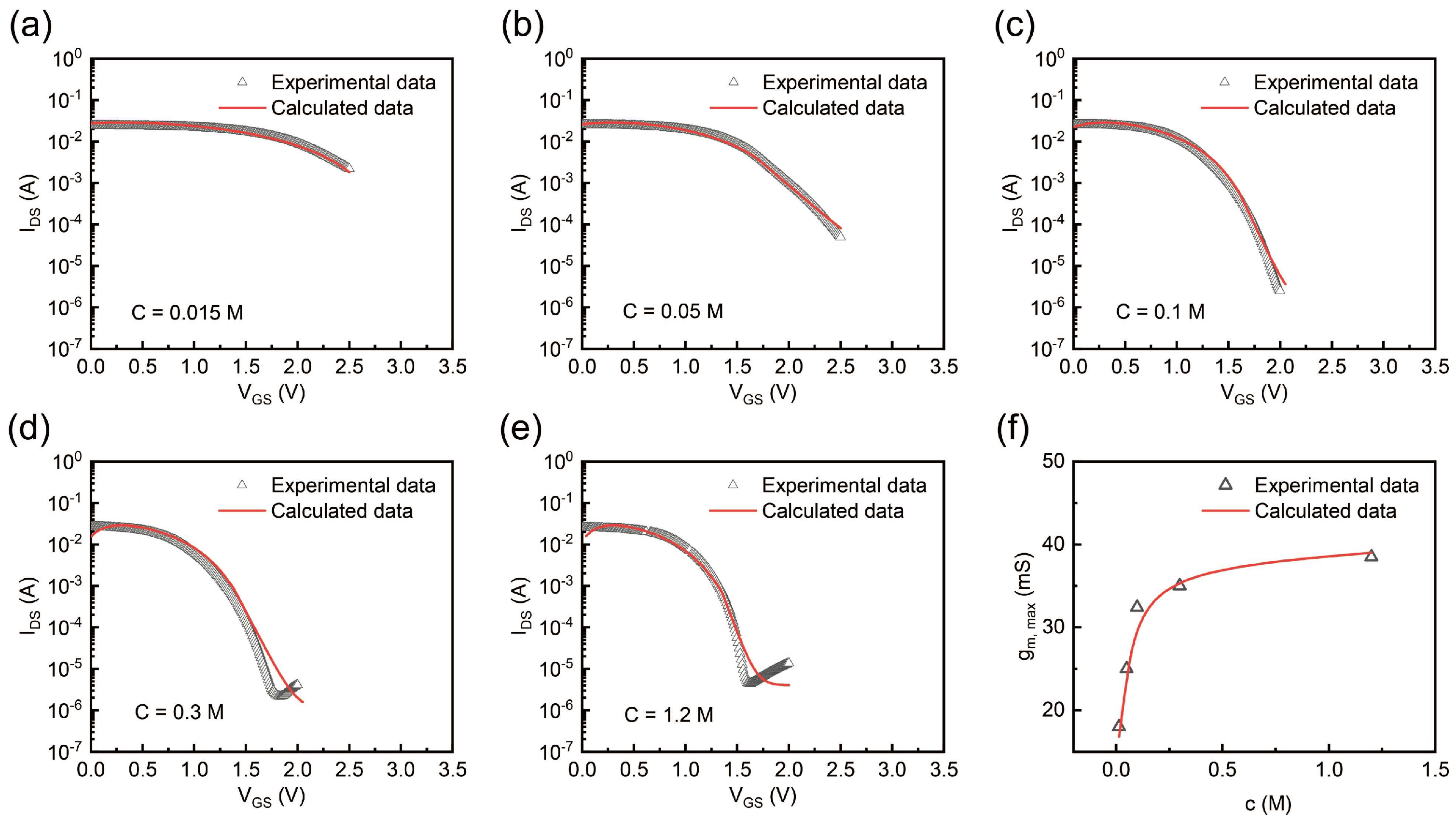
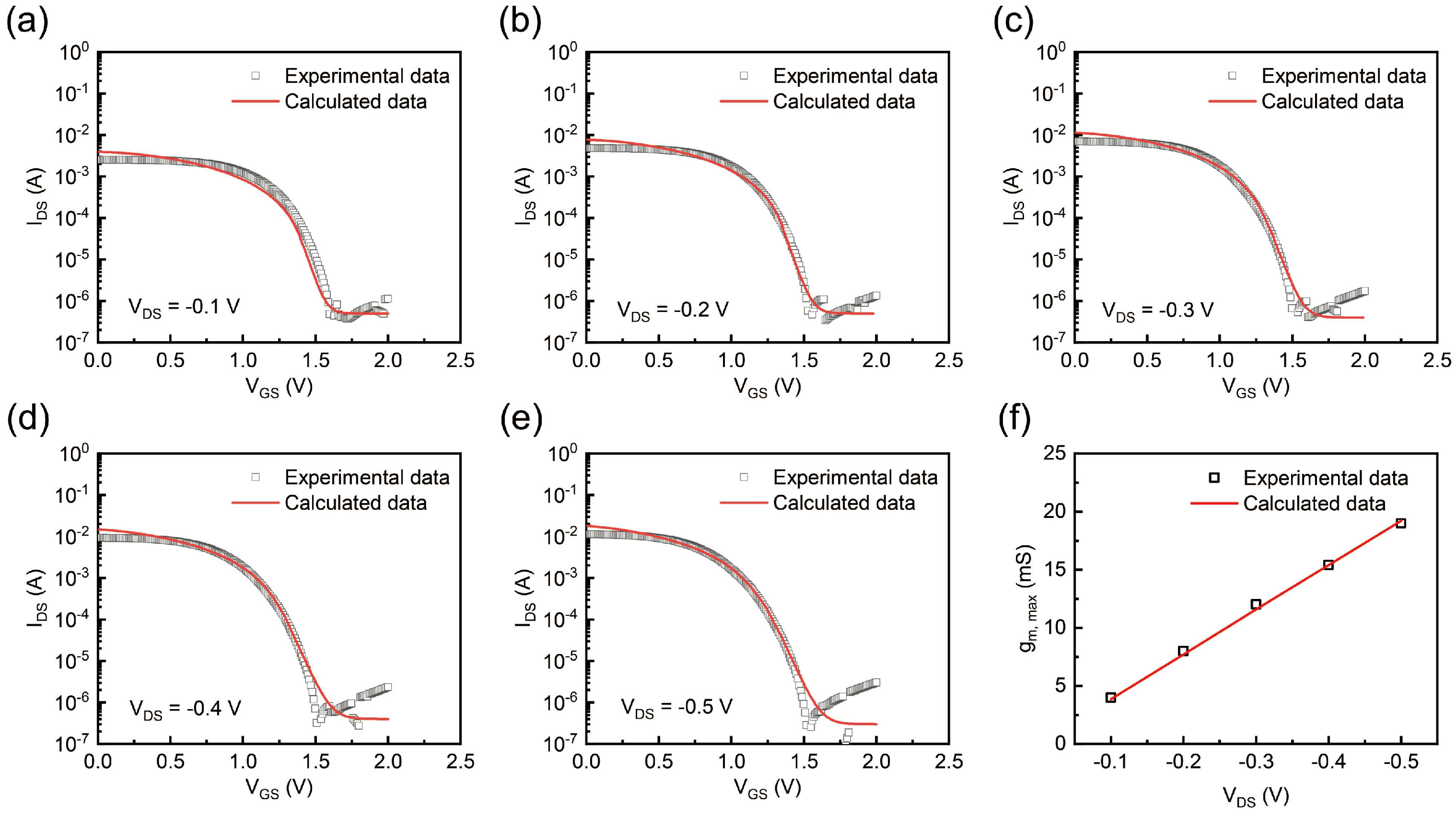
2.4. Experimental and Theoretical Calculation Analyses of Transfer Curve and Transconductance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Method
4.3. Simulation Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamada, S.; Toshiyoshi, H. A biodegradable ionic gel for stretchable ionics. Sens. Actuators A-Phys. 2023, 361, 114574. [Google Scholar] [CrossRef]
- Tavares, F.C.; Cholant, C.M.; Kohlrausch, E.C.; Bolzan, G.R.; Goncalves, P.F.B.; Gil, E.S.; Khan, S.; Dupont, J.; Avellaneda, C.O.; Santos, M.J.L. Ionic Liquid Boosted Conductivity of Biopolymer Gel Electrolyte. J. Electrochem. Soc. 2023, 170, 084501. [Google Scholar] [CrossRef]
- Kuddushi, M.; Xu, B.B.; Malek, N.; Zhang, X. Review of ionic liquid and ionogel-based biomaterials for advanced drug delivery. Adv. Colloid Interface Sci. 2024, 331, 103244. [Google Scholar] [CrossRef]
- Li, H.; Hao, Z.; Zhang, S.; Li, B.; Wang, Y.; Wu, X.; Hu, Y.; Chen, R.; Chen, T.; Li, J. “Smart” Stimuli-responsive Injectable Gels for Bone Tissue Engineering Application. Macromol. Biosci. 2023, 23, 2200481. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.E.; Lim, T.; Kim, S.H.; Lee, J.H. Wearable ion gel based pressure sensor with high sensitivity and ultra-wide sensing range for human motion detection. Chem. Eng. J. 2024, 484, 149464. [Google Scholar] [CrossRef]
- Lu, M.; Shen, L.; Su, H.; Li, B.; Wang, L.; Yu, W.W. Highly ionic conductive, elastic, and biocompatible double-network composite gel for epidermal biopotential monitoring and wearable sensing. J. Colloid Interface Sci. 2025, 684, 272–282. [Google Scholar] [CrossRef]
- Azimi, M.; Subramanian, A.; Fan, J.; Soavi, F.; Cicoira, F. Electrical and mechanical stability of flexible, organic electrolyte-gated transistors based on iongel and hydrogels. J. Mater. Chem. C 2023, 11, 4623–4633. [Google Scholar] [CrossRef]
- Lee, C.M.; Kim, Y.; Kim, W.; Lee, E.; Lee, E.K. High-Performance Synaptic Devices Based on Cross-linked Organic Electrochemical Transistors with Dual Ion Gel. Adv. Funct. Mater. 2024, 2417539. [Google Scholar] [CrossRef]
- Ren, G.; He, S.; Zhang, Y.; Zhu, C.; Gong, Z.; Wang, K.; Zhang, L.; Li, Z.; Lu, G.; Yu, H.-D. Assessment of the Testing Methods for Evaluating the Performance of Organic Electrochemical Transistors. ACS Appl. Electron. Mater. 2023, 5, 4437–4444. [Google Scholar] [CrossRef]
- Ghittorelli, M.; Lingstedt, L.; Romele, P.; Craciun, N.I.; Kovacs-Vajna, Z.M.; Blom, P.W.M.; Torricelli, F. High-sensitivity ion detection at low voltages with current-driven organic electrochemical transistors. Nat. Commun. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Yang, A.; Song, J.; Liu, H.; Zhao, Z.; Li, L.; Yan, F. Wearable Organic Electrochemical Transistor Array for Skin-Surface Electrocardiogram Mapping Above a Human Heart. Adv. Funct. Mater. 2023, 33, 2215037. [Google Scholar] [CrossRef]
- Romele, P.; Gkoupidenis, P.; Koutsouras, D.A.; Lieberth, K.; Kovacs-Vajna, Z.M.; Blom, P.W.M.; Torricelli, F. Multiscale real time and high sensitivity ion detection with complementary organic electrochemical transistors amplifier. Nat. Commun. 2020, 11, 3743. [Google Scholar] [CrossRef]
- van De Burgt, Y.; Melianas, A.; Keene, S.T.; Malliaras, G.; Salleo, A. Organic electronics for neuromorphic computing. Nat. Electron. 2018, 1, 386–397. [Google Scholar] [CrossRef]
- Ji, X.; Paulsen, B.D.; Chik, G.K.K.; Wu, R.; Yin, Y.; Chan, P.K.L.; Rivnay, J. Mimicking associative learning using an ion-trapping non-volatile synaptic organic electrochemical transistor. Nat. Commun. 2021, 12, 2480. [Google Scholar] [CrossRef] [PubMed]
- Azimi, M.; Kim, C.-h.; Fan, J.; Cicoira, F. Effect of ionic conductivity of electrolyte on printed planar and vertical organic electrochemical transistors. Faraday Discuss. 2023, 246, 540–555. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-state and transient behavior of organic electrochemical transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Romele, P.; Ghittorelli, M.; Kovacs-Vajna, Z.M.; Torricelli, F. Ion buffering and interface charge enable high performance electronics with organic electrochemical transistors. Nat. Commun. 2019, 10, 3044. [Google Scholar] [CrossRef]
- Skowrons, M.; Dahal, D.; Paudel, P.R.; Luessem, B. Depletion Type Organic Electrochemical Transistors and the Gradual Channel Approximation. Adv. Funct. Mater. 2024, 34, 2303324. [Google Scholar] [CrossRef]
- Wang, X.; Shapiro, B.; Smela, E. Development of a Model for Charge Transport in Conjugated Polymers. J. Phys. Chem. C 2009, 113, 382–401. [Google Scholar] [CrossRef]
- Kaphle, V.; Paudel, P.R.; Dahal, D.; Krishnan, R.K.R.; Lussem, B. Finding the equilibrium of organic electrochemical transistors. Nat. Commun. 2020, 11, 2515. [Google Scholar] [CrossRef]
- Paudel, P.R.; Kaphle, V.; Dahal, D.; Radha Krishnan, R.K.; Lussem, B. Tuning the Transconductance of Organic Electrochemical Transistors. Adv. Funct. Mater. 2021, 31, 2004939. [Google Scholar] [CrossRef]
- Weissbach, A.; Cucchi, M.; Tseng, H.; Leo, K.; Kleemann, H. Unraveling the Electrochemical Electrode Coupling in Integrated Organic Electrochemical Transistors. Adv. Funct. Mater. 2023, 33, 2302205. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Khodagholy, D.; Rivnay, J.; Sessolo, M.; Gurfinkel, M.; Leleux, P.; Jimison, L.H.; Stavrinidou, E.; Herve, T.; Sanaur, S.; Owens, R.M.; et al. High transconductance organic electrochemical transistors. Nat. Commun. 2013, 4, 2133. [Google Scholar] [CrossRef]
- Rivnay, J.; Leleux, P.; Sessolo, M.; Khodagholy, D.; Herve, T.; Fiocchi, M.; Malliaras, G.G. Organic Electrochemical Transistors with Maximum Transconductance at Zero Gate Bias. Adv. Mater. 2013, 25, 7010–7014. [Google Scholar] [CrossRef]
- Kaphle, V.; Liu, S.; Al-Shadeedi, A.; Keum, C.-M.; Lussem, B. Contact Resistance Effects in Highly Doped Organic Electrochemical Transistors. Adv. Mater. 2016, 28, 8766–8770. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.M.; Malliaras, G.G. Organic Electronics at the Interface with Biology. MRS Bull. 2010, 35, 449–456. [Google Scholar] [CrossRef]
- Tybrandt, K.; Zozoulenko, I.V.; Berggren, M. Chemical potential-electric double layer coupling in conjugated polymer-polyelectrolyte blends. Sci. Adv. 2017, 3, eaao3659. [Google Scholar] [CrossRef]
- Friedlein, J.T.; Shaheen, S.E.; Malliaras, G.G.; McLeod, R.R. Optical Measurements Revealing Nonuniform Hole Mobility in Organic Electrochemical Transistors. Adv. Electron. Mater. 2015, 1, 1500189. [Google Scholar] [CrossRef]
- Wu, J. Understanding the Electric Double-Layer Structure, Capacitance, and Charging Dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef]
- Le, J.-B.; Fan, Q.-Y.; Li, J.-Q.; Cheng, J. Molecular origin of negative component of Helmholtz capacitance at electrified Pt(111)/water interface. Sci. Adv. 2020, 6, eabb1219. [Google Scholar] [CrossRef]
- Allagui, A.; Benaoum, H.; Olendski, O. On the Gouy-Chapman-Stern model of the electrical double-layer structure with a generalized Boltzmann factor. Phys. A-Stat. Mech. Its Appl. 2021, 582, 126252. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Wu, J. Demystifying the Stern layer at a metal-electrolyte interface: Local dielectric constant, specific ion adsorption, and partial charge transfer. J. Chem. Phys. 2021, 154, 124701. [Google Scholar] [CrossRef] [PubMed]
- Buth, F.; Kumar, D.; Stutzmann, M.; Garrido, J.A. Electrolyte-gated organic field-effect transistors for sensing applications. Appl. Phys. Lett. 2011, 98, 153302. [Google Scholar] [CrossRef]
- Liang, X.; Luo, Y.; Pei, Y.; Wang, M.; Liu, C. Multimode transistors and neural networks based on ion-dynamic capacitance. Nat. Electron. 2022, 5, 859–869. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liang, X.; Liu, C.; Han, S. Revealing the Impact of Gel Electrolytes on the Performance of Organic Electrochemical Transistors. Gels 2025, 11, 202. https://doi.org/10.3390/gels11030202
Li M, Liang X, Liu C, Han S. Revealing the Impact of Gel Electrolytes on the Performance of Organic Electrochemical Transistors. Gels. 2025; 11(3):202. https://doi.org/10.3390/gels11030202
Chicago/Turabian StyleLi, Mancheng, Xiaoci Liang, Chuan Liu, and Songjia Han. 2025. "Revealing the Impact of Gel Electrolytes on the Performance of Organic Electrochemical Transistors" Gels 11, no. 3: 202. https://doi.org/10.3390/gels11030202
APA StyleLi, M., Liang, X., Liu, C., & Han, S. (2025). Revealing the Impact of Gel Electrolytes on the Performance of Organic Electrochemical Transistors. Gels, 11(3), 202. https://doi.org/10.3390/gels11030202






