Activation Energy of SDS–Protein Complexes in Capillary Electrophoresis with Tetrahydroxyborate Cross-Linked Agarose Gels
Abstract
1. Introduction
2. Results and Discussion
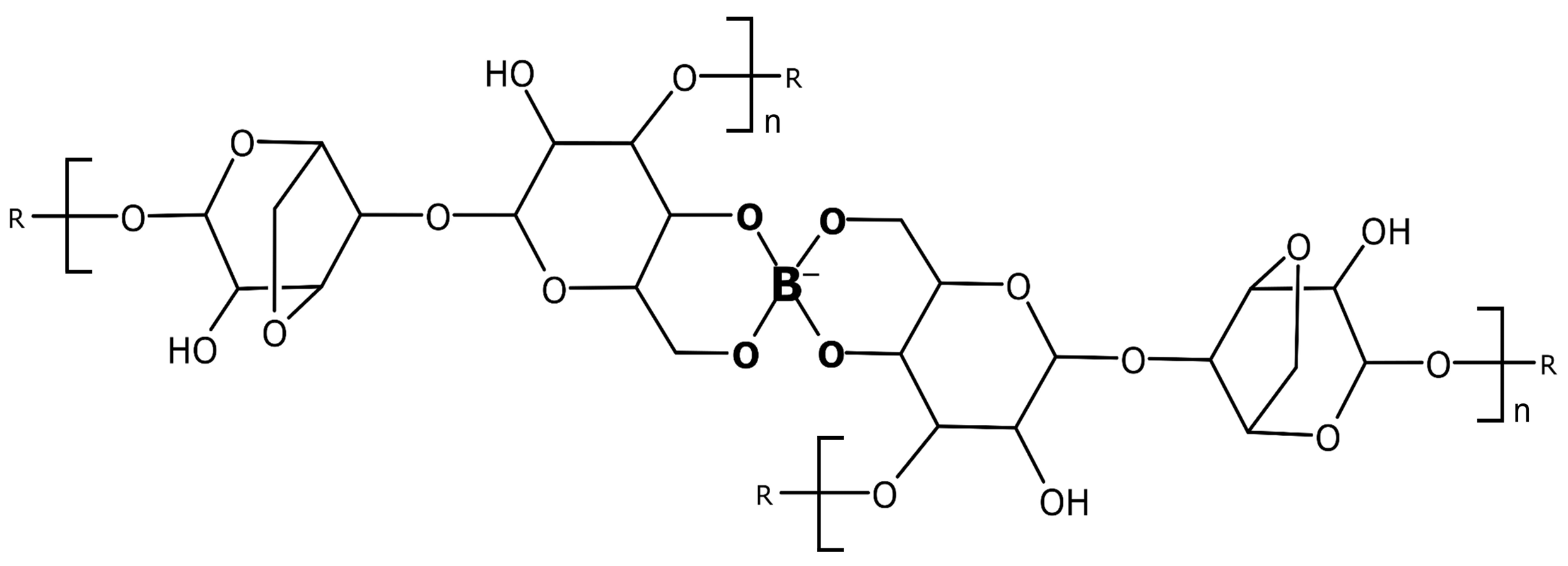
2.1. Theoretical Considerations
2.2. Reproducibility Study
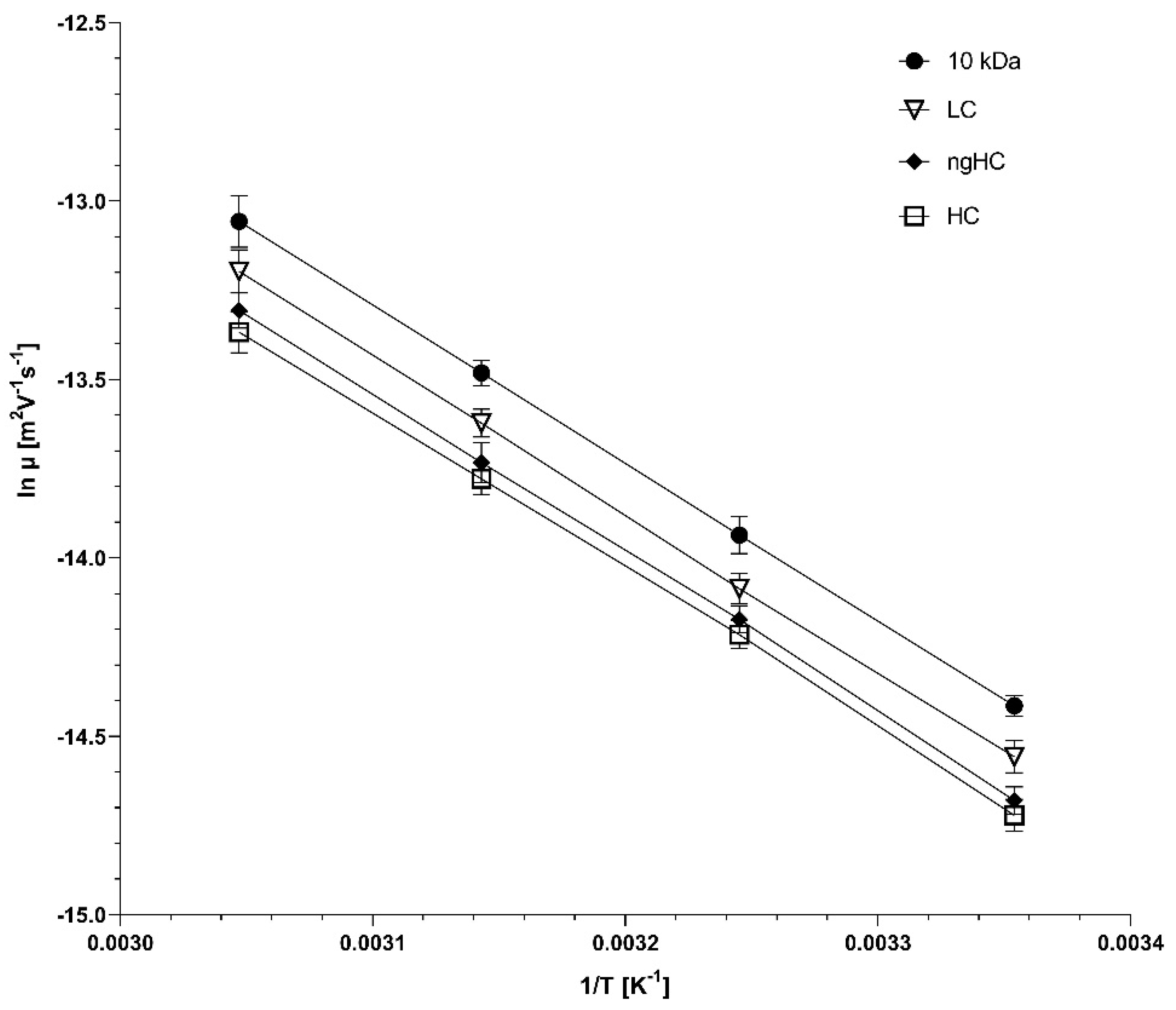
2.3. Temperature Dependence of the Separation in Borate Cross-Linked Agarose Gels
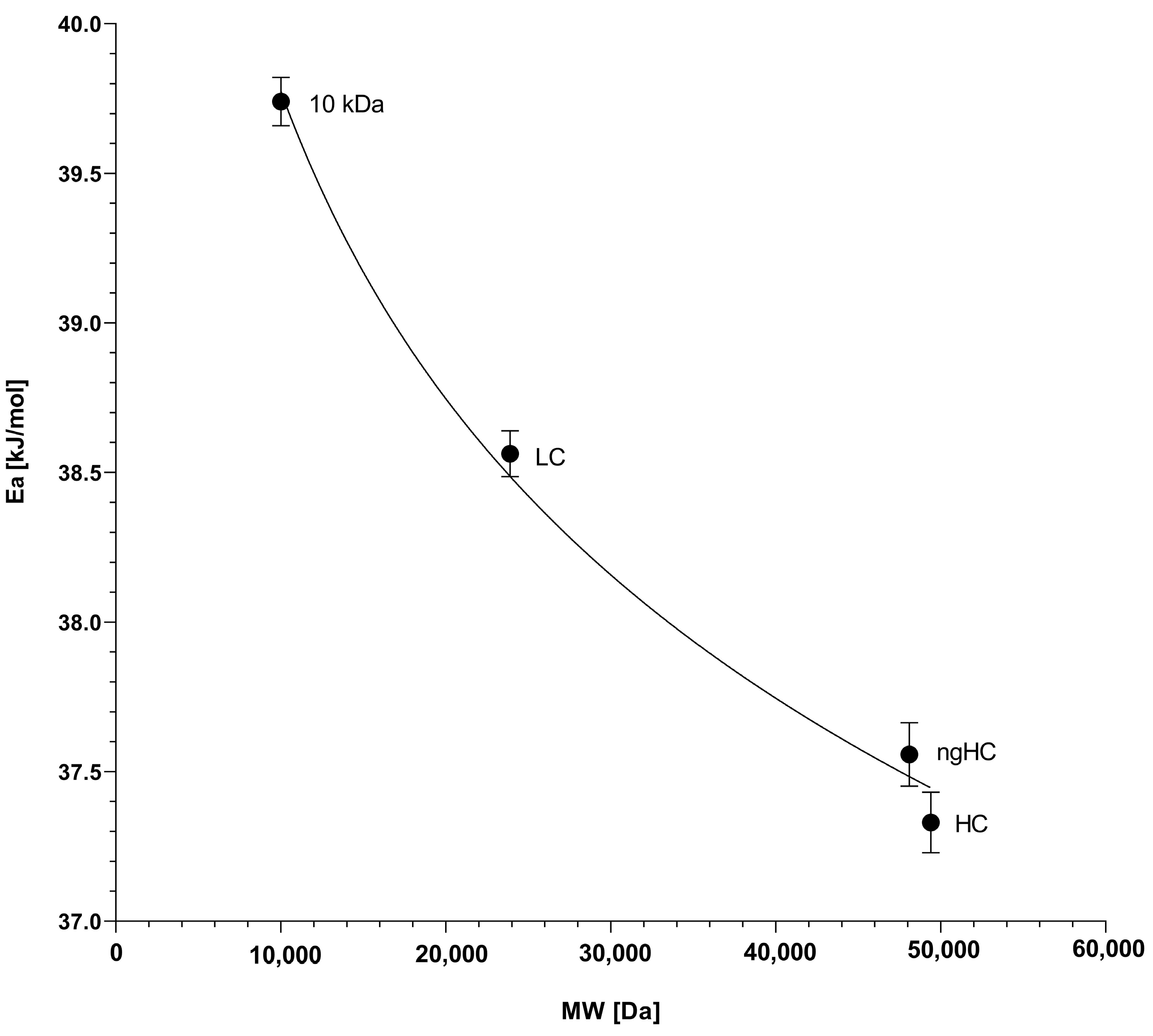
2.4. The Activation Energy Plot
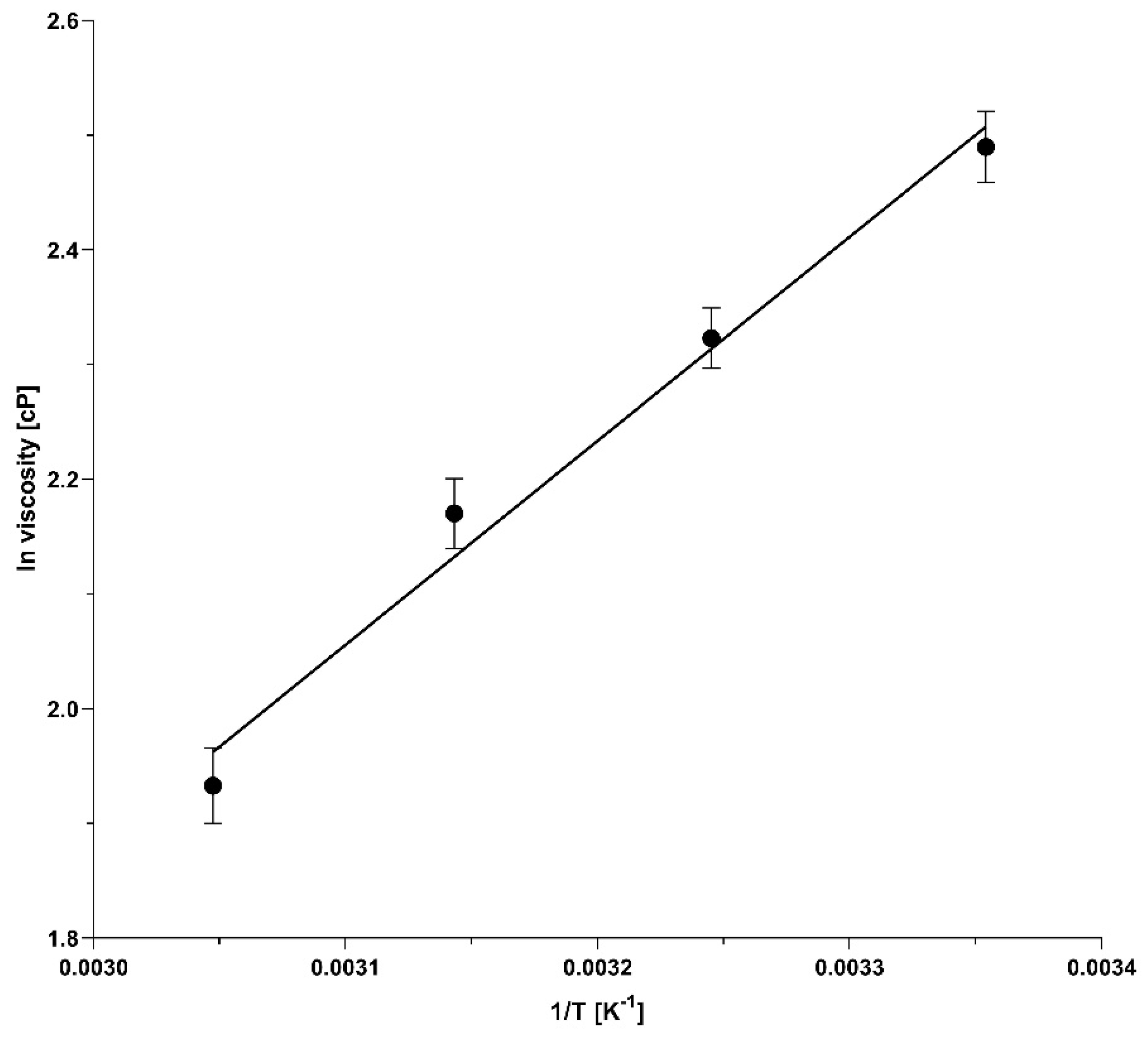
2.5. Temperature-Dependent Viscosity of the Borate Cross-Linked Agarose Gels
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Agarose Gel and Sample Preparation
4.3. SDS–Capillary Agarose Gel Electrophoresis (SDS-CAGE)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDS | sodium lauryl sulfate |
| CE | capillary electrophoresis |
| A | agarose |
| CAGE | capillary agarose gel electrophoresis |
| TBEG | Tris-borate-EDTA-glycerol |
| LC | light chain |
| ngHC | non-glycosylated heavy chain |
| HC | heavy chain |
| mAb | monoclonal antibody |
| Ea | activation energy |
| 2-ME | 2-mercaptoethanol |
References
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Physics of agarose fluid gels: Rheological properties and microstructure. Curr. Res. Food Sci. 2021, 4, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 2012, 62, 3923. [Google Scholar] [CrossRef]
- Guttman, A.; Hajba, L. Capillary Gel Electrophoresis; Elsevier Science: Amsterdam, The Netherlands, 2021; Available online: https://www.sciencedirect.com/science/article/pii/S0165993623001115 (accessed on 1 November 2024).
- Li, C.; Arakawa, T. Agarose native gel electrophoresis of proteins. Int. J. Biol. Macromol. 2019, 140, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Aymard, P.; Martin, D.R.; Plucknett, K.; Foster, T.J.; Clark, A.H.; Norton, I.T. Influence of thermal history on the structural and mechanical properties of agarose gels. Biopolymers 2001, 59, 131–144. [Google Scholar] [CrossRef]
- Gordon, A.H.; Keil, B.; Åebesta, K. Electrophoresis of proteins in agar jelly. Nature 1949, 164, 498–499. [Google Scholar] [CrossRef]
- Hjerten, S. Agarose as an anticonvection agent in zone electrophoresis. Biochim. Biophys. Acta 1961, 53, 514–517. [Google Scholar] [CrossRef]
- Serwer, P.; Hayes, S.J. Exclusion of spheres by agarose gels during agarose gel electrophoresis: Dependence on the sphere’s radius and the gel’s concentration. Anal. Biochem. 1986, 158, 72–78. [Google Scholar] [CrossRef]
- Chiari, M.; D’Alesio, L.; Consonni, R.; Righetti, P.G. New types of large-pore polyacrylamide-agarose mixed-bed matrices for DNA electrophoresis: Pore size estimation from Ferguson plots of DNA fragments. Electrophoresis 1995, 16, 1337–1344. [Google Scholar] [CrossRef]
- Warren, C.M.; Krzesinski, P.R.; Greaser, M.L. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 2003, 24, 1695–1702. [Google Scholar] [CrossRef]
- Pezron, E.; Ricard, A.; Lafuma, F.; Audebert, R. Reversible gel formation induced by ion complexation. 1. Borax-galactomannan interactions. Macromolecules 1988, 21, 1121–1125. [Google Scholar] [CrossRef]
- Sarkozy, D.; Guttman, A. Capillary Sodium Dodecyl Sulfate Agarose Gel Electrophoresis of Proteins. Gels 2022, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, N.C. Electrophoresis of DNA in agarose gels, polyacrylamide gels and in free solution. Electrophoresis 2009, 30 (Suppl. 1), S188–S195. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Furlani, F.; Cok, M.; Travan, A.; Borgogna, M.; Marsich, E.; Paoletti, S.; Donati, I. Boric Acid Induced Transient Cross-Links in Lactose-Modified Chitosan (Chitlac). Biomacromolecules 2017, 18, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Guttman, A.; Filep, C.; Karger, B.L. Fundamentals of Capillary Electrophoretic Migration and Separation of SDS Proteins in Borate Cross-Linked Dextran Gels. Anal. Chem. 2021, 93, 9267–9276. [Google Scholar] [CrossRef] [PubMed]
- Righetti, P.G. Electrophoresis: The march of pennies, the march of dimes. J. Chromatogr. A 2005, 1079, 24–40. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. für Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef]
- Eyring, H.; Polanyi, M. Über einfache gasreaktionen. Z. Phys. Chem. B 1931, 12, 279–311. [Google Scholar]
- Cottet, H.; Gareil, P. On the use of the activation energy concept to investigate analyte and network deformations in entangled polymer solution capillary electrophoresis of synthetic polyelectrolytes. Electrophoresis 2001, 22, 684–691. [Google Scholar] [CrossRef]
- Demorest, D.; Dubrow, R. Factors influencing the resolution and quantitation of oligonucleotides separated by capillary electrophoresis on a gel-filled capillary. J. Chromatogr. A 1991, 559, 43–56. [Google Scholar] [CrossRef]
- Lu, H.; Arriaga, E.; Da, Y.C.; Figeys, D.; Dovichi, N.J. Activation energy of single-stranded DNA moving through cross-linked polyacrylamide gels at 300 V/cm. Effect of temperature on sequencing rate in high-electric-field capillary gel electrophoresis. J. Chromatogr. A 1994, 680, 503–510. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.Z.; Hou, J.Y.; Lu, H.; Dovichi, N.J. Activation energy of the separation of DNA sequencing fragments in denaturing noncross-linked polyacrylamide by capillary elecrophoresis. Electrophoresis 1996, 17, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Burki, T. 2024 Nobel Prize awarded for work on microRNAs. Lancet 2024, 404, 1507–1508. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, M.; Guttman, A. Sample Preparation Scale-Up for Deep N-glycomic Analysis of Human Serum by Capillary Electrophoresis and CE-ESI-MS. Mol. Cell. Proteom. 2019, 18, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
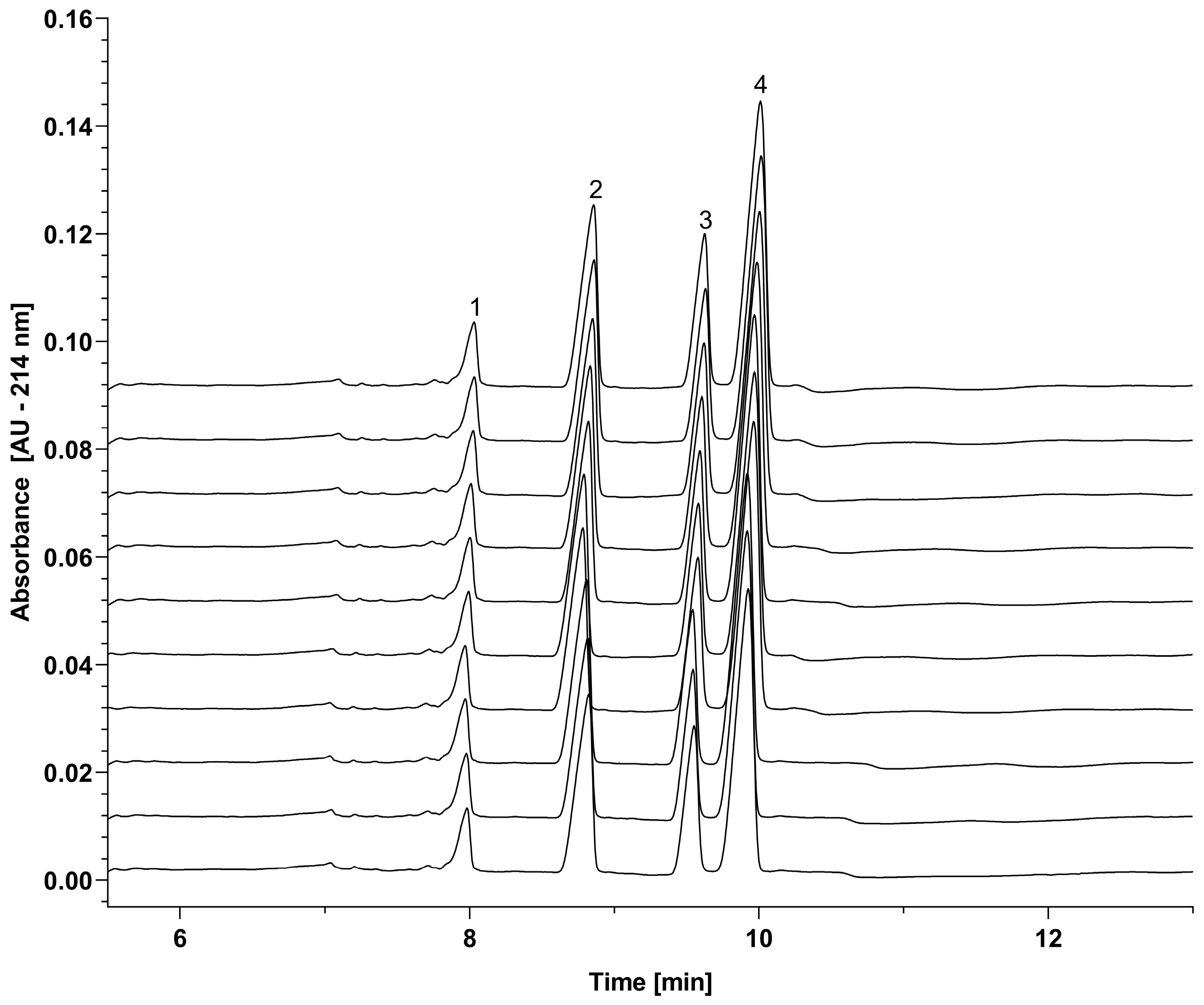

| Run | LC | ngHC | HC | |||
|---|---|---|---|---|---|---|
| Migration Time [min] | Peak Area % | Migration Time [min] | Peak Area % | Migration Time [min] | Peak Area % | |
| 1 | 8.832 | 27.689 | 9.564 | 17.495 | 9.936 | 47.537 |
| 2 | 8.814 | 28.973 | 9.541 | 16.826 | 9.921 | 47.313 |
| 3 | 8.765 | 28.047 | 9.503 | 16.422 | 9.877 | 48.232 |
| 4 | 8.878 | 28.425 | 9.679 | 19.094 | 10.054 | 45.595 |
| 5 | 8.893 | 27.442 | 9.687 | 19.341 | 10.071 | 46.397 |
| 6 | 8.804 | 27.069 | 9.578 | 18.290 | 9.954 | 48.052 |
| 7 | 8.827 | 28.786 | 9.601 | 18.535 | 9.977 | 45.762 |
| 8 | 8.827 | 27.521 | 9.605 | 19.362 | 9.991 | 45.681 |
| 9 | 8.842 | 27.763 | 9.614 | 17.931 | 9.996 | 47.377 |
| 10 | 8.841 | 27.943 | 9.611 | 19.294 | 9.992 | 45.649 |
| Average | 8.832 | 27.966 | 9.598 | 18.259 | 9.977 | 46.760 |
| %RSD | 0.386 | 2.054 | 0.560 | 5.572 | 0.558 | 2.139 |
| Activation Energy (kJ/mol) | 10 kDa | LC (23.89 kDa) | ngHC (47.87 kDa) | HC (49.37 kDa) |
|---|---|---|---|---|
| Ea total activation energy | 39.75 | 38.50 | 37.48 | 37.24 |
| Eav (viscous flow) | 14.59 | 14.59 | 14.59 | 14.59 |
| Ead (distortion /conformation) | 25.17 | 23.92 | 22.89 | 22.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sárközy, D.; Guttman, A. Activation Energy of SDS–Protein Complexes in Capillary Electrophoresis with Tetrahydroxyborate Cross-Linked Agarose Gels. Gels 2024, 10, 805. https://doi.org/10.3390/gels10120805
Sárközy D, Guttman A. Activation Energy of SDS–Protein Complexes in Capillary Electrophoresis with Tetrahydroxyborate Cross-Linked Agarose Gels. Gels. 2024; 10(12):805. https://doi.org/10.3390/gels10120805
Chicago/Turabian StyleSárközy, Dániel, and András Guttman. 2024. "Activation Energy of SDS–Protein Complexes in Capillary Electrophoresis with Tetrahydroxyborate Cross-Linked Agarose Gels" Gels 10, no. 12: 805. https://doi.org/10.3390/gels10120805
APA StyleSárközy, D., & Guttman, A. (2024). Activation Energy of SDS–Protein Complexes in Capillary Electrophoresis with Tetrahydroxyborate Cross-Linked Agarose Gels. Gels, 10(12), 805. https://doi.org/10.3390/gels10120805








