Diagnosis of Fungal Keratitis in Low-Income Countries: Evaluation of Smear Microscopy, Culture, and In Vivo Confocal Microscopy in Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design
2.3. Study Setting and Participants
2.4. Clinical Findings
2.5. Microbiological Diagnosis
2.6. In Vivo Confocal Microscopy
2.7. Disease Definition
2.8. Statistical Analysis
3. Results
3.1. Sensitivity—All Investigations Compared to Composite Referents
3.2. Sensitivity—Different Smear Microscopy Stains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, M.J.; Pithuwa, J.; Okello, E.; Afwamba, I.; Onyango, J.J.; Oates, F.; Chevallier, C.; Hall, A.B. Microbial Keratitis in East Africa: Why are the Outcomes so Poor? Ophthalmic Epidemiol. 2011, 18, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Allan, B.D.; Dart, J.K. Strategies for the management of microbial keratitis. Br. J. Ophthalmol. 1995, 79, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Thomas, P.A.; Leck, A.K.; Myatt, M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br. J. Ophthalmol. 2005, 89, 1554–1558. [Google Scholar] [CrossRef]
- McLeod, S.D. The role of cultures in the management of ulcerative keratitis. Cornea 1997, 16, 381–382. [Google Scholar] [CrossRef]
- Levey, S.B.; Katz, H.R.; Abrams, D.A.; Hirschbein, M.J.; Marsh, M.J. The role of cultures in the management of ulcerative keratitis. Cornea 1997, 16, 383–386. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Dart, J.K.G.; De, S.K.; Carnt, N.; Cleary, G.; Hau, S. Comparison of culture, confocal microscopy and PCR in routine hospital use for microbial keratitis diagnosis. Eye 2021. [Google Scholar] [CrossRef]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef]
- Bharathi, M.J.; Ramakrishnan, R.; Meenakshi, R.; Mittal, S.; Shivakumar, C.; Srinivasan, M. Microbiological diagnosis of infective keratitis: Comparative evaluation of direct microscopy and culture results. Br. J. Ophthalmol. 2006, 90, 1271–1276. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Larke, N.L.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Lalitha, P.; Carnt, N.; Vesaluoma, M.H.; et al. Prospective Study of the Diagnostic Accuracy of the In Vivo Laser Scanning Confocal Microscope for Severe Microbial Keratitis. Ophthalmology 2016, 123, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Chidambaram, J.D.; Prajna, N.V.; Larke, N.; Macleod, D.; Srikanthi, P.; Lanjewar, S.; Shah, M.; Lalitha, P.; Elakkiya, S.; Burton, M.J. In vivo confocal microscopy appearance of Fusarium and Aspergillus species in fungal keratitis. Br. J. Ophthalmol. 2017, 101, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kunimoto, D.Y.; Gopinathan, U.; Athmanathan, S.; Garg, P.; Rao, G.N. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: A survey of eight years of laboratory experience. Cornea 2002, 21, 643–647. [Google Scholar] [CrossRef]
- Sharma, S.; Silverberg, M.; Mehta, P.; Gopinathan, U.; Agrawal, V.; Naduvilath, T.J. Early diagnosis of mycotic keratitis: Predictive value of potassium hydroxide preparation. Indian J. Ophthalmol. 1998, 46, 31–35. [Google Scholar] [PubMed]
- Liesegang, T.J.; Forster, R.K. Spectrum of microbial keratitis in South Florida. Am. J. Ophthalmol. 1980, 90, 38–47. [Google Scholar] [CrossRef]
- Xie, L.; Dong, X.; Shi, W. Treatment of fungal keratitis by penetrating keratoplasty. Br. J. Ophthalmol. 2001, 85, 1070–1074. [Google Scholar] [CrossRef]
- Robin, J.B.; Nielson, S.; Trousdale, M.D. Fluorescein-conjugated lectin identification of a case of human keratomycosis. Am. J. Ophthalmol. 1986, 102, 797–798. [Google Scholar] [CrossRef]
- Hau, S.C.; Dart, J.K.G.; Vesaluoma, M.; Parmar, D.N.; Claerhout, I.; Bibi, K.; Larkin, D.F.P. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br. J. Ophthalmol. 2010, 94, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Kanavi, M.R.; Javadi, M.; Yazdani, S.; Mirdehghanm, S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea 2007, 26, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.W.Y.; Harrison, R.; Hau, S.; Alexander, C.L.; Tole, D.M.; Avadhanam, V.S. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea 2018, 37, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.J.; Yadav, R.; Das Sanyam, S.; Chaudhary, P.; Roshan, A.; Singh, S.K.; Arunga, S.; Matayan, E.; Macleod, D.; Weiss, H.A.; et al. Topical chlorhexidine 0.2% versus topical natamycin 5% for fungal keratitis in Nepal: Rationale and design of a randomised controlled non-inferiority trial. BMJ Open 2020, 10, e038066. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Yadav, R.; Sanyam, S.D.; Chaudhary, P.; Roshan, A.; Singh, S.K.; Singh, S.K.; Mishra, S.K.; Arunga, S.; Hu, V.H.; et al. Topical chlorhexidine 0.2% versus topical natamycin 5% for the treatment of fungal keratitis in Nepal: A randomised controlled non-inferiority trial. Ophthalmology 2021, 10, e038066. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Yadav, R.; Sanyam, S.D.; Chaudhary, P.; Roshan, A.; Singh, S.K.; Arunga, S.; Hu, V.H.; Macleod, D.; Leck, A.; et al. Microbial Keratitis in Nepal: Predicting the Microbial Aetiology from Clinical Features. J. Fungi 2022, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Bakken, I.M.; Jackson, C.J.; Utheim, T.P.; Villani, E.; Hamrah, P.; Kheirkhah, A.; Nielsen, E.; Hau, S.; Lagali, N.S. The use of in vivo confocal microscopy in fungal keratitis—Progress and challenges. Ocul. Surf. 2022, 24, 103–118. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, H.; Jiang, L.; Han, L.; Wang, L. Use of potassium hydroxide, Giemsa and calcofluor white staining techniques in the microscopic evaluation of corneal scrapings for diagnosis of fungal keratitis. J. Int. Med. Res. 2010, 38, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.J.; Burton, M.J.; Leck, A. Mycotic Keratitis—A Global Threat from the Filamentous Fungi. J. Fungi 2021, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. GDP Per Capita (Current US$)—Nepal. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=NP&most_recent_value_desc=false (accessed on 27 January 2022).
- Dahlgren, M.A.; Lingappan, A.; Wilhelmus, K.R. The Clinical Diagnosis of Microbial Keratitis. Am. J. Ophthalmol. 2007, 143, 940–944.e941. [Google Scholar] [CrossRef]
- Dalmon, C.; Porco, T.C.; Lietman, T.M.; Prajna, N.V.; Prajna, L.; Das, M.R.; Kumar, J.A.; Mascarenhas, J.; Margolis, T.P.; Whitcher, J.P.; et al. The Clinical Differentiation of Bacterial and Fungal Keratitis: A Photographic Survey. Investig. Opthalmol. Vis. Sci. 2012, 53, 1785–1787. [Google Scholar] [CrossRef]
- Khanal, B.; Deb, M.; Panda, A.; Sethi, H.S. Laboratory diagnosis in ulcerative keratitis. Ophthalmic Res. 2005, 37, 123–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.Q.; Deng, S.J.; Tian, L.; Liang, Q.F. Diagnostic value of fungal fluorescence staining on corneal scrapings for fungal keratitis. Zhonghua Yan Ke Za Zhi 2019, 55, 601–608. [Google Scholar] [CrossRef]
- Cariello, A.; Passos, R.; Yu, M.; Hofling-lima, A.L. Microbial keratitis at a referral center in Brazil. Int. Ophthalmol. 2011, 31, 197–204. [Google Scholar] [CrossRef]
- Fong, C.F.; Tseng, C.H.; Hu, F.R.; Wang, I.J.; Chen, W.L.; Hou, Y.C. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am. J. Ophthalmol. 2004, 137, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Hopping, G.C.; Vaidyanathan, U.; Liu, H.; Somani, A.N.; Ronquillo, Y.C.; Hoopes, P.C. Biological Staining and Culturing in Infectious Keratitis: Controversy in Clinical Utility. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 145–151. [Google Scholar] [PubMed]
- Marines, H.M.; Osato, M.S.; Font, R.L. The value of calcofluor white in the diagnosis of mycotic and Acanthamoeba infections of the eye and ocular adnexa. Ophthalmology 1987, 94, 23–26. [Google Scholar] [CrossRef]
- Haghani, I.; Amirinia, F.; Nowroozpoor Dailami, K.; Shokohi, T. Detection of fungi by conventional methods and semi-nested PCR in patients with presumed fungal keratitis. Curr. Med. Mycol. 2015, 1, 31–38. [Google Scholar] [CrossRef]
- Vaddavalli, P.K.; Garg, P.; Sharma, S.; Sangwan, V.S.; Rao, G.N.; Thomas, R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology 2011, 118, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Leck, A. Taking a corneal scrape and making a diagnosis. Community Eye Health/Int. Cent. Eye Health 2015, 28, 8–9. [Google Scholar]
- Leck, A.; Burton, M. Distinguishing fungal and bacterial keratitis on clinical signs. Community Eye Health 2015, 28, 6–7. [Google Scholar]
- Donnenfeld, E.D.; Schrier, A.; Perry, H.D.; Aulicino, T.; Gombert, M.E.; Snyder, R. Penetration of topically applied ciprofloxacin, norfloxacin, and ofloxacin into the aqueous humor. Ophthalmology 1994, 101, 902–905. [Google Scholar] [CrossRef]

| Diagnostic Methods | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Composite † | Microscopy | IVCM | Culture | ||||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Number of subjects tested by each method | 642 | 631 | 638 | 624 | |||||
| Positive tests (any organism) | 565 | (88.0) | 533 | (84.5) | 476 | (74.6) | 443 | (71.0) | |
| Positive test by organism type | |||||||||
| Monomicrobial | Bacteria ‡ | 32 | (5.0) | n/a | n/a | 32/32 | (100) | ||
| Filamentous fungi | 468 | (72.9) | 425/464 | (91.6) | 421/466 | (90.3) | 345/458 | (75.3) | |
| Yeast | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | |
| Acanthamoeba § | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | |
| Polymicrobial‡ | Bacteria WITH | 64 | (10.0) | n/a | n/a | 64/64 | (100) | ||
| filamentous fungi ¶ | 54/64 | (84.4) | 55/64 | (85.9) | 50/64 | (78.1) | |||
| Bacteria WITH | 1 | (0.2) | n/a | n/a | 1/1 | (100) | |||
| yeast | 0/1 | (0) | 0/1 | (0) | 1/1 | (100) | |||
| Diagnostic Method | Composite Diagnosis Reference Standard † | Totals ‡ | Value (% CI) | |||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Microscopy | ||||||
| Positive | 479 | 0 | 479 | Sensitivity % | 90.7 | (87.9–93.1) |
| Negative | 49 | 103 | 152 | |||
| Total | 528 | 103 | 631 | |||
| IVCM | ||||||
| Positive | 476 | 0 | 476 | Sensitivity % | 89.8 | (86.9–92.3) |
| Negative | 54 | 108 | 162 | |||
| Total | 530 | 108 | 638 | |||
| Culture | ||||||
| Positive | 395 | 0 | 395 | Sensitivity % | 75.7 | (71.8–79.3) |
| Negative | 127 | 100 | 227 | |||
| Total | 522 | 100 | 622 | |||
| Diagnostic Method | Composite Diagnosis Reference Standard † | Totals | Value (% CI) | |||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| KOH | ||||||
| Positive | 413 | 0 | 413 | Sensitivity % | 85.3 | (81.9–88.4) |
| Negative | 71 | 95 | 166 | |||
| Total | 484 | 95 | 579 | |||
| Gram stain | ||||||
| Positive | 417 | 0 | 417 | Sensitivity % | 83.2 | (79.7–86.4) |
| Negative | 84 | 59 | 143 | |||
| Total | 501 | 59 | 560 | |||
| Calcofluor white | ||||||
| Positive | 417 | 0 | 417 | Sensitivity % | 79.1 | (75.4–82.5) |
| Negative | 110 | 101 | 211 | |||
| Total | 527 | 101 | 628 | |||
| 1. High index of suspicion. Fungal keratitis should be considered at first presentation, particularly if there are clinical features that are more commonly seen in fungal keratitis such as serrated/feathery margins, satellite lesions, raised slough and pigment [25,37,38]. |
| 2. Perform confocal microscopy (if available). However, most settings will not have access to IVCM and therefore, clinicians should proceed directly to step 3. |
| 3. Perform corneal scrapes. Follow previously published techniques on corneal scraping including how to streak the material on slides and culture media [2,37]. The yield from corneal scrapes increases with increasing ulcer size [9]. Any infiltrates larger than 2 mm must be scraped, and ideally, infiltrates between 1 and 2 mm should also be scraped. However, a negative result in a smaller ulcer may be false–negative and so should be interpreted with caution. Ideally, corneal material should be smeared thinly and evenly onto each microscope slide; if the smear preparation is too thick, this may affect the staining process and will be difficult to interpret. Corneal scraping itself may be of some therapeutic benefit by improving the penetration of topical medications and reducing the infectious burden [2,39]. As a minimum, two slides should be sent (one for Gram stain and one for KOH) for smear microscopy. |
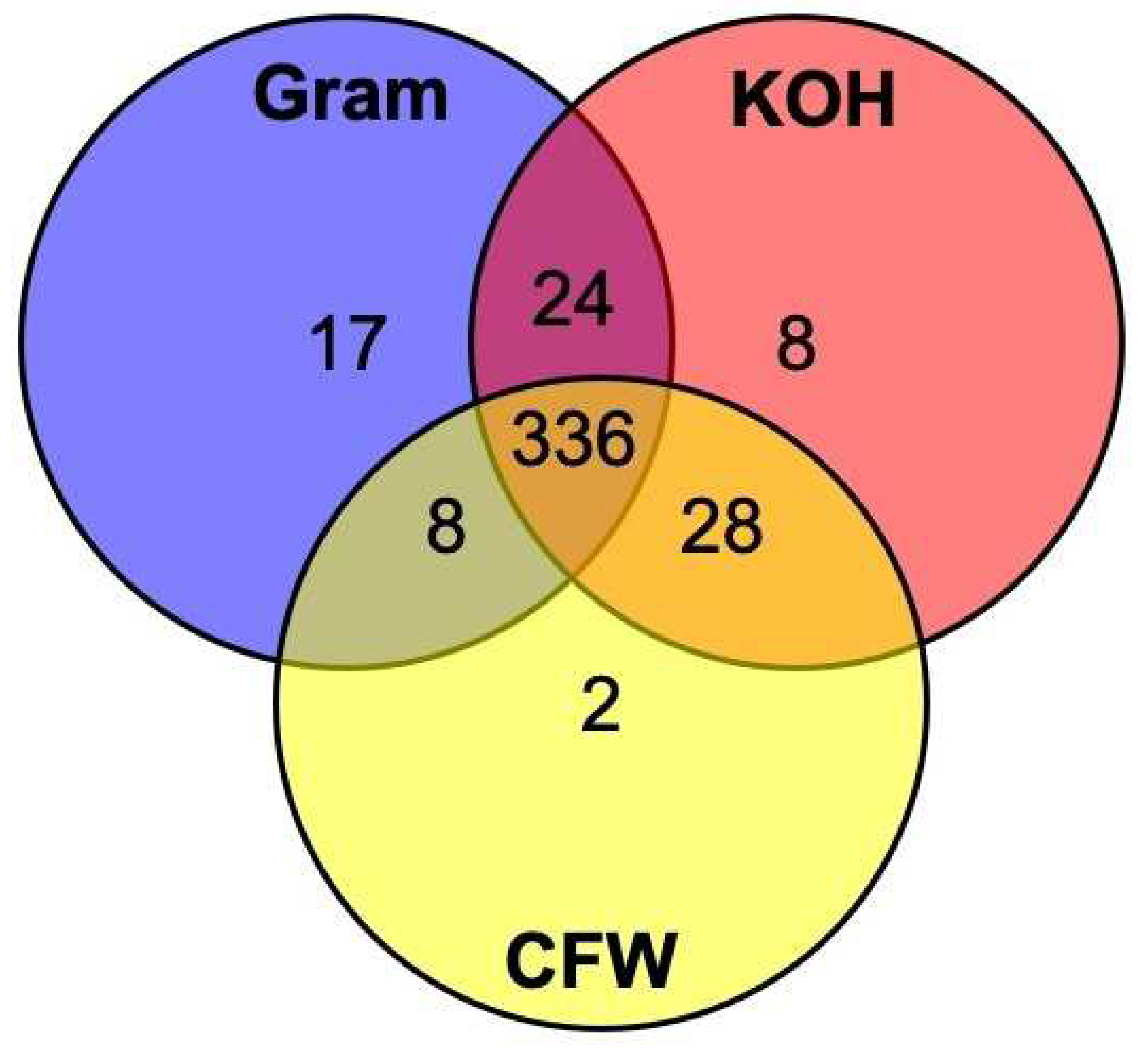
| 4. Microbiology testing. We recommend the use of KOH and Gram stain on two separate specimen slides prior to inoculating culture media. Culture is also recommended, especially to diagnose bacterial cases, but facilities may not be available. The use of CFW is a helpful addition if a UV microscope is available as it allows for the hyphae to be clearly visible in relation to the septae. Smear microscopy should be performed as soon as possible after taking the specimen, to enable a diagnosis to be made and treatment to be started before the patient leaves the facility. A strong working partnership with the hospital laboratory is desirable. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, J.J.; Yadav, R.; Sanyam, S.D.; Chaudhary, P.; Roshan, A.; Singh, S.K.; Arunga, S.; Hu, V.H.; Macleod, D.; Leck, A.; et al. Diagnosis of Fungal Keratitis in Low-Income Countries: Evaluation of Smear Microscopy, Culture, and In Vivo Confocal Microscopy in Nepal. J. Fungi 2022, 8, 955. https://doi.org/10.3390/jof8090955
Hoffman JJ, Yadav R, Sanyam SD, Chaudhary P, Roshan A, Singh SK, Arunga S, Hu VH, Macleod D, Leck A, et al. Diagnosis of Fungal Keratitis in Low-Income Countries: Evaluation of Smear Microscopy, Culture, and In Vivo Confocal Microscopy in Nepal. Journal of Fungi. 2022; 8(9):955. https://doi.org/10.3390/jof8090955
Chicago/Turabian StyleHoffman, Jeremy J., Reena Yadav, Sandip Das Sanyam, Pankaj Chaudhary, Abhishek Roshan, Sanjay Kumar Singh, Simon Arunga, Victor H. Hu, David Macleod, Astrid Leck, and et al. 2022. "Diagnosis of Fungal Keratitis in Low-Income Countries: Evaluation of Smear Microscopy, Culture, and In Vivo Confocal Microscopy in Nepal" Journal of Fungi 8, no. 9: 955. https://doi.org/10.3390/jof8090955
APA StyleHoffman, J. J., Yadav, R., Sanyam, S. D., Chaudhary, P., Roshan, A., Singh, S. K., Arunga, S., Hu, V. H., Macleod, D., Leck, A., & Burton, M. J. (2022). Diagnosis of Fungal Keratitis in Low-Income Countries: Evaluation of Smear Microscopy, Culture, and In Vivo Confocal Microscopy in Nepal. Journal of Fungi, 8(9), 955. https://doi.org/10.3390/jof8090955







