Penicillium digitatum as a Model Fungus for Detecting Antifungal Activity of Botanicals: An Evaluation on Vietnamese Medicinal Plant Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Samples
2.2. Fungal Strains and Spore Preparation
2.3. Preparation of Plant Extracts
2.4. In Vitro Antifungal Activity Assays
2.5. Antifungal Activity Assays of Plant Extracts against P. digitatum on Citrus Fruits
2.6. Enrichment and Isolation of Alkaloids from M. bealei Ethanolic Extract
2.7. Quantitation of Berberine and Palmatine
2.8. Statistical Analysis
3. Results
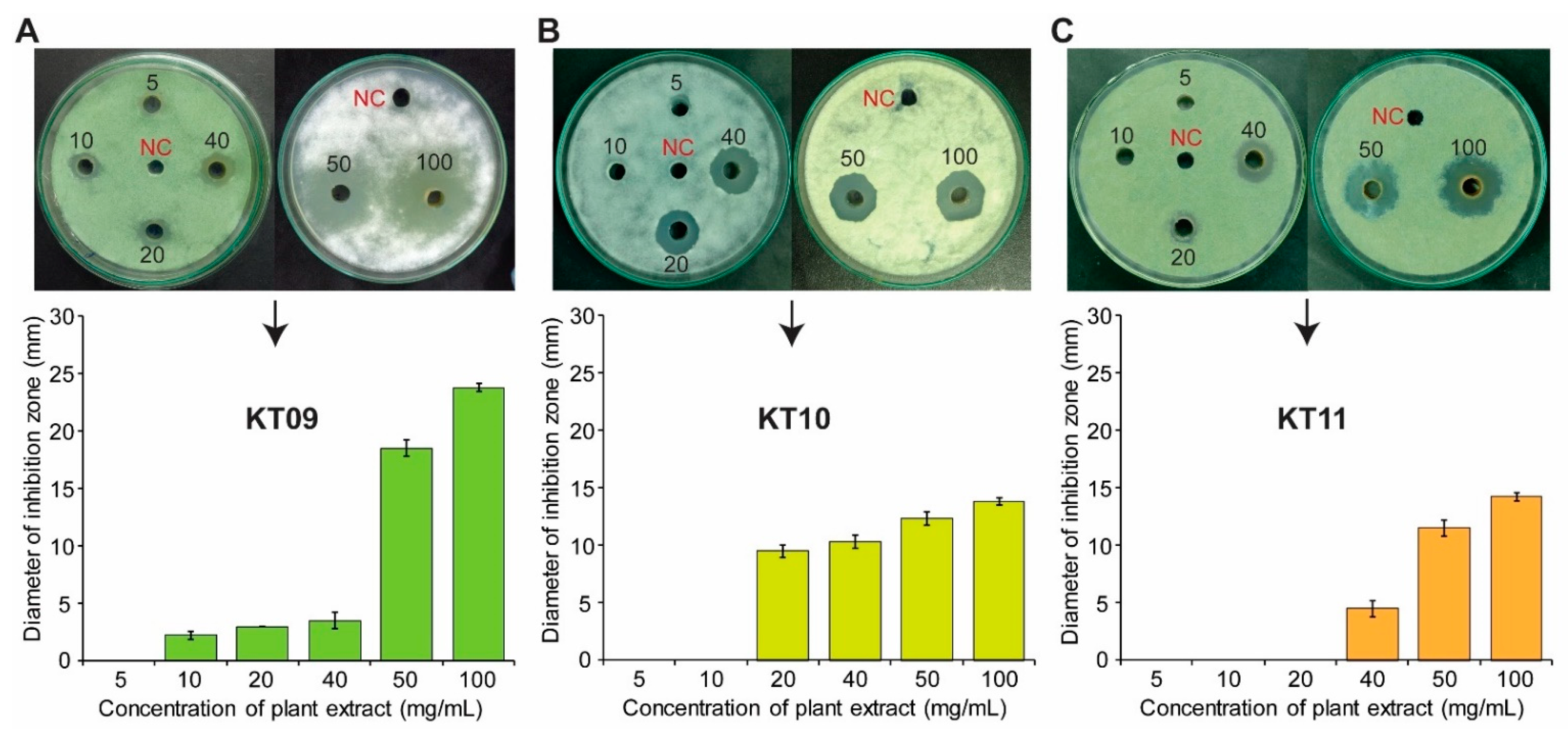
3.1. Three Medicinal Plant Species Possess Antifungal Activity
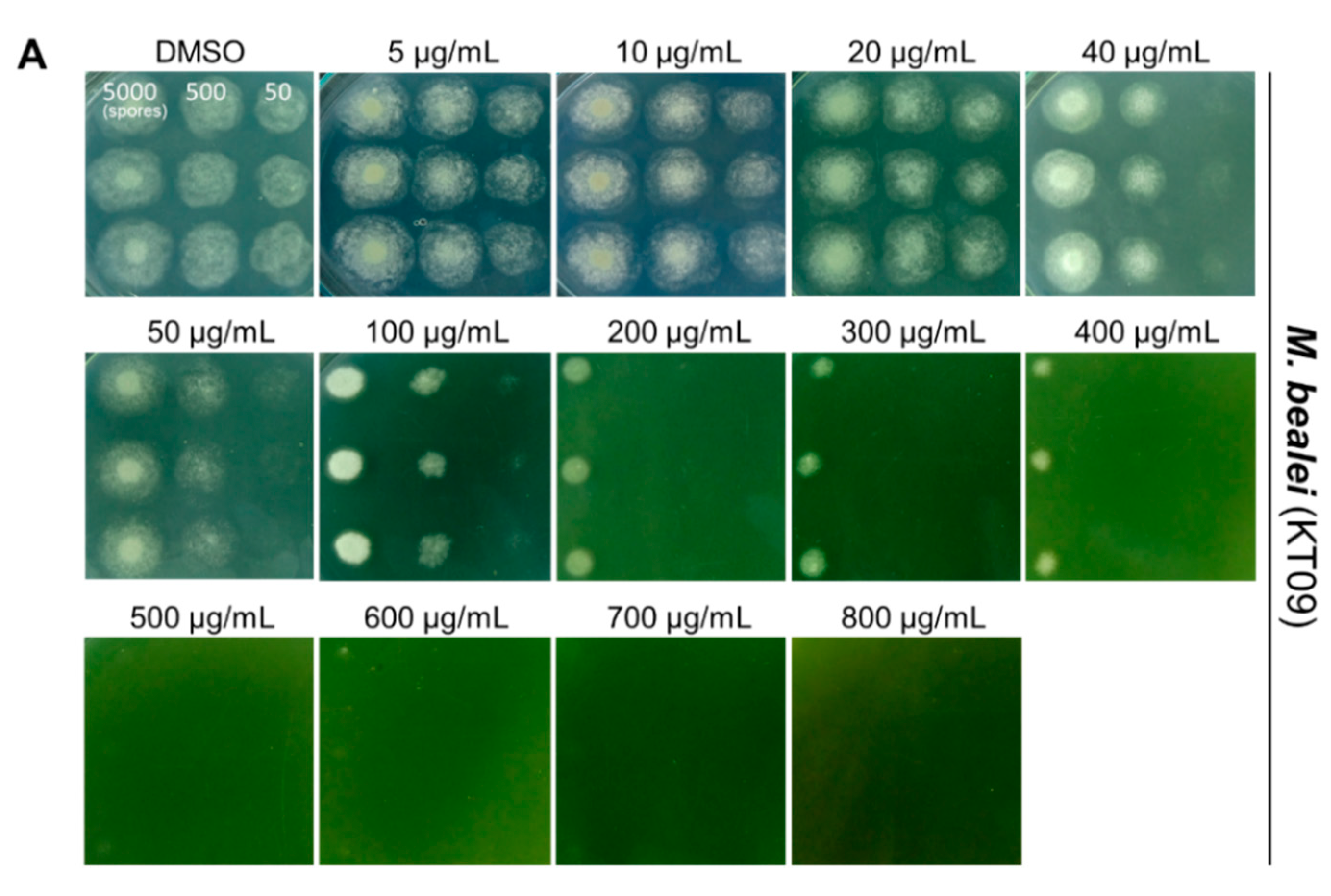
3.2. Evaluating Antifungal Activity of Three Plant Extracts against P. digitatum
3.3. Three Selected Plant Extracts Suppress the Invasion of P. digitatum on Citrus Fruits
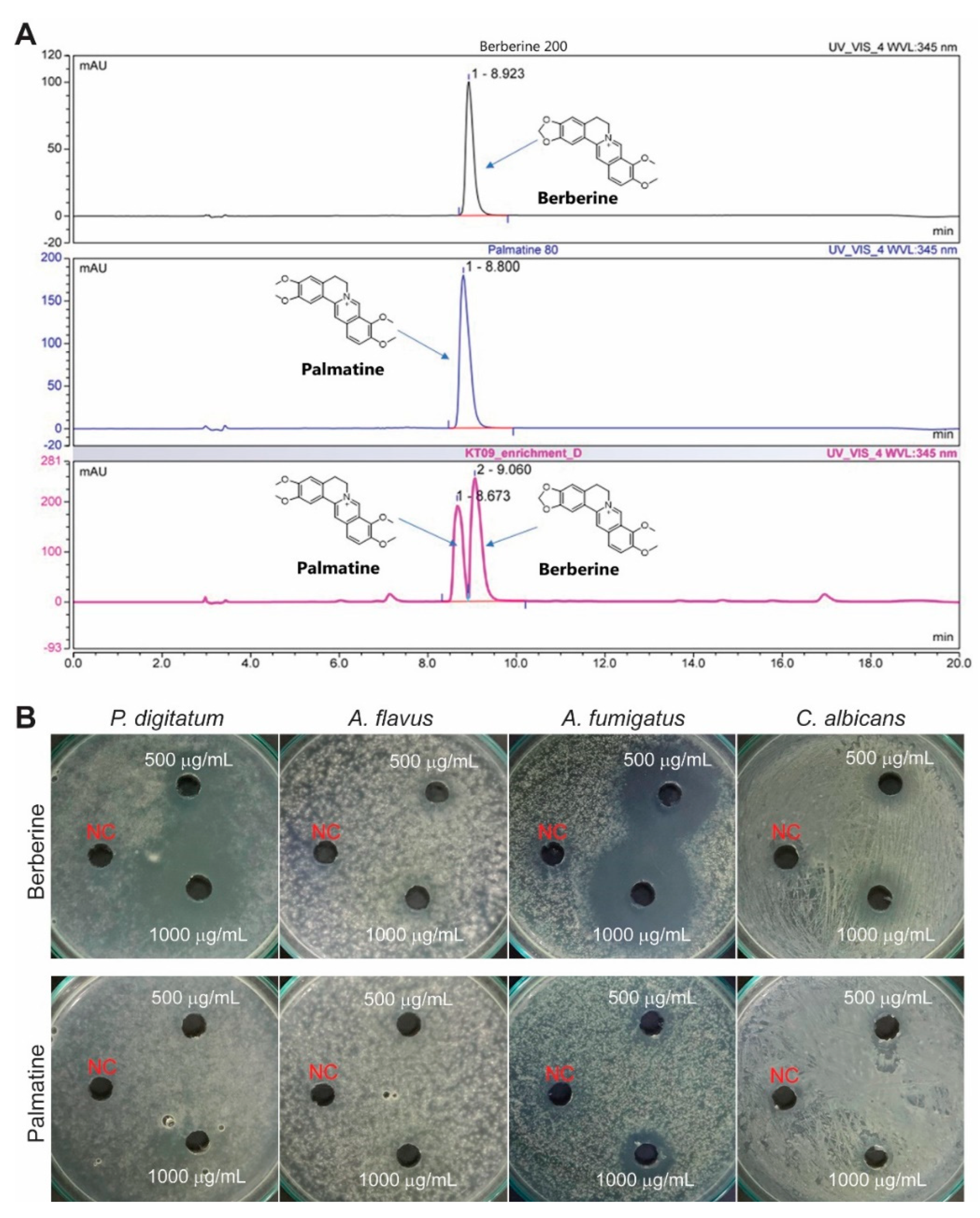
3.4. Berberine of the M. bealei Ethanolic Extract as a Major Antifungal Compound
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurnaz, M.L.; Kurnaz, I.A. Commercialization of medicinal bioeconomy resources and sustainability. Sustain. Chem. Pharm. 2021, 22, 100484. [Google Scholar] [CrossRef]
- Astutik, S.; Pretzsch, J.; Kimengsi, J.N. Asian medicinal plants’ production and utilization potentials: A review. Sustainability 2019, 11, 5483. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Lee, S.; Kang, S.S.; Shin, H.S. Selected commercial plants: A review of extraction and isolation of bioactive compounds and their pharmacological market value. Trends Food Sci. Technol. 2018, 82, 89–109. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Yang, L.; Yang, C.; Li, C.; Zhao, Q.; Liu, L.; Fang, X.; Chen, X.Y. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. 2016, 61, 3–17. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 2020, 121, 3495–3560. [Google Scholar] [CrossRef]
- Geddes-McAlister, J.; Shapiro, R.S. New pathogens, new tricks: Emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef]
- Bhatta, U.K. Alternative management approaches of citrus diseases caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Front. Plant Sci. 2022, 12, 833328. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Yaghmour, R.M.R.; Faidi, Y.R.; Salem, K.; Al-Nuri, M.A. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef]
- Giordani, C.; Simonetti, G.; Natsagdorj, D.; Choijamts, G.; Ghirga, F.; Calcaterra, A.; Quaglio, D.; De Angelis, G.; Toniolo, C.; Pasqua, G. Antifungal activity of Mongolian medicinal plant extracts. Nat. Prod. Res. 2019, 34, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.; Taschereau, P.; Belland, R.J.; Sand, C.; Rennie, R.P. Antifungal activity of medicinal plant extracts; preliminary screening studies. J. Ethnopharmacol. 2008, 115, 140–146. [Google Scholar] [CrossRef]
- Hoffman, B.R.; DelasAlas, H.; Blanco, K.; Wiederhold, N.; Lewis, R.E.; Williams, L. Screening of antibacterial and antifungal activities of ten medicinal plants from Ghana. Pharm. Biol. 2008, 42, 13–17. [Google Scholar] [CrossRef]
- Dabur, R.; Singh, H.; Chhillar, A.K.; Ali, M.; Sharma, G.L. Antifungal potential of Indian medicinal plants. Fitoterapia 2004, 75, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Tillhon, M.; Ortiz, L.G.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Le Pape, P.; Ganesan, A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2021, 71, 150–169. [Google Scholar] [CrossRef]
- Khetabi, A.E.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; Ghadraoui, L.E.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Makhuvele, R.; Naidu, K.; Gbashi, S.; Thipe, V.C.; Adebo, O.A.; Njobeh, P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon 2020, 6, e05291. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, D.; Gao, C.; Li, J.; Du, B.; Wang, Z.; Qian, S. Recent advances for alkaloids as botanical pesticides for use in organic agriculture. Int. J. Pest Manag. 2021, 1–11. [Google Scholar] [CrossRef]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural products from medicinal plants against phytopathogenic Fusarium species: Current research endeavours, challenges and prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.H.; Bazioli, J.M.; de Moraes Pontes, J.G.; Fill, T.P. Penicillium digitatum infection mechanisms in citrus: What do we know so far? Fungal Biol. 2019, 123, 584–593. [Google Scholar] [CrossRef]
- Sánchez-Torres, P. Molecular mechanisms underlying fungicide resistance in citrus postharvest green mold. J. Fungi 2021, 7, 783. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Do, T.L.; Nguyen, X.D. Native drugs of Vietnam: Which traditional and scientific approaches? J. Ethnopharmacol. 1991, 32, 51–56. [Google Scholar] [CrossRef]
- Hoang, S.V.; Baas, P.; Keßler, P.J.A. Traditional medicinal plants in Ben En National Park, Vietnam. Blumea 2008, 53, 569–601. [Google Scholar] [CrossRef]
- On, T.V.; Quyen, D.; Bich, L.D.; Jones, B.; Wunder, J.; Russell-Smith, J. A survey of medicinal plants in BaVi National Park, Vietnam: Methodology and implications for conservation and sustainable use. Biol. Conserv. 2001, 97, 295–304. [Google Scholar] [CrossRef]
- Giang, P.M.; Otsuka, H. New compounds and potential candidates for drug discovery from medicinal plants of Vietnam. Chem. Pharm. Bull. 2018, 66, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, S.Y.; Eum, S.; Paik, J.H.; Bach, T.T.; Darshetkar, A.M.; Choudhary, R.K.; Hai, D.V.; Quang, B.H.; Thanh, N.T.; et al. Ethnobotanical study on medicinal plants used by local Van Kieu ethnic people of Bac Huong Hoa nature reserve, Vietnam. J. Ethnopharmacol. 2019, 231, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.M.A.; Bun, S.S.; Ollivier, E.; Dang, T.P.T. Ethnobotanical study of medicinal plants used by K’Ho-Cil people for treatment of diarrhea in Lam Dong Province, Vietnam. J. Herb. Med. 2020, 19, 100320. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Le Tran, Q.; Kadota, S. Chemical constituents and biological activities of Vietnamese medicinal plants. Curr. Top. Med. Chem. 2003, 3, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.Y.; Tezuka, Y.; Banskota, A.H.; Tran, Q.L.; Tran, Q.K.; Harimaya, Y.; Saiki, I.; Kadota, S. Antiproliferative activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2002, 25, 753–760. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Tran, Q.L.; Watanabe, H.; Kadota, S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2004, 27, 1414–1421. [Google Scholar] [CrossRef]
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Nguyen, V.B.; Eun, J.B.; Wang, S.L.; Nguyen, D.H.; Tran, T.N.; Nguyen, A.D. Anti-oxidant and antidiabetic effect of some medicinal plants belong to Terminalia species collected in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2016, 42, 5859–5871. [Google Scholar] [CrossRef]
- Vu, T.T.; Kim, H.; Tran, V.K.; Dang, Q.L.; Nguyen, H.T.; Kim, H.; Kim, I.S.; Choi, G.J.; Kim, J.C. In vitro antibacterial activity of selected medicinal plants traditionally used in Vietnam against human pathogenic bacteria. BMC Complementary Altern. Med. 2016, 16, 32. [Google Scholar] [CrossRef]
- Nguyen-Vo, T.H.; Le, T.; Pham, D.; Nguyen, T.; Le, P.; Nguyen, A.; Nguyen, T.; Nguyen, T.N.; Nguyen, V.; Do, H.; et al. VIETHERB: A database for Vietnamese herbal species. J. Chem. Inf. Modeling 2018, 59, 1–9. [Google Scholar] [CrossRef]
- Vu, X.T.; Ngo, T.T.; Mai, T.D.L.; Bui, T.T.; Le, H.D.; Bui, T.V.H.; Nguyen, Q.H.; Ngo, X.B.; Tran, V.T. A highly efficient Agrobacterium tumefaciens-mediated transformation system for the postharvest pathogen Penicillium digitatum using DsRed and GFP to visualize citrus host colonization. J. Microbiol. Methods 2018, 144, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Nierman, W.C.; Yu, J.; Fedorova-Abrams, N.D.; Losada, L.; Cleveland, T.E.; Bhatnagar, D.; Bennett, J.W.; Dean, R.; Payne, G.A. Genome sequence of Aspergillus flavus NRRL 3357, a strain that causes aflatoxin contamination of food and feed. Genome Announc. 2015, 3, e00168-15. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, T.; Jiang, Z. Fast analysis of alkaloids from different parts of Mahonia bealei (Fort.) Carr. studied for their anti-Alzheimer’s activity using supercritical fluid chromatography. J. Sep. Sci. 2021, 44, 2006–2014. [Google Scholar] [CrossRef]
- He, J.M.; Mu, Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 175, 668–683. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Liu, Q.; Luyten, W.; Pellens, K.; Wang, Y.; Wang, W.; Thevissen, K.; Liang, Q.; Cammue, B.P.A.; Schoofs, L.; Luo, G. Antifungal activity in plants from Chinese traditional and folk medicine. J. Ethnopharmacol. 2012, 143, 772–778. [Google Scholar] [CrossRef]
- Shi, Y.; Mon, A.M.; Fu, Y.; Zhang, Y.; Wang, C.; Yang, X.; Wang, Y. The genus Ficus (Moraceae) used in diet: Its plant diversity, distribution, traditional uses and ethnopharmacological importance. J. Ethnopharmacol. 2018, 226, 185–196. [Google Scholar] [CrossRef]
- Wan, C.; Chen, C.; Li, M.; Yang, Y.; Chen, M.; Chen, J. Chemical constituents and antifungal activity of Ficus hirta Vahl. fruits. Plants 2017, 6, 44. [Google Scholar] [CrossRef]
- Martin, F.; Grkovic, T.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J.; Davis, R.A. Alkaloids from the Chinese Vine Gnetum montanum. J. Nat. Prod. 2011, 74, 2425–2430. [Google Scholar] [CrossRef]
- Xiang, W.; Jiang, B.; Li, X.M.; Zhang, H.J.; Zhao, Q.S.; Li, S.H.; Sun, H.D. Constituents of Gnetum montanum. Fitoterapia 2002, 73, 40–42. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: A review. Plants 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yu, L.; Wang, M.H. Antioxidant and antiproliferative properties of water extract from Mahonia bealei (Fort.) Carr. leaves. Food Chem. Toxicol. 2011, 49, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, L.; Qiang, Q.; Ji, L.; Wang, X.; Luo, H.; Wu, H.; Jiang, Y.; Wang, G.; Shen, T. The dichloromethane fraction from Mahonia bealei (Fort.) Carr. leaves exerts an anti-inflammatory effect both in vitro and in vivo. J. Ethnopharmacol. 2016, 188, 134–143. [Google Scholar] [CrossRef]
- Wu, L.; Shen, T.; Zhou, Y.; Wu, J.; Ji, X.Y.; Si, C.L.; Hu, W.C. Secondary metabolites of Mahonia bealei branches. Chem. Nat. Compd. 2018, 54, 564–566. [Google Scholar] [CrossRef]
- Singh, I.P.; Mahajan, S. Berberine and its derivatives: A patent review (2009–2012). Expert Opin. Ther. Pat. 2012, 23, 215–231. [Google Scholar] [CrossRef]


| Plant Species (Family) | Plant Sample Codes | Parts for Extraction | Period of Sample Collection | Location of Sample Collection | Specimen Collection Codes |
|---|---|---|---|---|---|
| Clerodendrum cyrtophyllum Turcz. (Verbenaceae) | KT07 | Leaf | December 2017 | Ha Giang | HNU 024106 |
| Mahonia bealei (Fortune) Carrière (Berberidaceae) | KT09 | Stem | October 2017 | Lao Cai | HNU 024779 |
| Ficus semicordata Buch.-Ham. ex Sm. (Moraceae) | KT10 | Leaf, stem | May 2017 | Ha Giang | HNU 024780 |
| Gnetum montanum Markgr. (Gnetaceae) | KT11 | Stem | May 2017 | Ha Giang | HNU 024781 |
| Tacca chantrieri André (Dioscoreaceae) | KT12 | Root | May 2017 | Ha Giang | HNU 024782 |
| Crinum asiaticum L. (Amaryllidaceae) | KT13 | Leaf | June 2017 | Quang Ninh | HNU 024783 |
| Mallotus barbatus Müll.Arg. (Euphorbiaceae) | KT14 | Root | June 2017 | Hoa Binh | HNU 024784 |
| Aganope balansae (Gagnep.) P.K.Lôc (Fabaceae) | KT15 | Stem | October 2018 | Ha Giang | HNU 024785 |
| Hedyotis capitellata Wall. ex G.Don (Rubiaceae) | KT16 | Leaf, stem | July 2018 | Ha Giang | HNU 024786 |
| Stixis scandens Lour. (Capparaceae) | KT17 | Leaf | August 2019 | Ha Giang | HNU 024787 |
| Cymbidium aloifolium (L.) Sw. (Orchidaceae) | KT18 | Leaf, stem | August 2018 | Tuyen Quang | HNU 024788 |
| Tinospora sinensis (Lour.) Merr. (Menispermaceae) | KT20 | Stem | November 2018 | Hoa Binh | HNU 024790 |
| Plant Species | Plant Sample Codes | Water Extract | Ethanolic Extract | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pd | Afl | Afu | Ca | Pd | Afl | Afu | Ca | ||
| C. cyrtophyllum | KT07 | − | − | − | − | − | − | − | − |
| M. bealei | KT09 | − | − | − | − | +++ | + | +++ | ++ |
| F. semicordata | KT10 | ++ | − | − | − | − | − | − | − |
| G. montanum | KT11 | − | − | − | − | ++ | + | + | − |
| T. chantrieri | KT12 | − | − | − | − | − | − | − | − |
| C. asiaticum | KT13 | − | − | − | − | − | − | − | − |
| M. barbatus | KT14 | − | − | − | − | − | − | − | − |
| A. balansae | KT15 | − | − | − | − | − | − | − | − |
| H. capitellata | KT16 | − | − | − | − | − | − | − | − |
| S. scandens | KT17 | − | − | − | − | − | − | − | − |
| C. aloifolium | KT18 | − | − | − | − | − | − | − | − |
| T. sinensis | KT20 | − | − | − | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, H.M.; Le, D.H.; Nguyen, V.-A.T.; Vu, T.X.; Thanh, N.T.K.; Giang, D.H.; Dat, N.T.; Pham, H.T.; Muller, M.; Nguyen, H.Q.; et al. Penicillium digitatum as a Model Fungus for Detecting Antifungal Activity of Botanicals: An Evaluation on Vietnamese Medicinal Plant Extracts. J. Fungi 2022, 8, 956. https://doi.org/10.3390/jof8090956
Tran HM, Le DH, Nguyen V-AT, Vu TX, Thanh NTK, Giang DH, Dat NT, Pham HT, Muller M, Nguyen HQ, et al. Penicillium digitatum as a Model Fungus for Detecting Antifungal Activity of Botanicals: An Evaluation on Vietnamese Medicinal Plant Extracts. Journal of Fungi. 2022; 8(9):956. https://doi.org/10.3390/jof8090956
Chicago/Turabian StyleTran, Hanh My, Diep Hong Le, Van-Anh Thi Nguyen, Tao Xuan Vu, Nguyen Thi Kim Thanh, Do Hoang Giang, Nguyen Tien Dat, Hai The Pham, Marc Muller, Huy Quang Nguyen, and et al. 2022. "Penicillium digitatum as a Model Fungus for Detecting Antifungal Activity of Botanicals: An Evaluation on Vietnamese Medicinal Plant Extracts" Journal of Fungi 8, no. 9: 956. https://doi.org/10.3390/jof8090956
APA StyleTran, H. M., Le, D. H., Nguyen, V.-A. T., Vu, T. X., Thanh, N. T. K., Giang, D. H., Dat, N. T., Pham, H. T., Muller, M., Nguyen, H. Q., & Tran, V.-T. (2022). Penicillium digitatum as a Model Fungus for Detecting Antifungal Activity of Botanicals: An Evaluation on Vietnamese Medicinal Plant Extracts. Journal of Fungi, 8(9), 956. https://doi.org/10.3390/jof8090956








