Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI
Abstract
1. Introduction
2. Aetiology of Obesity
3. Pathophysiology of Obesity
4. Appetite Suppressing Effect of Mushrooms
5. Alteration of Adipocyte Function
6. Effect of Mushroom Consumption on Gut Microbiota
7. Mushrooms as Potential Anti-Obesity Agents
8. Discussion
9. Recommendations and Implications for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The Medical Risks of Obesity. Postgrad. Med. 2009, 121, 21. [Google Scholar] [CrossRef]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals with Type 2 Diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Sande, D.; de Oliveira, G.P.; Moura, M.A.F.e.; Martins, B.d.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Feeney, M.J.; Miller, A.M.; Roupas, P. Mushrooms—Biologically distinct and nutritionally unique: Exploring a “third food kingdom”. Nutr. Today 2014, 49, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Rincão, V.P.; Yamamoto, K.A.; Silva Ricardo, N.M.P.; Soares, S.A.; Paccola Meirelles, L.D.; Nozawa, C.; Carvalho Linhares, R.E. Polysaccharide and extracts from Lentinula edodes: Structural features and antiviral activity. Virol. J. 2012, 9, 37. [Google Scholar] [CrossRef]
- Roldan-Deamicis, A.; Alonso, E.; Brie, B.; Braico, D.A.; Balogh, G.A. Maitake Pro4X has anti-cancer activity and prevents oncogenesis in BALBc mice. Cancer Med. 2016, 5, 2427–2441. [Google Scholar] [CrossRef]
- Asanovic, S. Maitake Mushrooms as an Anti-Cancer Agent. J. Am. Diet. Assoc. 1996, 96, A44. [Google Scholar] [CrossRef]
- Landi, N.; Pacifico, S.; Ragucci, S.; Di Giuseppe, A.M.A.; Iannuzzi, F.; Zarrelli, A.; Piccolella, S.; Di Maro, A. Pioppino mushroom in southern Italy: An undervalued source of nutrients and bioactive compounds. J. Sci. Food Agric. 2017, 97, 5388–5397. [Google Scholar] [CrossRef]
- Ragucci, S.; Landi, N.; Russo, R.; Valletta, M.; Pedone, P.V.; Chambery, A.; Di Maro, A. Ageritin from pioppino mushroom: The prototype of ribotoxin-like proteins, a novel family of specific ribonucleases in edible mushrooms. Toxins 2021, 13, 263. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Mohamed Yahaya, N.F.; Rahman, M.A.; Abdullah, N. Therapeutic potential of mushrooms in preventing and ameliorating hypertension. Trends Food Sci. Technol. 2014, 39, 104–115. [Google Scholar] [CrossRef]
- Zhang, S.; Sugawara, Y.; Chen, S.; Beelman, R.B.; Tsuduki, T.; Tomata, Y.; Matsuyama, S.; Tsuji, I. Mushroom consumption and incident risk of prostate cancer in Japan: A pooled analysis of the Miyagi Cohort Study and the Ohsaki Cohort Study. Int. J. Cancer 2020, 146, 2712–2720. [Google Scholar] [CrossRef]
- Ba, D.; Ssentongo, P.; Beelman, R.; Gao, X.; Richie, J. Mushroom Consumption Is Associated with Low Risk of Cancer: A Systematic Review and Meta-Analysis of Observation Studies. Curr. Dev. Nutr. 2020, 4, 307. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Perricone, N.V. Maitake mushroom extracts ameliorate progressive hypertension and other chronic metabolic perturbations in aging female rats. Int. J. Med. Sci. 2010, 7, 169–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, L.; Niu, Z. A mushroom diet reduced the risk of pregnancy-induced hyper tension and macrosomia: A randomized clinical trial. Food Nutr. Res. 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef]
- Grotto, D.; Camargo, I.F.; Kodaira, K.; Mazzei, L.G.; Castro, J.; Vieira, R.A.L.; Bergamaschi, C.D.C.; Lopes, L.C. Effect of mushrooms on obesity in animal models: Study protocol for a systematic review and meta-analysis. Syst. Rev. 2019, 8, 288. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Nabatanzi, A.; Steinmann, C.M.L.; Eloff, J.N. Phytochemical, cytotoxicity, antioxidant and anti-inflammatory effects of psilocybe natalensis magic mushroom. Plants 2020, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhu, F.; Xu, B. An insight into the anti-inflammatory properties of edible and medicinal mushrooms. J. Funct. Foods 2018, 47, 334–342. [Google Scholar] [CrossRef]
- Hetland, G.; Tangen, J.M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Sakthivel, M.; Thomas, P.A.; Geraldine, P. Pleurotus ostreatus, an oyster mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain. Chem. Biol. Interact. 2008, 176, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Bobek, P.; Ozdín, L.; Galbavý, Š. Dose- and Time-Dependent Hypocholesterolemic Effect of Oyster Mushroom (Pleurotus ostreatus) in Rats. Nutrition 1998, 14, 282–286. [Google Scholar] [CrossRef]
- Piskov, S.; Timchenko, L.; Grimm, W.D.; Rzhepakovsky, I.; Avanesyan, S.; Sizonenko, M.; Kurchenko, V. Effects of various drying methods on some physico-chemical properties and the antioxidant profile and ACE inhibition activity of oyster mushrooms (Pleurotus ostreatus). Foods 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M. Effect of aqueous extracts of Pleurotus ostreatus and Lentinus subnudus on activity of adenosine deaminase, arginase, cholinergic enzyme, and angiotensin-1-converting enzyme. J. Food Biochem. 2021, 45, e13490. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; Rahman, T.; Kakon, A.; Hoque, N.; Akhtaruzzaman, M.; Begum, M.; Choudhuri, M.; Hossain, M. Effects of Pleurotus ostreatus on Blood Pressure and Glycemic Status of Hypertensive Diabetic Male Volunteers. Bangladesh J. Med. Biochem. 2013, 6, 5–10. [Google Scholar] [CrossRef]
- Chen, C.H.; Wu, J.Y.; Chen, C.H.; Chang, W.H.; Chung, K.T.; Liu, Y.W.; Lu, F.J. Anti-cancer effects of protein extracts from Calvatia lilacina, Pleurotus ostreatus and Volvariella volvacea. Evid. Based Complement. Altern. Med. 2011, 2011, 982368. [Google Scholar] [CrossRef]
- Sarangi, I.; Ghosh, D.; Bhutia, S.K.; Mallick, S.K.; Maiti, T.K. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int. Immunopharmacol. 2006, 6, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flores, H.E.; Contreras-Chávez, R.; Garnica-Romo, M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1406–1418. [Google Scholar] [CrossRef]
- Sekan, A.S.; Myronycheva, O.S.; Karlsson, O.; Gryganskyi, A.P.; Blume, Y. Green potential of Pleurotus spp. in biotechnology. PeerJ 2019, 7, e6664. [Google Scholar] [CrossRef]
- Jeong, Y.U.; Park, Y.J. Ergosterol peroxide from the medicinal mushroom Ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. Int. J. Mol. Sci. 2020, 21, 460. [Google Scholar] [CrossRef]
- Huang, H.T.; Ho, C.H.; Sung, H.Y.; Lee, L.Y.; Chen, W.P.; Chen, Y.W.; Chen, C.C.; Yang, C.S.; Tzeng, S.F. Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein. Sci. Rep. 2021, 11, 6551. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Thyagarajan-Sahu, A.; Lane, B.; Sliva, D. ReishiMax, mushroom based dietary supplement, inhibits adipocyte differentiation, stimulates glucose uptake and activates AMPK. BMC Complement. Altern. Med. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.L.; Sun, H.Q.; Zhang, X.J.; Wu, L.R.; Zhu, Z.Y. A novel polysaccharide from Pleurotus citrinopileatus mycelia: Structural characterization, hypoglycemic activity and mechanism. Food Biosci. 2020, 37, 100735. [Google Scholar] [CrossRef]
- Chen, P.H.; Weng, Y.M.; Yu, Z.R.; Koo, M.; Wang, B.J. Extraction temperature affects the activities of antioxidation, carbohydrate-digestion enzymes, and angiotensin-converting enzyme of Pleurotus citrinopileatus extract. J. Food Drug Anal. 2016, 24, 548–555. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, L.L.; Liu, H.P.; Wu, Y.R.; Li, M.Y.; Jia, Q. Structural characterization of a novel polysaccharide from Pleurotus citrinopileatus and its antitumor activity on H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and hypolipidemic effect of water extract from Pleurotus citrinopileatus in C57BL/6J mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef]
- Seaman, D.R. Weight gain as a consequence of living a modern lifestyle: A discussion of barriers to effective weight control and how to overcome them. J. Chiropr. Humanit. 2013, 20, 27–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panahi, S.; Tremblay, A. Sedentariness and Health: Is Sedentary Behavior More Than Just Physical Inactivity? Front. Public Health 2018, 6, 258. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Ghimire, P.; Chimoriya, R.; Chimoriya, R. Trends in the Prevalence of Overweight and Obesity and Associated Socioeconomic and Household Environmental Factors among Women in Nepal: Findings from the Nepal Demographic and Health Surveys. Obesities 2021, 1, 113–135. [Google Scholar] [CrossRef]
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Kovacs, P.; Guiu-Jurado, E. Genetics of Obesity in East Asians. Front. Genet. 2020, 11, 575049. [Google Scholar] [CrossRef]
- Choquet, H.; Meyre, D. Genetics of Obesity: What have we Learned? Curr. Genom. 2012, 12, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, D.; Nóbrega, C.; Manco, L.; Padez, C. The contribution of genetics and environment to obesity. Br. Med. Bull. 2017, 123, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, A.; Novalbos Ruiz, J.P.; Martínez Nieto, J.M.; Escobar Jiménez, L. Life-style factors associated with overweight and obesity among Spanish adults. Nutr. Hosp. 2009, 24, 144–151. [Google Scholar] [PubMed]
- Bilger, M.; Kruger, E.J.; Finkelstein, E.A. Measuring Socioeconomic Inequality in Obesity: Looking Beyond the Obesity Threshold. Heal. Econ. 2017, 26, 1052–1066. [Google Scholar] [CrossRef]
- Kjellberg, J.; Tange Larsen, A.; Ibsen, R.; Højgaard, B. The socioeconomic burden of obesity. Obes. Facts 2017, 10, 493–502. [Google Scholar] [CrossRef]
- McLaren, L. Socioeconomic status and obesity. Epidemiol. Rev. 2007, 29, 29–48. [Google Scholar] [CrossRef]
- Bhurosy, T.; Jeewon, R. Overweight and obesity epidemic in developing countries: A problem with diet, physical activity, or socioeconomic status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and Diabetes in the Developing World—A Growing Challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Venema, K. The art of targeting gut microbiota for tackling human obesity. Genes Nutr. 2015, 10, 20. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Harris, K.; Kassis, A.; Major, G.; Chou, C.J. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J. Obes. 2012, 2012, 879151. [Google Scholar] [PubMed]
- Harakeh, S.M.; Khan, I.; Kumosani, T.; Barbour, E.; Almasaudi, S.B.; Bahijri, S.M.; Alfadul, S.M.; Ajabnoor, G.M.A.; Azhar, E.I. Gut microbiota: A contributing factor to obesity. Front. Cell. Infect. Microbiol. 2016, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Oddo, V.M.; Maehara, M.; Izwardy, D.; Sugihantono, A.; Ali, P.B.; Rah, J.H. Risk factors for nutrition-related chronic disease among adults in Indonesia. PLoS ONE 2019, 14, e0221927. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
- Garaulet, M.; Ordovás, J.M.; Madrid, J.A. The chronobiology, etiology and pathophysiology of obesity. Int. J. Obes. 2010, 34, 1667–1683. [Google Scholar] [CrossRef]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The role of the autonomic nervous system in the pathophysiology of obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, S.; Wu, R.; Su, X.; Peng, D.; Zhao, M.; Su, Y. New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 2019, 18, 171. [Google Scholar] [CrossRef]
- Suárez-Carmona, W.; Sánchez-Oliver, A.J.; González-Jurado, J.A. Pathophysiology of obesity: Current view. Rev. Chil. Nutr. 2017, 44, 226–233. [Google Scholar] [CrossRef]
- Hess, J.M.; Wang, Q.; Kraft, C.; Slavin, J.L. Impact of Agaricus bisporus mushroom consumption on satiety and food intake. Appetite 2017, 117, 179–185. [Google Scholar] [CrossRef]
- Greenhill, C. Obesity: Celastrol identified as a leptin sensitizer and potential novel treatment for obesity. Nat. Rev. Endocrinol. 2015, 11, 444. [Google Scholar] [CrossRef]
- Yimam, M.; Jiao, P.; Hong, M.; Brownell, L.; Lee, Y.C.; Hyun, E.J.; Kim, H.J.; Kim, T.W.; Nam, J.B.; Kim, M.R.; et al. Appetite Suppression and Antiobesity Effect of a Botanical Composition Composed of Morus alba, Yerba mate, and Magnolia officinalis. J. Obes. 2016, 2016, 4670818. [Google Scholar] [CrossRef]
- Yimam, M.; Jiao, P.; Hong, M.; Brownell, L.; Lee, Y.C.; Kim, H.J.; Nam, J.B.; Kim, M.R.; Jia, Q. Morus alba, a Medicinal Plant for Appetite Suppression and Weight Loss. J. Med. Food 2019, 22, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Yoon, S.J.; Pyun, Y.R. Polysaccharides from edible mushroom Hinmogi (Tremella fuciformis) inhibit differentiation of 3T3-L1 adipocytes by reducing mRNA expression of PPARγ, C/EBPα, and leptin. Food Sci. Biotechnol. 2008, 17, 267–273. [Google Scholar]
- Fukushima, M.; Nakano, M.; Morii, Y.; Ohashi, T.; Fujiwara, Y.; Sonoyama, K. Hepatic LDL receptor mRNA in rats is increased by dietary mushroom (Agaricus bisporus) fiber and sugar beet fiber. J. Nutr. 2000, 130, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Ma, Y.; Geng, L.; Zhao, A.; Zheng, J.; Xu, C.P. Fermentation characteristics in stirred-tank reactor of exopolysaccharides with hypolipidemic activity produced by Pleurotus geesteranus 5#. An. Acad. Bras. Cienc. 2013, 85, 1473–1481. [Google Scholar] [CrossRef]
- Hiwatashi, K.; Kosaka, Y.; Suzuki, N.; Hata, K.; Mukaiyama, T.; Sakamoto, K.; Shirakawa, H.; Komai, M. Yamabushitake mushroom (Hericium erinaceus) improved lipid metabolism in mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010, 74, 1447–1451. [Google Scholar] [CrossRef]
- Yang, B.K.; Park, J.B.; Song, C.H. Hypolipidemic effect of an exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci. Biotechnol. Biochem. 2003, 67, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hwang, I.; Kim, S.; Hong, E.J.; Jeung, E.B. Lentinus edodes promotes fat removal in hypercholesterolemic mice. Exp. Ther. Med. 2013, 6, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.N.; Lee, J.S.; Kim, H.Y.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Alam, N.; Ha, T.M.; Lee, T.S. Appraisal of antihyperlipidemic activities of Lentinus lepideus in hypercholesterolemic rats. Mycobiology 2011, 39, 283–289. [Google Scholar] [CrossRef]
- Spim, S.R.V.; de Oliveira, B.G.C.C.; Leite, F.G.; Gerenutti, M.; Grotto, D. Effects of Lentinula edodes consumption on biochemical, hematologic and oxidative stress parameters in rats receiving high-fat diet. Eur. J. Nutr. 2017, 56, 2255–2264. [Google Scholar] [CrossRef]
- Zheng, L.; Zhai, G.; Zhang, J.; Wang, L.; Ma, Z.; Jia, M.; Jia, L. Antihyperlipidemic and hepatoprotective activities of mycelia zinc polysaccharide from Pholiota nameko SW-02. Int. J. Biol. Macromol. 2014, 70, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Yoon, K.N.; Lee, J.S.; Cho, H.J.; Shim, M.J.; Lee, T.S. Dietary effect of Pleurotus eryngii on biochemical function and histology in hypercholesterolemic rats. Saudi J. Biol. Sci. 2011, 18, 403–409. [Google Scholar] [CrossRef]
- Alam, N.; Yoon, K.N.; Lee, T.S. Antihyperlipidemic activities of Pleurotus ferulae on biochemical and histological function in hypercholesterolemic rats. J. Res. Med. Sci. 2011, 16, 776–786. [Google Scholar]
- Alam, N.; Yoon, K.N.; Lee, T.S.; Lee, U.Y. Hypolipidemic activities of dietary Pleurotus ostreatus in hypercholesterolemic rats. Mycobiology 2011, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.N.; Alam, N.; Shim, M.J.; Lee, T.S. Hypolipidemic and antiatherogenesis effect of culinary-medicinal pink oyster mushroom, pleurotus salmoneostramineus L. Vass. (higher Basidiomycetes), in hypercholesterolemic rats. Int. J. Med. Mushrooms 2012, 14, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Korivi, M.; Yang, H.T.; Huang, C.C.; Chaing, Y.Y.; Tsai, Y.C. Effect of Pleurotus tuber-regium polysaccharides supplementation on the progression of diabetes complications in obese-diabetic rats. Chin. J. Physiol. 2014, 57, 198–208. [Google Scholar] [CrossRef]
- Bobek, P.; Ozdín, L.; Kuniak, L.; Hromadová, M. Regulation of cholesterol metabolism with dietary addition of oyster mushrooms (Pleurotus ostreatus) in rats with hypercholesterolemia. Casopis Lekaru Ceskych 1997, 136, 186–190. [Google Scholar]
- Kasabri, V.; Al-Hallaq, E.K.; Bustanji, Y.K.; Abdul-Razzak, K.K.; Abaza, I.F.; Afifi, F.U. Antiobesity and antihyperglycaemic effects of adiantum capillus-veneris extracts: In vitro and in vivo evaluations. Pharm. Biol. 2017, 55, 164–172. [Google Scholar] [CrossRef]
- Kim, S.J.; Bang, C.Y.; Guo, Y.R.; Choung, S.Y. Anti-Obesity Effects of Aster spathulifolius Extract in High-Fat Diet-Induced Obese Rats. J. Med. Food 2016, 19, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, H.; Liu, H.; Xiong, L.; Gao, X.; Jia, H.; Lian, Z.; Tong, N.; Han, T. Hypocholesterolemic effects of Kluyveromyces marxianus M3 isolated from Tibetan mushrooms on diet-induced hypercholesterolemia in rat. Braz. J. Microbiol. 2015, 46, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.A.; Hossain, M.A.; Damte, D.; Jo, W.S.; Hsu, W.H.; Park, S.C. Hypolipidemic and hepatic steatosis preventing activities of the wood ear medicinal mushroom auricularia auricula-judae (Higher basidiomycetes) ethanol extract in vivo and in vitro. Int. J. Med. Mushrooms 2015, 17, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.K.; Jeong, S.C.; Lee, H.J.; Sohn, D.H.; Song, C.H. Antidiabetic and hypolipidemic effects of Collybia confluens mycelia produced by submerged culture in streptozotocin-diabetic rats. Arch. Pharm. Res. 2006, 29, 73–79. [Google Scholar] [CrossRef]
- Wang, L.; Xu, N.; Zhang, J.; Zhao, H.; Lin, L.; Jia, S.; Jia, L. Antihyperlipidemic and hepatoprotective activities of residue polysaccharide from Cordyceps militaris SU-12. Carbohydr. Polym. 2015, 131, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.Y.; Ko, W.C.; Lin, L.Y. Hypolipidemic and Antioxidant Activity of Enoki Mushrooms (Flammulina velutipes). Biomed. Res. Int. 2014, 2014, 352385. [Google Scholar] [CrossRef]
- Fukushima, M.; Ohashi, T.; Fujiwara, Y.; Sonoyama, K.; Nakano, M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. 2001, 226, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Rong, C.; Liu, Y.; Xu, F.; Wang, S.; Duan, C.; Chen, J.; Wu, X. Extraction of a soluble polysaccharide from Auricularia polytricha and evaluation of its anti-hypercholesterolemic effect in rats. Carbohydr. Polym. 2015, 122, 39–45. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Evaluation of the antioxidative and hypo-cholesterolemic effects of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), in ameliorating cardiovascular disease. Int. J. Med. Mushrooms 2018, 20, 961–969. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.W.; Kim, S.; Kim, S.J. In vitro antioxidant and in vivo hypolipidemic effects of the king oyster culinary-medicinal mushroom, pleurotus eryngii var. Ferulae DDL01 (agaricomycetes), in rats with high-fat diet–induced fatty liver and hyperlipidemia. Int. J. Med. Mushrooms 2017, 19, 107–119. [Google Scholar] [CrossRef]
- Khatun, M.A.; Sato, S.; Konishi, T. Obesity preventive function of novel edible mushroom, Basidiomycetes-X (Echigoshirayukidake): Manipulations of insulin resistance and lipid metabolism. J. Tradit. Complement. Med. 2020, 10, 245–251. [Google Scholar] [CrossRef]
- Mfopa, A.; Mediesse, F.K.; Mvongo, C.; Nkoubatchoundjwen, S.; Lum, A.A.; Sobngwi, E.; Kamgang, R.; Boudjeko, T. Antidyslipidemic Potential of Water-Soluble Polysaccharides of Ganoderma applanatum in MACAPOS-2-Induced Obese Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 2452057. [Google Scholar] [CrossRef]
- Im, K.-H.; Baek, S.-A.; Choi, J.; Lee, T.-S. Anti-hyperlipidemic and anti-obesity effects of Sparassis latifolia fruiting bodies in high-fat and cholesterol-diet-induced hyperlipidemic rats. J. Mushroom 2021, 19, 23–32. [Google Scholar] [CrossRef]
- Kanwal, S.; Aliya, S.; Xin, Y. Anti-Obesity Effect of Dictyophora indusiata Mushroom Polysaccharide (DIP) in High Fat Diet-Induced Obesity via Regulating Inflammatory Cascades and Intestinal Microbiome. Front. Endocrinol. 2020, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, N.; Yoshimoto, H.; Kurihara, S.; Hamaya, T.; Eguchi, F. Improvement of diet-induced obesity by ingestion of mushroom chitosan prepared from flammulina velutipes. J. Oleo Sci. 2018, 67, 245–254. [Google Scholar] [CrossRef]
- Harada, E.; Morizono, T.; Kanno, T.; Saito, M.; Kawagishi, H. Medicinal mushroom, grifola gargal (Agaricomycetes), lowers triglyceride in animal models of obesity and diabetes and in adults with prediabetes. Int. J. Med. Mushrooms 2020, 22, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, D.; Nan, C.; Mori, K.; Hanayama, M.; Kikuchi, H.; Hirai, S.; Egashira, Y. Effect of mushroom polysaccharides from Pleurotus eryngii on obesity and gut microbiota in mice fed a high-fat diet. Eur. J. Nutr. 2020, 59, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, G.; Peng, X.; Hu, G.; Su, H.; Liu, J.; Chen, X.; Qiu, M. Triterpenoids from functional mushroom Ganoderma resinaceum and the novel role of Resinacein S in enhancing the activity of brown/beige adipocytes. Food Res. Int. 2020, 136, 109303. [Google Scholar] [CrossRef]
- Ricquier, D.; Bouillaud, F. Mitochondrial uncoupling proteins: From mitochondria to the regulation of energy balance. J. Physiol. 2000, 529, 3. [Google Scholar] [CrossRef]
- Horowitz, J.F. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol. Metab. 2003, 14, 386–392. [Google Scholar] [CrossRef]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef]
- Pongkunakorn, T.; Watcharachaisoponsiri, T.; Chupeerach, C.; On-Nom, N.; Suttisansanee, U. Inhibitions of key enzymes relevant to obesity and diabetes of thai local mushroom extracts. Curr. Appl. Sci. Technol. 2017, 17, 181–190. [Google Scholar]
- Yang, S.F.; Zhuang, T.F.; Si, Y.M.; Qi, K.Y.; Zhao, J. Coriolus versicolor mushroom polysaccharides exert immunoregulatory effects on mouse B cells via membrane Ig and TLR-4 to activate the MAPK and NF-κB signaling pathways. Mol. Immunol. 2015, 64, 144–151. [Google Scholar] [CrossRef]
- Maheshwari, G.; Gessner, D.K.; Neuhaus, K.; Most, E.; Zorn, H.; Eder, K.; Ringseis, R. Influence of a Biotechnologically Produced Oyster Mushroom (Pleurotus sajor-caju) on the Gut Microbiota and Microbial Metabolites in Obese Zucker Rats. J. Agric. Food Chem. 2021, 69, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Myrmel, L.S.; Fjære, E.; Liaset, B.; Kristiansen, K. Links between dietary protein sources, the gut microbiota, and obesity. Front. Physiol. 2017, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Zhao, C.N.; Xu, X.Y.; Tang, G.Y.; Corke, H.; Gan, R.Y.; Li, H. Bin Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, D.; Goossens, G.H.; Hermes, G.D.A.; Neis, E.P.J.G.; van der Beek, C.M.; Most, J.; Holst, J.J.; Lenaerts, K.; Kootte, R.S.; Nieuwdorp, M.; et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016, 24, 63–74. [Google Scholar] [CrossRef]
- Shimizu, T.; Mori, K.; Ouchi, K.; Kushida, M.; Tsuduki, T. Effects of dietary intake of Japanese mushrooms on visceral fat accumulation and gut microbiota in mice. Nutrients 2018, 10, 610. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.; Li, X.-A.; Liao, W.; Leong, W.K.; Xia, W.; Bian, X.; Wu, J.; Hsiao, W.W. Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in ApcMin/+ mice. Pharmacol. Res. 2019, 148, 104448. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, Q.; Chen, Y.; Lu, Y.; Wang, Y.; Jia, Y.; Zhang, M.; Chen, H. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020, 129, 108870. [Google Scholar] [CrossRef]
- Sang, H.; Xie, Y.; Su, X.; Zhang, M.; Zhang, Y.; Liu, K.; Wang, J. Mushroom Bulgaria inquinans Modulates Host Immunological Response and Gut Microbiota in Mice. Front. Nutr. 2020, 7, 144. [Google Scholar] [CrossRef]
- Lv, X.C.; Guo, W.L.; Li, L.; Yu, X.D.; Liu, B. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J. Funct. Foods 2019, 57, 48–58. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef]
- Hu, R.; Guo, W.; Huang, Z.; Li, L.; Liu, B.; Lv, X. Extracts of Ganoderma lucidum attenuate lipid metabolism and modulate gut microbiota in high-fat diet fed rats. J. Funct. Foods 2018, 46, 403–412. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.-L.; Zhang, W.; Xu, J.-X.; Qian, M.; Bai, W.-D.; Zhang, Y.-Y.; Rao, P.-F.; Ni, L.; Lv, X.-C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.L.; Shi, F.F.; Liu, G.L.; Qian, M.; Bai, W.D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wan, X.; Zeng, F.; Zhong, R.; Guo, W.; Lv, X.C.; Zhao, C.; Liu, B. Regulatory effect of Grifola frondosa extract rich in polysaccharides and organic acids on glycolipid metabolism and gut microbiota in rats. Int. J. Biol. Macromol. 2020, 155, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-Y.; Zeng, F.; Guo, W.-L.; Li, T.-T.; Jia, R.-B.; Huang, Z.-R.; Lv, X.-C.; Zhang, J.; Liu, B. Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct. 2018, 9, 6268–6278. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Li, J.; Li, T.; Zhang, Y.; Liang, Y.; Mei, Y. Phellinus linteus polysaccharide extract improves insulin resistance by regulating gut microbiota composition. FASEB J. 2020, 34, 1065–1078. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Diling, C.; Xin, Y.; Chaoqun, Z.; Jian, Y.; Xiaocui, T.; Jun, C.; Ou, S.; Yizhen, X. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 2017, 8, 85838–85857. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Su, L.; Li, D.; Shuai, O.; Zhang, Y.; Liang, H.; Jiao, C.; Xu, Z.; Lai, Y.; Xie, Y. Antitumor Activity of Extract From the Sporoderm-Breaking Spore of Ganoderma lucidum: Restoration on Exhausted Cytotoxic T Cell with Gut Microbiota Remodeling. Front. Immunol. 2018, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Pelinescu, D. Effects of mushroom consumption on the microbiota of different target groups—Impact of polyphenolic composition and mitigation on the microbiome fingerprint. LWT Food Sci. Technol. 2017, 85, 262–268. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of rich in B-glucans edible mushrooms on aging gut microbiota characteristics: An in vitro study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef]
- Poddar, K.H.; Ames, M.; Hsin-Jen, C.; Feeney, M.J.; Wang, Y.; Cheskin, L.J. Positive effect of mushrooms substituted for meat on body weight, body composition, and health parameters. A 1-year randomized clinical trial. Appetite 2013, 71, 379–387. [Google Scholar] [CrossRef]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef]
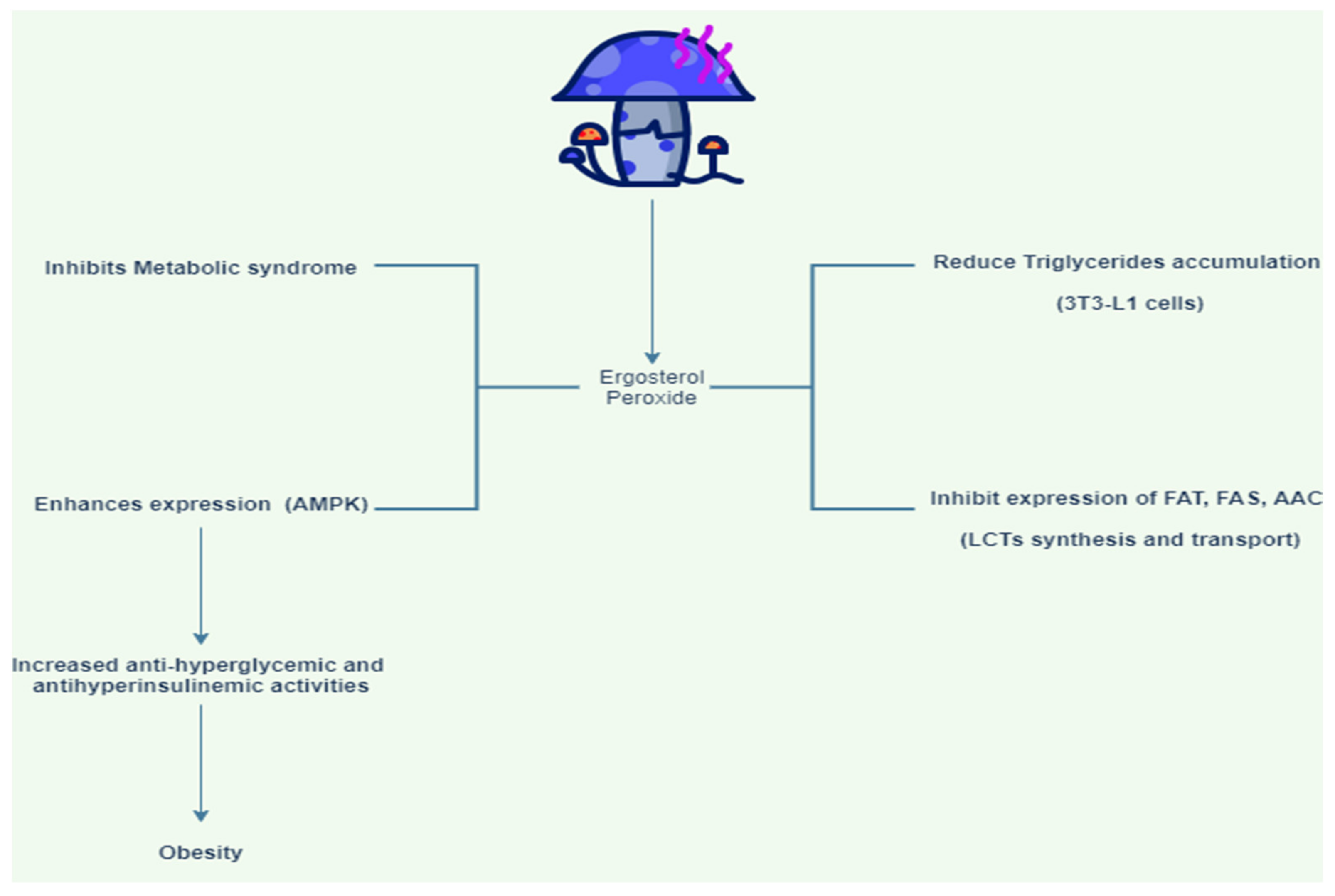
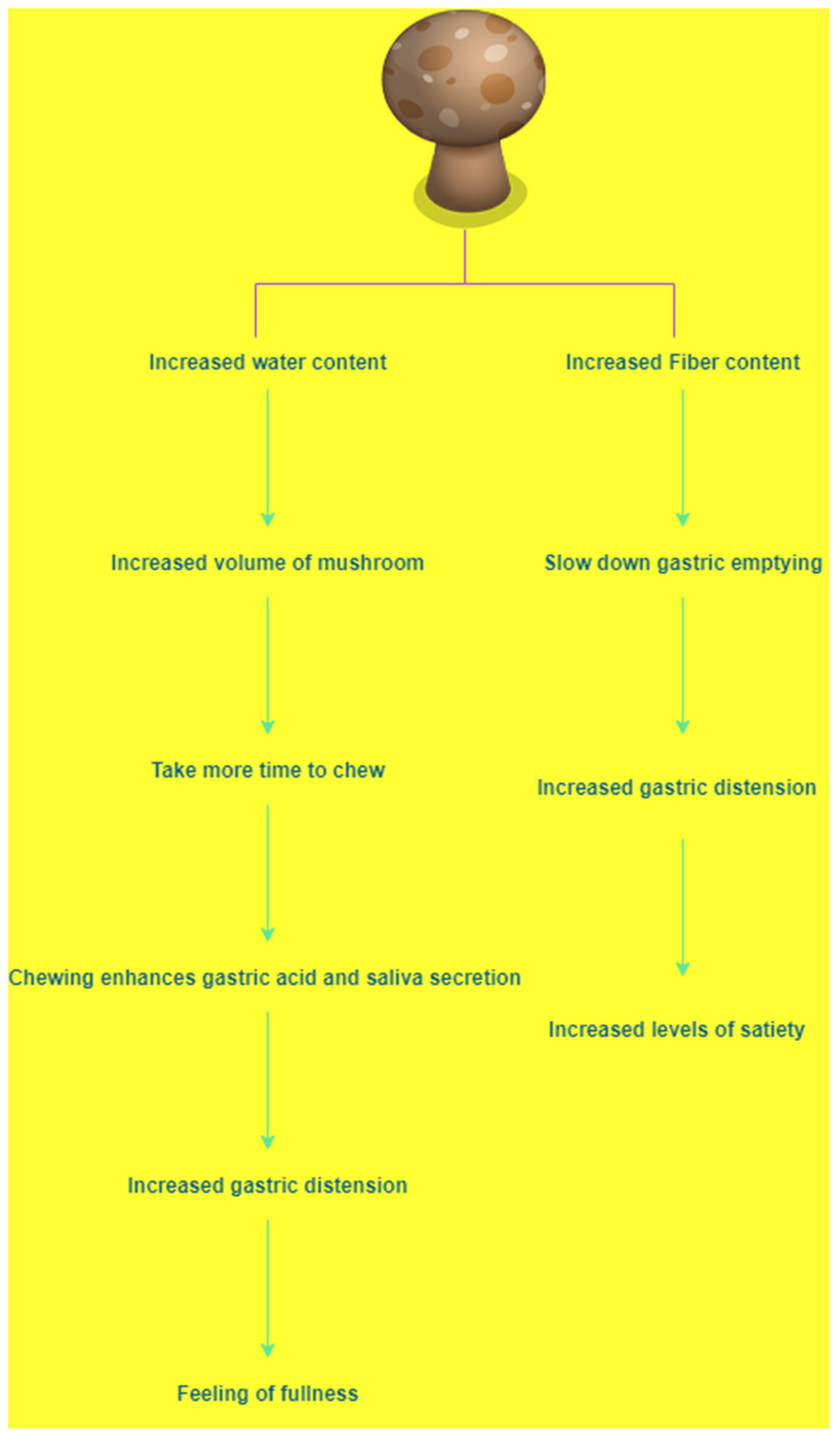
| Name of Mushroom | Summary of Methods | Outcome of Study | References |
|---|---|---|---|
| Tremella fuciformis | Water-soluble fraction obtained by water extraction and polysaccharides from ethanol extraction | The differentiation of 3T3-L1 adipocytes was inhibited by mushroom | [74] |
| Agaricus bisporus | Equivalent amounts of mushroom fibre and sugar beet fibre-fed to rats for 4fourweeks; liver weight studied for both groups of rats | The cellulose powder group should higher HDL cholesterol concentration than the mushroom fibre group. | [75] |
| Pleurotus geesteranus | Exopolysaccharides were extracted from mushrooms and tested on diabetes-induced mice | The hypolipidemic impact of the polysaccharide explored in streptozotocin-prompted diabetic mice, diminished plasma glucose levels, all-out triacylglycerol and cholesterol focuses by 17.1%, 18.8%, and 12.0% | [76] |
| Hericium erinaceus | Mice were fed a high-fat diet along with extracts of Yamabushitake mushroom | A substantial diminution in increased body mass, fat weight, and triacyl-glycerol level in serum and hepatic were observed after 28 days of a high fat diet. | [77] |
| Exobiopolymer extracted from mycelial culture of mushroom was studied on hyperlipidemic mice | A major reduction in the overall plasma cholesterol (32.9%), cholesterol (45.4%), Low-Density Lipoprotein (LDL) atherogenic index (58.7%), triglyceride (34.3%), phospholipid (18.9%), and hepatic HMG-CoA reductase activity (20.2%) was observed after administration of 200 mg/kg dose. | [78] | |
| Lentinula edodes | The diet containing varying proportions of mushroom with a high-fat diet was fed to mice for 4 weeks against a normal diet and high-fat diet control. | The mRNA expression of cholesterol 7-α-hydroxylase 1 (CYP7A1) was reduced in hypercholesterolemic mice and amplified by eritadenine and L. edodes (5, 10, and 20%) supplementation. Treatments with eritadenine and L. edodes were shown to decrease lipid build-up in hepatic tissues. | [79] |
| Hypercholesterolemia Albino rats were fed a diet containing fruiting bodies of mushrooms and checked for plasma and faeces biochemistry and liver histology. | A diet containing 5% L. lepideus fruiting bodies decreased total plasma cholesterol, triglyceride, LDL, total lipid, phospholipids, and LDL to HDL. | [80] | |
| Wister rats were fed a high-fat diet and mushroom extract for 30 days, and then biochemical parameters, including the stress markers, were determined. | Lentinula edodes decreased levels of glucose and urea. Lipid peroxidation was augmented in rats receiving the HFD, and L. edodes reduced malondialdehyde levels, thus preventing fatty acid oxidation. | [81] | |
| Lentinus lepideus | Hypercholesterolemia Albino rats were fed a diet containing mushroom fruiting bodies and checked for plasma and faeces biochemistry and liver histology. | Total plasma cholesterol (TC), triglyceride (TG), LDL, total lipid, phospholipids, and the LDL/HDL ratio was decreased in hypercholesterolemic rats after a diet containing 5% Lentinus edodes fruiting bodies. | [59] |
| Pholiota nameko SW-02 | The mice hyperlipidemic model was established to study the effects of mycelia zinc polysaccharide (containing zinc, glucose, mannose, galactose, and arabinose) on lipid profile and oxidative stress. | The supplementation of mycelia zinc polysaccharide might progress blood lipid levels (TC, TG, HDL-C, LDL-C, and VLDL-C), liver lipid levels (TC and TG), and antioxidant status. | [82] |
| Pleurotus eryngii | Hypercholesterolemia Albino rats were fed a diet containing fruiting bodies of mushrooms and checked for plasma and faeces biochemistry and liver histology. | Total plasma cholesterol (TC), triglyceride (TG), LDL, total lipid, phospholipids, and the LDL/HDL ratio was decreased in hypercholesterolemic rats after a diet containing 5% Pleurotus eryngii fruiting bodies | [83] |
| Pleurotus ferulae | Hypercholesterolemia Albino rats were fed a diet containing mushroom fruiting bodies and checked for plasma and faeces biochemistry and liver histology. | Supplementation with 5% P. ferulae fruiting bodies to hypercholesterolemic rat decreased low-density lipoprotein (LDL), total plasma cholesterol, triglyceride, total lipid, phospholipids, and LDL/high-density lipoprotein ratio by 71.15%, 30.02, 49.31, 30.23, 21.93, and 65.31%, correspondingly. | [84] |
| Pleurotus ostreatus | Hypercholesterolemia Albino rats were fed a diet containing fruiting bodies of mushrooms and checked for plasma and faeces biochemistry and liver histology. | Total plasma cholesterol, triglyceride, low-density lipoprotein (LDL), total lipid, phospholipids, And LDL/HDL ratio was reduced in hypercholesterolemic rats after 5% powder of Pleurotus ostreatus fruiting bodies. | [85] |
| Pleurotus salmoneostramineus L. Vass | Hyper and normo cholesterolemia rats were fed a diet containing fruiting bodies of mushrooms and checked for plasma and faeces biochemistry and liver histology. | P. Salmoneostramineus fruiting bodies (5% administration) in hypercholesterolemic rats reduced LDL/HDL ratio, total plasma cholesterol, triglyceride, LDL, total lipids, and phospholipids. | [86] |
| Pleurotus tuber-regium | Mushroom extracellular polysaccharides were orally administered to obese diabetes-induced mice for 8 weeks, and liver PPAR-α expression was studied. | Serum TG, LDL, and total cholesterol concentration were decreased, and HDL level was increased after P. tuber-regium. | [87] |
| Pleurotus ostreatus | Hypercholesterolemic Wistar rats were fed a 5% dried mushroom diet and studied for biochemical markers of cholesterol metabolism. | Pleurotus ostreatus administration reduced serum and liver cholesterol level, LDL production, cholesterol absorption, HMG-CoA activity in the liver, and redistribution of cholesterol in favor of HDL. | [88] |
| Adiantum capillus-veneris L. | Pharmacological modulation of pancreatic lipase and α-amylase/α-glucosidase studied using in-vitro and in vivo study on high cholesterol diet fed Wistar rats | capillus-veneris showed antiobesity and triacylglycerol-reducing effects compared to rats fed with a high cholesterol diet for 10 weeks. | [89] |
| Aster spathulifolius Maxim | Rats fed a diet with mushroom extract supplementation for 4.5 weeks were tested for hepatic and serum lipid levels. | Aster spathulifolius Maxim extract (ASE) treatment includes fat intake and lipogenesis-related genes. It also increases the level of phosphorylated AMPKα in obese rats. | [90] |
| Kluyveromyces marxianus | Hyperlipedimic rats were fed a diet supplemented with three different dosages of mushroom extract and measured for serum and hepatocyte lipid concentrations. | K. Marxianus administration significantly reduced serum and liver total cholesterol, triglyceride, LDL cholesterol, and atherogenic index in rats while HDL cholesterol level and the anti-atherogenic index were increased. | [91] |
| Auricularia auricula-judae | Rats were fed with high-fat diet along with mushroom extract. The impacts on preventing hepatic steatosis were studied. In vitro study was carried out for the mechanistic study of mice adipocytes | Plasma lipid and liver enzymes were reduced after supplementation of Auricularia auricula-judae. | [92] |
| Collybia confluens | The effects of three weeks of mycelial powder administration on plasma glucose and biochemistry were studied on diabetic mice. | TG and TC level in the liver was decreased by Collybia confluens. AST and ALT activity was also reduced. | [93] |
| Cordyceps militaris SU-12 | The structure of residue polysaccharides of mushrooms was studied using gas chromatography. Rat study was carried out to see its impact on plasma lipid profile and anti-oxidant potential. | Residue polysaccharide reduced blood and liver lipid levels, improving glutamate pyruvate transaminase and antioxidant activity. | [94] |
| Flammulina velutipes | The effect of active components in the mushroom extract was studied through administration for eight weeks into diets of hamsters. The outcomes investigated included serum and liver lipid profiling. | Flammulina velutipes (3%) powder and extract reduced the concentration of TC, TG, LDL, and HDL in the serum and liver. | [95] |
| Grifola frondosa | The cholesterol-lowering effects of mushroom fibre were investigated after feeding the cholesterol-free supplemented diet for four weeks. Serum cholesterol concentration and LDL receptor mRNA were determined. | Grifola frondosa fiber depressed the serum total cholesterol level by augmentation of faecal cholesterol excretion. | [96] |
| Auricularia polytricha | anti-hypercholesterolemic effects of the mushroom extract on hypercholesterolemic mice models were studied. | The total cholesterol in the Soluble Polysaccharide Auricularia polytricha ingestion groups considerably reduced 34.6 ± 7.6% and 33.3 ± 7.9% with doses of 4.5 and 9.0 mg/kg BW on the 29th day. | [97] |
| Ganoderma lucidum | Invitro analysis of mushroom extracts was carried out to determine free radical scavenging potential. In vivo antioxidant potential was determined through blood levels of stress markers in mice fed with the supplemented diet. Cardiovascular risk factors were determined through serum lipid profiling of mice | Hot water extract at 200 mg/kg b.w. lowered plasma levels of total cholesterol, triacylglycerol, and LDL cholesterol and increased HDL cholesterol. | [98] |
| Ergosterol peroxide potential to inhibit triglyceride synthesis was determined at protein and mRNA levels and through differentiation of 3T3-L1 adipocytes | The mitotic clonal expansion (MCE) stage blocked the phosphorylation of mitogen-activated protein kinases (MAPKs), which play a part in cell production and the stimulation of early differentiation transcription factors. Ergosterol peroxide also significantly reduced triglyceride production and differentiation in 3T3-L1 cells. | [38] | |
| Pleurotus eryngii | Invitro analysis was performed on DPPH and hydroxyl radical scavenging potential. Three-week administration of supplemented diet on hyperlipidemic mice model was carried out to investigate the antiatherogenic potential (through lipid profiling and inflammatory enzyme markers) | Hepatic lipid accumulation was significantly reduced by Pleurotus eryngii administration. | [99] |
| Echigoshirayukidake | Feeding supplemented diet to rat models for 15 weeks on obesity (weight gain), and insulin resistance was investigated. | Supplementation to the eating routine altogether (p < 0.01) smothered the body weight gain and furthermore instinctive fat aggregation throughout the taking care of period contrasted with the control diet | [100] |
| Ganoderma applanatum | The effect of feeding diet supplemented with mushroom polysaccharides for two months on serum, and tissue lipid profile and weight gain were determined | Organization of Ganoderma applanatum remove at various portion levels essentially diminished the all-out cholesterol, TG, LDL, cholesterol levels, and the atherogenic file from 50 to 150 mg/kg body weight. | [101] |
| Sparassis latifolia | Six weeks trial through feeding the diet supplemented with the fruiting body of mushroom was carried out. Outcome measures were weight gain, food efficiency ratio and serum lipid profile. | Significantly suppressed the occurrence of non-alcoholic fat deposits in the liver | [102] |
| Dictyophora indusiata | The modulatory impact of mushroom polysaccharide on obese mice model fed a high-fat diet were determined through studying the lipid profile and inflammatory markers. | Bodyweight, adipocyte size, fat accumulation, adipogenic and liver-associated markers, glucose levels, endotoxin (Lipopolysaccharide, LPS) levels, and inflammatory cytokines were diminished significantly. Furthermore, the study exposed that Dictyophora indusiata polysaccharide treatment inverted the dynamic variations of the gut microbiome community by causing a decrease in the Firmicutes to Bacteroidetes ratio | [103] |
| Flammulina velutipes | Mushroom chitosan fed for five weeks to rats was tested for its effects on serum lipid profile, liver function enzyme markers, and weight gain. | Mushroom chitosan complex acted to stifle amplification of the liver from fat affidavit coming about due to a high-fat eating routine and re-establish hepatic capacity. The lipid content of dung indicated a stamped increment corresponded with the mushroom chitosan portion. | [104] |
| Grifola gargal | A human clinical trial was performed to study the effect of four weeks of feeding the mushroom extract on Triglyceride levels. The mice model was also used to study blood glucose, triglyceride, and adipose tissues. | Decreased blood glucose and fatty oil levels, and fat tissue. Grifola gargal (2.0 mg/mL) essentially stifled the expression of the cytokine interleukin-6 in 3T3-L1 cells contrasted and control cells. | [105] |
| F. velutipes, H. marmoreus, L. edodes, G. frondosa and P. eryngii | Lipid metabolism was investigated in mice fed with Japanese mushrooms. | Utilization advanced the corruption of lipids in instinctive fat and restricted the ingestion of food lipids. Also, the high-fat eating routine that took care of gathering exhibited higher convergences of phospholipids; some of them had odd-chain unsaturated fats. | |
| Pleurotus eryngii | Effect of feeding mushroom supplemented diet to mice models was investigated on obesity (adipose tissues and blood parameters) and gut microbiota (gene sequencing) | Serum all out cholesterol and LDL cholesterol levels diminished, and lipid and complete bile acids in dung expanded | [106] |
| Ganoderma resinaceum | The antiobesity effect of the biologically active component was determined using extensive spectroscopic analysis. In vitro analysis was also performed on brown adipocytes. | Resinacein S reduced lipid drops size by overseeing lipid absorption anyway didn’t impact the detachment of C3H10T1/2 cells. Resinacein S extended the assertion of brown and beige adipocytes markers and updated the activity of brown and beige adipocytes in isolated C3H10T1/2 cells. | [107] |
| Name of Mushroom | Effect on Gut Microbiota | References |
|---|---|---|
| Pleurotus eryngii | P. eryngii polysaccharides altered the abundance of SCFA producing gut bacteria | [106] |
| Pleurotus sajor-caju | Growth of SCFA producing bacteria was reduced, and E.Shigella was decreased by Pleurotus sajor-caju. | [113] |
| Flammulina velutipes | increase in lactic acid-producing bacteria (Lactobacillus, Lactococcus, and Streptococcus) and SCFA-producing bacteria (Allobaculum, Bifidobacterium, and Ruminococcus) | [119] |
| Hypsizygus marmoreus | ||
| Lentinusedodes | ||
| Grifola frondosa | ||
| Pleurotus eryngii | ||
| Ganoderma lucidum | G. lucidum enhanced SCFAs producing bacteria and abridged sulfate-reducing bacteria in a time-dependent manner | [120] |
| Lentinula edodes | LESDF-3 was found to stimulate the synthesisof Bacteroides | [121] |
| Bulgaria inquinans | increase of Faecalibaculum and Parabacteroides abundance and the decrease of Allobaculum, Candidatus_Saccharimonas, and Rikenella abundance at the genus level | [122] |
| Ganoderma lucidum | There was an increase in Bacteroides/Firmicutes ratio, Clostridium clusters IV, XVIII, XIVa (Roseburia spp.), Eubacterium spp.) SCFAs production bacteria, reduction in Oscillibacter spp. and E. fergusonii. | [40] |
| Increase in Alloprevotella, Barnesiella, Parabacteroides, Bacteroides, Bacteroidales S24-7 and Alistipe. Decrease in Blautia, Roseburia, and Enterorhabdus. | [123] | |
| Increase in Blautia, Bacteroides Dehalobacterium, and Parabacteroides, Decrease in Proteus, Aerococcus, Ruminococcus, and Corynebactrium. | [124] | |
| Increase in Alloprevotella, Prevotella, Ruminococcus and, Alistipes, Peptococcaceae, Alloprevotella, and Defluviitalea,; Decrease in Turicibacter, Clostridium XVIII and Phascolarctobacterium. | [125] | |
| Grifola frondosa | Increase in Akkermansia muciniphila, Bacteroidetes/Firmicutes, Porphyromonas gingivalis, Lactobacillus acidophilus, Roseburia intestinalis, Tannerella forsythia, and Bacteroides acidifaciens. | [124] |
| Increase in Barnesiella Helicobater, Intestinimonas, Defluvitalea, Flavonifractor and Paraprevotella and Ruminococcus. Decrease in Butyricicoccus, Clostridium-XVI, and Turicibacter. | [126] | |
| Increase in Alistipes. Decrease in Streptococcus, Enterococcus, Staphlococcus, and Aerococcus. | [127] | |
| An increase in Bacteroidetes/Firmicutes ratio increased the abundance of Oscillibacter, Defluvitalea, and Barnesiella. | [128] | |
| Increase in Intestinimonas and Butyricimonas. Decrease in Turicibacter and Clostridium XVIII. | [129] | |
| Phellinus linteus | Increase in Lachnospiraceae-NK4A136, Roseburia, Prevotella Lachnospiraceae-UCG-006, Anaerotruncus, Blautia, Eubacterium_xylanophilum, Ruminiclostridium-9, and Oscillibacter. | [130] |
| Coriolus versicolor | Increase in Akkermansia muciniphila | [131] |
| Hericium erinaceus | Increase in Bifidobacterium, Coprococcus, Desulfovibrio, Lactobacillus, Parabacteroides, Prevotella; Decrease in Corynebacterium, Dorea, Roseburia, Ruminococcus, Staphylococcus, Sutterella | [132] |
| Ganoderma lucidum | Increase in Firmicutes, Proteobacteria (Helicobacter), Rikenella; Decrease in Acinetobacter, Actinobacteria (Arthrobacter, Corynebacterium), Bacteroidetes (Bacteroides, Parabacteroides, Prevotella), Blautia, Brevundimonas, Clostridium, Coprobacillus, Cyanobacteria, Facklamia, Jeotgalicoccus, Sporosarcina, Staphylococcus, Streptococcus | [133] |
| Boletus edulis, Boletus pinophilus, Boletus aureus (Porcini), Armillaria mellea(Honey fungus), Lactarius piperatus (blancaccio), Pleurotus eryngii (King oyster) | Increase in Bifidobacterium and Lactobacillus genera | [134] |
| Cyclocybe cylindracea (poplar mushroom), Hericium erinaceus, Pleurotus eryngii, Pleurotus ostreatus (Oyster mushroom) | Increase in Bifidobacterium spp. Faecalibacterium prausnitzii (Ruminococcaceae), Eubacterium rectale/Roseburia spp. | [135] |
| Flammulina velutipes (Enoki), Hypsizygus marmoreus, (White beech mushroom), Lentinula edodes (Shiitake), Grifola frondosa, (Maitake) Pleurotus eryngii | Increase in Allobaculum, Bifidobacterium, Ruminococcus, Lactobacillus, Lactococcus, Streptococcus | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, F.; Chopra, H.; Baig, A.A.; Avula, S.K.; Kumari, S.; Mohanta, T.K.; Saravanan, M.; Mishra, A.K.; Sharma, N.; Mohanta, Y.K. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. J. Fungi 2022, 8, 211. https://doi.org/10.3390/jof8020211
Mustafa F, Chopra H, Baig AA, Avula SK, Kumari S, Mohanta TK, Saravanan M, Mishra AK, Sharma N, Mohanta YK. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. Journal of Fungi. 2022; 8(2):211. https://doi.org/10.3390/jof8020211
Chicago/Turabian StyleMustafa, Faheem, Hitesh Chopra, Atif Amin Baig, Satya Kumar Avula, Sony Kumari, Tapan Kumar Mohanta, Muthupandian Saravanan, Awdhesh Kumar Mishra, Nanaocha Sharma, and Yugal Kishore Mohanta. 2022. "Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI" Journal of Fungi 8, no. 2: 211. https://doi.org/10.3390/jof8020211
APA StyleMustafa, F., Chopra, H., Baig, A. A., Avula, S. K., Kumari, S., Mohanta, T. K., Saravanan, M., Mishra, A. K., Sharma, N., & Mohanta, Y. K. (2022). Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. Journal of Fungi, 8(2), 211. https://doi.org/10.3390/jof8020211










