High Efficiency In Vitro Wound Healing of Dictyophora indusiata Extracts via Anti-Inflammatory and Collagen Stimulating (MMP-2 Inhibition) Mechanisms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Dictyophora indusiata
2.1.1. Specific Primers Designing and Selection
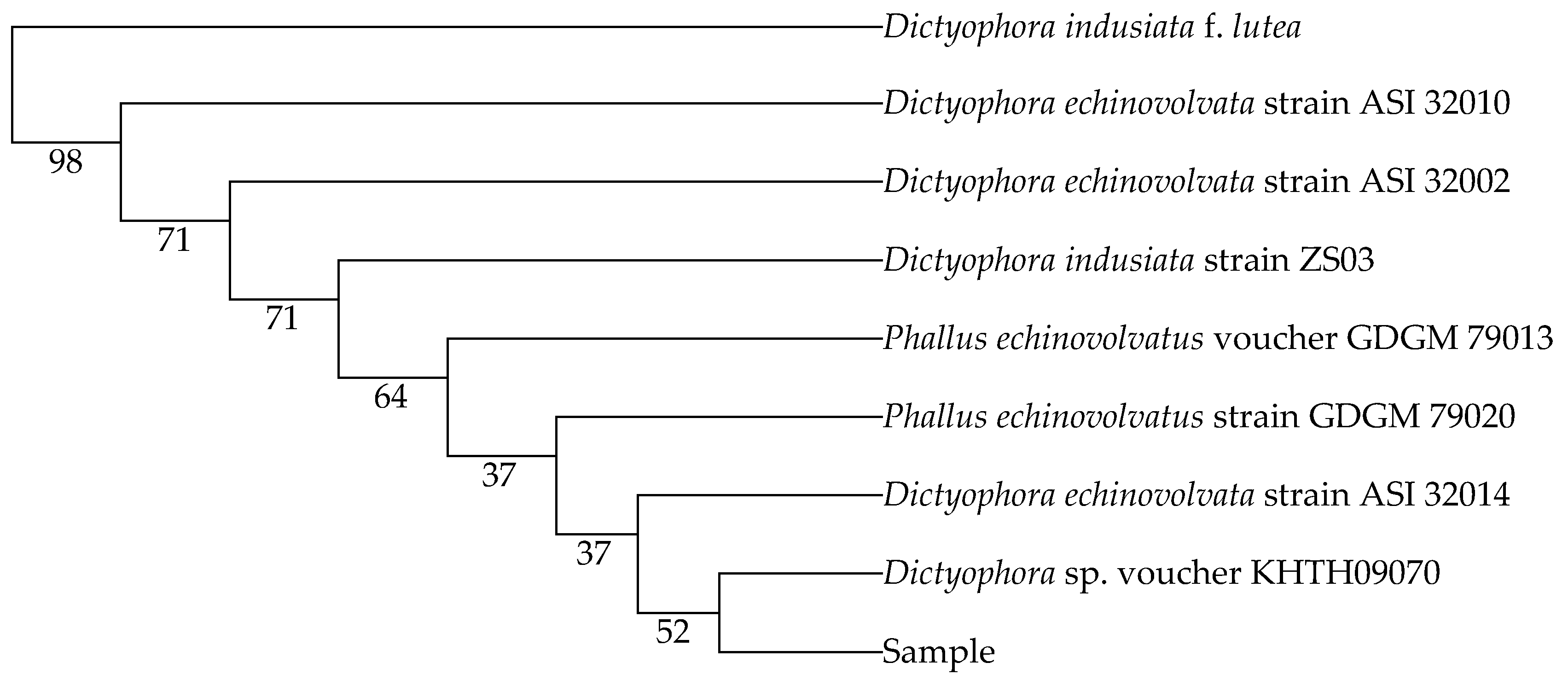
2.1.2. Nucleotide Sequences and Phylogenetic Relationships
2.2. Bioactive Compounds and Antioxidant Activities
2.3. Anti-Inflammatory Activity of Dictyophora indusiata Aqueous Extracts
2.3.1. Non-Cytotoxic Concentration by the Sulforhodamine B (SRB) Assay on Macrophages
2.3.2. Anti-Inflammatory Activity
2.4. Wound Healing Activity of Dictyophora indusiata Aqueous Extracts
2.4.1. Non-Cytotoxic Concentration by the Sulforhodamine B (SRB) Assay on Fibroblasts
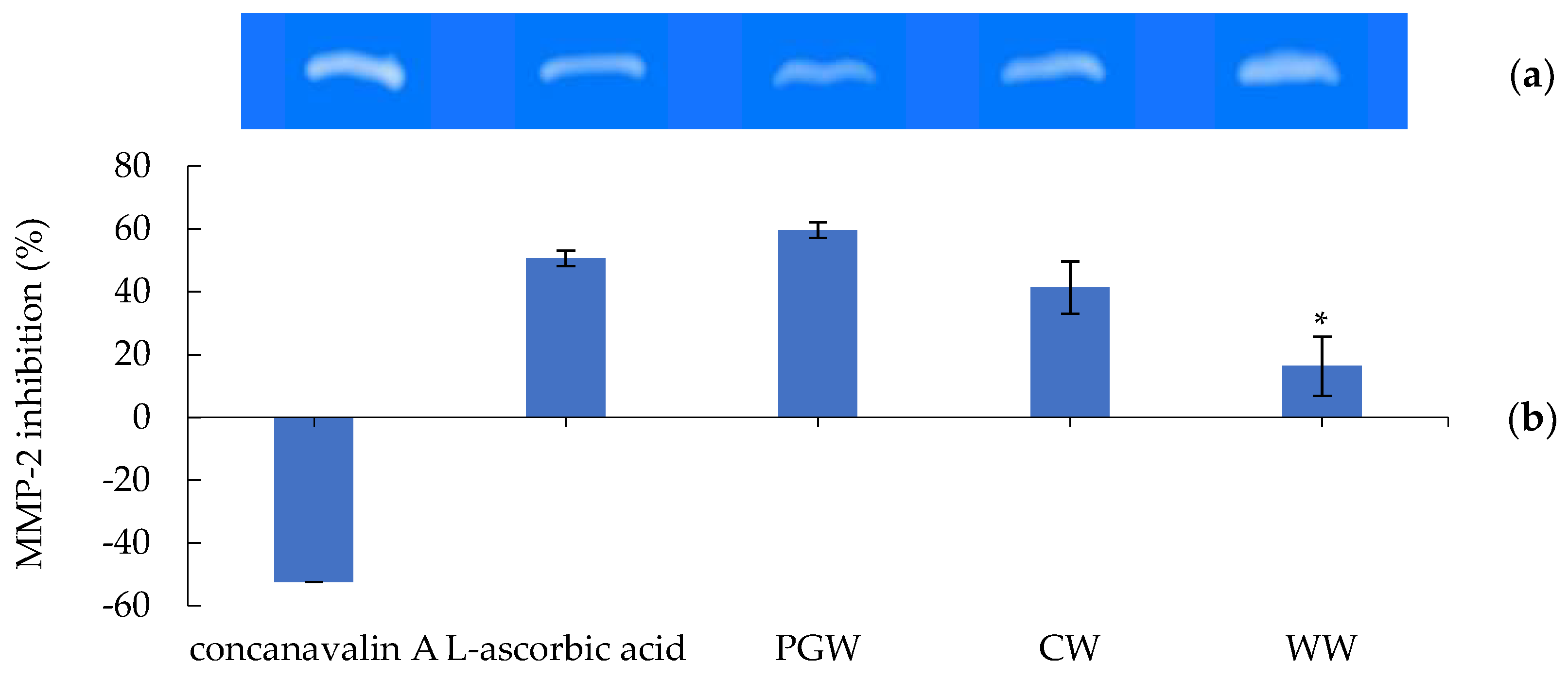
2.4.2. Gelatinolytic Activity (Zymography) of MMP-2 Inhibition
3. Materials and Methods
3.1. Materials
3.2. Identification of Dictyophora indusiata Using Polymerase Chain Reaction (PCR) Based on Ribosomal DNA Internal Transcribed Spacers (rDNA-ITS)
3.2.1. Defining Specific Primers
3.2.2. DNA Extraction and PCR Reactions
3.2.3. Sequencing and Phylogenetic Analysis
3.3. Determination of Bioactive Compounds
3.3.1. Total Phenolic Content
3.3.2. Total Flavonoid Content
3.3.3. Total Polysaccharide Content
3.3.4. Quantitative Analysis of Phenolic and Flavonoid by Liquid Chromatography–Electrospray Ionization/Mass Spectrometry (LC-ESI/MS)
3.4. Antioxidant Assay of Dictyophora indusiata Aqueous Extracts
3.4.1. DPPH Radical Scavenging Activity
3.4.2. ABTS+ Scavenging Activity
3.4.3. Metal Chelating Activity
3.4.4. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Anti-inflammatory Activity of Dictyophora indusiata Aqueous Extracts
3.5.1. Cell Culture
3.5.2. Determination of Non-Cytotoxic Concentration by the Sulforhodamine B (SRB) Assay on Macrophages
3.5.3. Determination of NO, IL-1, IL-6, and TNF-α Levels
3.6. Collagen Stimulating Activity of Dictyophora indusiata Aqueous Extracts
3.6.1. Cell Culture
3.6.2. Determination of Non-Cytotoxic Concentration by the Sulforhodamine B (SRB) Assay on Fibroblasts
3.6.3. Gelatinolytic Activity (Zymography) of MMP-2 Inhibition
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom cosmetics: The present and future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Srisuk, N.; Jirasatid, N. Characteristics co-encapsulation of Lactobacillus acidophilus with Dictyophora indusiata. Curr. Res. Nutr. Food Sci. 2020, 8, 1013. [Google Scholar] [CrossRef]
- Burapapadh, K.; Changsan, N.; Sinsuebpol, C.; Saokham, P. An evaluation of Dictyophora indusiata mucilage as a binder in tablet formulations. Key Eng. Mater. 2021, 901, 22–27. [Google Scholar] [CrossRef]
- Habtemariam, S. The chemistry, pharmacology and therapeutic potential of the edible mushroom Dictyophora indusiata (Vent ex. Pers.) Fischer (Synn. Phallus indusiatus). Biomedicines 2019, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wen, X.; Zhang, Y.; Zou, P.; Cheng, L.; Gan, R.; Li, X.; Liu, D.; Geng, F. Quantitative proteomic and metabolomic analysis of Dictyophora indusiata fruiting bodies during post-harvest morphological development. Food Chem. 2021, 339, 127884. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Zhang, J.; Li, H.; Liu, M.; Gao, Z.; Wang, X.; Jia, L. Antioxidation, hepatic-and renal-protection of water-extractable polysaccharides by Dictyophora indusiata on obese mice. Int. J. Biol. Macromol. 2019, 134, 290–301. [Google Scholar] [CrossRef]
- Talebi, M.; Kakouri, E.; Talebi, M.; Tarantilis, P.A.; Farkhondeh, T.; İlgün, S.; Pourbagher-Shahri, A.M.; Samarghandian, S. Nutraceuticals-based therapeutic approach: Recent advances to combat pathogenesis of Alzheimer’s disease. Expert Rev. Neurother. 2021, 21, 625–642. [Google Scholar] [CrossRef]
- Oyetayo, V.; Dong, C.-H.; Yao, Y.-J. Antioxidant and antimicrobial properties of aqueous extract from Dictyophora indusiata. Open Mycol. J. 2009, 3, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Choi, J.; Sharma, N.; Choi, M.; Seo, S.Y. In vitro anti-tyrosinase activity of 5-(hydroxymethyl)-2-furfural isolated from Dictyophora indusiata. Phytother. Res. 2004, 18, 841–844. [Google Scholar] [CrossRef]
- Hua, Y.; Yang, B.; Tang, J.; Ma, Z.; Gao, Q.; Zhao, M. Structural analysis of water-soluble polysaccharides in the fruiting body of Dictyophora indusiata and their in vivo antioxidant activities. Carbohydr. Polym. 2012, 87, 343–347. [Google Scholar] [CrossRef]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef]
- Deng, C.; Shang, J.; Fu, H.; Chen, J.; Liu, H.; Chen, J. Mechanism of the immunostimulatory activity by a polysaccharide from Dictyophora indusiata. Int. J. Biol. Macromol. 2016, 91, 752–759. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, L.; Teng, L.; Li, Y.; Cheng, J.; Chen, J.; Deng, C. Mechanism of the anti-inflammatory activity by a polysaccharide from Dictyophora indusiata in lipopolysaccharide-stimulated macrophages. Int. J. Biol. Macromol. 2019, 126, 1158–1166. [Google Scholar] [CrossRef]
- Shih, B.; Garside, E.; McGrouther, D.A.; Bayat, A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010, 18, 139–153. [Google Scholar] [CrossRef]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2005, 23, 37–42. [Google Scholar]
- Buranasukhon, W.; Athikomkulchai, S.; Tadtong, S.; Chittasupho, C. Wound healing activity of Pluchea indica leaf extract in oral mucosal cell line and oral spray formulation containing nanoparticles of the extract. Pharm. Biol. 2017, 55, 1767–1774. [Google Scholar] [CrossRef] [Green Version]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Jantrawut, P.; Rajchasom, S. Optimization of placenta extraction for wound healing activity. Chiang Mai J. Sci. 2019, 46, 946–959. [Google Scholar]
- Menon, S.N.; Flegg, J.A.; McCue, S.W.; Schugart, R.C.; Dawson, R.A.; McElwain, D.S. Modelling the interaction of keratinocytes and fibroblasts during normal and abnormal wound healing processes. Proc. R. Soc. B Biol. Sci. 2012, 279, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Virtucio, C.; Zemska, O.; Baltazar, G.; Zhou, Y.; Baia, D.; Jones-Iatauro, S.; Sexton, H.; Martin, S.; Dee, J. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: Selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J. Pharmacol. Exp. Ther. 2016, 358, 413–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparecida Da Silva, A.; Leal-Junior, E.C.P.; Alves, A.C.A.; Rambo, C.S.; Dos Santos, S.A.; Vieira, R.P.; De Carvalho, P.D.T.C. Wound-healing effects of low-level laser therapy in diabetic rats involve the modulation of MMP-2 and MMP-9 and the redistribution of collagen types I and III. J. Cosmet. Laser Ther. 2013, 15, 210–216. [Google Scholar] [CrossRef]
- Dictyophora indusiata f. lutea 18S Ribosomal RNA Gene, Partial Sequence; Internal Transcribed Spacer 1, 5.8S Ribosomal RNA Gene, and Internal Transcribed Spacer 2, Complete Sequence; and 28S Ribosomal RNA Gene, Partial Sequence. Available online: https://www.ncbi.nlm.nih.gov/nuccore/329568060 (accessed on 14 October 2021).
- Dictyophora indusiata Strain ASI 32001 Internal Transcribed Spacer 1, Partial Sequence; 5.8S Ribosomal RNA Gene, Complete Sequence; and Internal Transcribed Spacer 2, Partial Sequence. Available online: https://www.ncbi.nlm.nih.gov/nuccore/305696830 (accessed on 14 October 2021).
- Ruamrungsri, N.; Siengdee, P.; Sringarm, K.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. In vitro cytotoxic screening of 31 crude extracts of Thai herbs on a chondrosarcoma cell line and primary chondrocytes and apoptotic effects of selected extracts. In Vitro Cell Dev. Biol. Anim. 2016, 52, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Arjin, C.; Pringproa, K.; Hongsibsong, S.; Ruksiriwanich, W.; Seel-Audom, M.; Mekchay, S.; Sringarm, K. In vitro screening antiviral activity of Thai medicinal plants against porcine reproductive and respiratory syndrome virus. BMC Vet. Res. 2020, 16, 102. [Google Scholar] [CrossRef]
- Ozcan, T.; Sahin, S.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. Assessment of antioxidant capacity by method comparison and amino acid characterisation in buffalo milk kefir. Int. J. Dairy Technol. 2019, 72, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Surin, S.; You, S.; Seesuriyachan, P.; Muangrat, R.; Wangtueai, S.; Jambrak, A.R.; Phongthai, S.; Jantanasakulwong, K.; Chaiyaso, T.; Phimolsiripol, Y. Optimization of ultrasonic-assisted extraction of polysaccharides from purple glutinous rice bran (Oryza sativa L.) and their antioxidant activities. Sci. Rep. 2020, 10, 10410. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Sakai, H.; Aburai, K.; Manosroi, W.; Manosroi, J. Physico-chemical properties of cationic niosomes loaded with fraction of rice (Oryza sativa) bran extract. J. Nanosci. Nanotechnol. 2012, 12, 7339–7345. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T. Recovery of polyphenolic fraction from arabica coffee pulp and its antifungal applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Mitra, I.; Saha, A.; Roy, K. Exploring quantitative structure–activity relationship studies of antioxidant phenolic compounds obtained from traditional Chinese medicinal plants. Mol. Simul. 2010, 36, 1067–1079. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Antioxidant role of catechin in health and disease. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 267–271. [Google Scholar]
- Muzolf-Panek, M.; Gliszczyńska-Świgło, A.; Szymusiak, H.; Tyrakowska, B. The influence of stereochemistry on the antioxidant properties of catechin epimers. Eur. Food Res. Technol. 2012, 235, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.-Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Buyukokuroglu, M.E.; Kufrevioglu, O.I. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 2003, 34, 278–281. [Google Scholar] [CrossRef]
- Phan, C.-W.; David, P.; Sabaratnam, V. Edible and medicinal mushrooms: Emerging brain food for the mitigation of neurodegenerative diseases. J. Med. Food 2017, 20, 1–10. [Google Scholar] [CrossRef]
- Surin, S.; Surayot, U.; Seesuriyachan, P.; You, S.; Phimolsiripol, Y. Antioxidant and immunomodulatory activities of sulphated polysaccharides from purple glutinous rice bran (Oryza sativa L.). Int. J. Food Sci. Technol. 2018, 53, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Leelapornpisid, P.; Chansakaow, S.; Na-Boonlong, S.; Jantrawut, P. Development of cream containing nanostructured lipid carriers loaded marigold (tagetes erecta linn) flowers extract for anti-wrinkles application. Int. J. Pharm. Pharm. Sci. 2014, 6, 313–314. [Google Scholar]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A polysaccharide isolated from Dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Jambrak, A.R.; Nazir, Y.; Yooin, W. Anti-inflammation of bioactive compounds from ethanolic extracts of edible bamboo mushroom (Dictyophora indusiata) as functional health promoting food ingredients. Int. J. Food Sci. Technol. 2021, 57, 110–122. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Yan, M.; Chen, X.; Kang, M.; Teng, L.; Wu, X.; Chen, J.; Deng, C. Protective effect and mechanism of polysaccharide from Dictyophora indusiata on dextran sodium sulfate-induced colitis in C57BL/6 mice. Int. J. Biol. Macromol. 2019, 140, 973–984. [Google Scholar] [CrossRef]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Surayot, U.; Wangtueai, S.; You, S.; Palanisamy, S.; Krusong, W.; Brennan, C.S.; Barba, F.J.; Phimolsiripol, Y.; Seesuriyachan, P. Extraction, structural characterisation, and immunomodulatory properties of edible Amanita hemibapha subspecies javanica (Corner and Bas) mucilage polysaccharide as a potential of functional food. J. Fungi 2021, 7, 683. [Google Scholar] [CrossRef]
- Surayot, U.; Wangtueai, S.; You, S.; Techapun, C.; Phimolsiripol, Y.; Leksawasdi, N.; Krusong, W.; Barba, F.J.; Seesuriyachan, P. Sulphation and hydrolysis improvements of bioactivities, and immuno-modulatory properties of edible Amanita hemibapha subspecies javanica (Corner and Bas) mucilage polysaccharide as a potential in personalized functional foods. J. Fungi 2021, 7, 847. [Google Scholar] [CrossRef]
- Arjin, C.; Hongsibsong, S.; Pringproa, K.; Seel-audom, M.; Ruksiriwanich, W.; Sutan, K.; Sommano, S.R.; Sringarm, K. Effect of ethanolic Caesalpinia sappan fraction on in vitro antiviral activity against porcine reproductive and respiratory syndrome virus. Vet. Sci. 2021, 8, 106. [Google Scholar] [CrossRef]
- Manosroi, A.; Lohcharoenkal, W.; Ruksiriwanich, W.; Kietthanakorn, B.-O.; Manosroi, W.; Manosroi, J. In vitro immunostimulating activity of the dried sap from fermented thai rice on human and murine neutrophils. Adv. Sci. Lett. 2012, 17, 306–311. [Google Scholar] [CrossRef]
- Grellner, W.; Georg, T.; Wilske, J. Quantitative analysis of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in human skin wounds. Forensic Sci. Int. 2000, 113, 251–264. [Google Scholar] [CrossRef]
- Wiegand, C.; Schönfelder, U.; Abel, M.; Ruth, P.; Kaatz, M.; Hipler, U.-C. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch. Dermatol. Res. 2010, 302, 419–428. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Kędzior, K.; Masłowski, L.; Mierzchała, M.; Bednarz-Misa, I.; Bronowicka-Szydełko, A.; Kubiak, J.; Gacka, M.; Płaczkowska, S.; Gamian, A. Impact of chronic wounds of various etiology on systemic profiles of key inflammatory cytokines, chemokines and growth factors, and their interplay. Adv. Clin. Exp. Med. 2019, 28, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, K.; Paris, I.; Pedretti, N.; Boniface, K.; Juchaux, F.; Huguier, V.; Guillet, G.; Bernard, F.-X.; Lecron, J.-C.; Morel, F. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1α, and TNF-α recapitulates some features of psoriasis. J. Immunol. 2010, 184, 5263–5270. [Google Scholar] [CrossRef] [Green Version]
- Van der Zee, H.; de Ruiter, L.; Van Den Broecke, D.; Dik, W.; Laman, J.; Prens, E. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: A rationale for targeting TNF-α and IL-1β. Br. J. Dermatol. 2011, 164, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Ozkanli, S.; Karadag, A.S.; Ozlu, E.; Uzuncakmak, T.K.; Takci, Z.; Zemheri, E.; Zindancı, I.; Akdeniz, N. A comparative study of MMP-1, MMP-2, and TNF-α expression in different acne vulgaris lesions. Int. J. Dermatol. 2016, 55, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Therapeutic agents and herbs in topical application for acne treatment. Int. J. Cosmet. Sci. 2011, 33, 289–297. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Nishikai-Yan Shen, T.; Kanazawa, S.; Kado, M.; Okada, K.; Luo, L.; Hayashi, A.; Mizuno, H.; Tanaka, R. Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS ONE 2017, 12, e0178232. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.; Yang, B.; Lim, C.; Kim, J.-H.; Kim, H.; Cho, S. Anti-inflammatory effects of Brassica oleracea Var. capitata L.(Cabbage) methanol extract in mice with contact dermatitis. Pharmacogn. Mag. 2018, 14, 174. [Google Scholar]
- Huang, W.-C.; Tsai, T.-H.; Huang, C.-J.; Li, Y.-Y.; Chyuan, J.-H.; Chuang, L.-T.; Tsai, P.-J. Inhibitory effects of wild bitter melon leaf extract on Propionibacterium acnes-induced skin inflammation in mice and cytokine production in vitro. Food Funct. 2015, 6, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef]
- Kanno, E.; Tanno, H.; Masaki, A.; Sasaki, A.; Sato, N.; Goto, M.; Shisai, M.; Yamaguchi, K.; Takagi, N.; Shoji, M. Defect of interferon γ leads to impaired wound healing through prolonged neutrophilic inflammatory response and enhanced MMP-2 activation. Int. J. Mol. Sci. 2019, 20, 5657. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, M.; Muragaki, Y.; Ooshima, A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br. J. Dermatol. 2005, 153, 295–300. [Google Scholar] [CrossRef]
- Ulrich, D.; Ulrich, F.; Unglaub, F.; Piatkowski, A.; Pallua, N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J. Plast Reconstr Aesthet Surg 2010, 63, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, E.; Morelli, P.; Bennardo, L.; Di Raimondo, C.; Nisticò, S.P. Cytokine pathways and investigational target therapies in Hidradenitis suppurativa. Int. J. Mol. Sci. 2020, 21, 8436. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Le Jan, S.; Muller, C.; François, C.; Renard, Y.; Durlach, A.; Bernard, P.; Reguiai, Z.; Antonicelli, F. Matrix remodelling and MMP expression/activation are associated with hidradenitis suppurativa skin inflammation. Exp. Dermatol. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.; Damiani, G.; Ceccherini, I.; Berti, E.; Gattorno, M.; Cugno, M. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br. J. Dermatol. 2017, 176, 1588–1598. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Piao, M.S.; Lee, J.-B.; Oh, J.S.; Kim, I.-G.; Lee, S.-C. Propionibacterium acnes stimulates pro-matrix metalloproteinase-2 expression through tumor necrosis factor-α in human dermal fibroblasts. J. Investig. Dermatol. 2008, 128, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Mozeika, E.; Pilmane, M.; Nürnberg, B.M.; Jemec, G.B. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm. Venereol. 2013, 93, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.O.; Ruksiriwanich, W.; Chaikul, P.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019, 46, 180–195. [Google Scholar]
- Ruksiriwanich, W.; Sirithunyalug, J.; Boonpisuttinant, K.; Jantrawut, P. Potent in vitro collagen biosynthesis stimulating and antioxidant activities of edible mushroom Volvariella volvacea aqueous extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–412. [Google Scholar]
- Vuong, T.T.; Rønning, S.B.; Ahmed, T.A.; Brathagen, K.; Høst, V.; Hincke, M.T.; Suso, H.-P.; Pedersen, M.E. Processed eggshell membrane powder regulates cellular functions and increase MMP-activity important in early wound healing processes. PLoS ONE 2018, 13, e0201975. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Lux, P.E.; Freiling, M.; Stuetz, W.; von Tucher, S.; Carle, R.; Steingass, C.B.; Frank, J. (Poly) phenols, carotenoids, and tocochromanols in corn (Zea mays L.) kernels as affected by phosphate fertilization and sowing time. J. Agric. Food Chem. 2020, 68, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Sringarm, K.; Sommano, S.; Jantrawut, P. Depigmented Centella asiatica extraction by pretreated with supercritical carbon dioxide fluid for wound healing application. Processes 2020, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Khantham, C.; Yooin, W.; Sringarm, K.; Sommano, S.R.; Jiranusornkul, S.; Carmona, F.D.; Nimlamool, W.; Jantrawut, P.; Rachtanapun, P.; Ruksiriwanich, W. Effects on steroid 5-alpha reductase gene expression of Thai rice bran extracts and molecular dynamics study on SRD5A2. Biology 2021, 10, 319. [Google Scholar] [CrossRef]
- Ko, W.-K.; Lee, S.-H.; Kim, S.J.; Jo, M.-J.; Kumar, H.; Han, I.-B.; Sohn, S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0180673. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Primer Code Name | Primer Sequences (5′-3′) | Size of Product (bp) | References |
|---|---|---|---|
| Dict 01: forward | AGGCCTCTCGAAAGAGGGTC | 20 | [23] |
| Dict 01: reverse | TCATCGATGCGAAAGCCAAG | 20 | |
| Dict 02: forward | TCGCGCGTGTCAGTGAAATA | 20 | [23] |
| Dict 02: reverse | CCAAGTCCGAAAGGGGTCTC | 20 | |
| Dict 03: forward | TGCCTGTTTGAGTGTCGTGA | 20 | [23] |
| Dict 03: reverse | ACGGACGACGCAAGACTTAT | 20 | |
| Dict 04: forward | GGAAGTAAAAGTCGTAACAAGG | 22 | [23] |
| Dict 04: reverse | TCCTCCGCTTATTGATATGC | 20 | |
| Dict 05: forward | AGGAGCATGCCTGTTTGAGT | 20 | [24] |
| Dict 05: reverse | TGGAAACCTCGCCGATGAAT | 20 | |
| Dict 06: forward | GTCATGAACGCCCGTTTCTC | 20 | [24] |
| Dict 06: reverse | ACCCTCCTTCCGATGAGACT | 20 |
| Specific Primer | Sequences (5′-3′) | Size (bp) |
|---|---|---|
| Dict 03 | TTACCGAAGGAGGCAGGACTAACAAGTTCGGAAGGGGGGTAAAGGGGAAGGG GGACCTTCGCCGATGAATTTGAAGACGAGCCTTCGACCGCAGGGGGATTCGAG GGCAAGACCGTCCAAGTCCGAAAAAAGGAGAAATCCGTTAA | 146 |
| Dict 04 | TTCCTTCCTTTCCTCCGCTTATTGATATGATTAAGTTGGGCGGGTAATCCTGCCTG ATTTGAGGTCAAGGCGTATAATGAATGACGGAACGAGAAGCCCACCCCGCCCT TTTTTTTCCCCCCAGGACGAAGCAAGACTTATCAAGTTTGGATGGGGGGTAAAG GGGAAGGGGGACCTTCGCCGATGAATTTGAAGACGAGCCTTCGACCGCAGGGG GATTGGAGGGCAAGACCGTCCAAGTCAAGAAAAAAGGGAGAAATCCTTTTTTT CGATGAGATTTCACGACACTCAAACAGGCATGCTCCTCGGAATACCGAGGAGC GCAAGATGCGTTCAAAGATTCGATGATTCATTGAATTCTGCAATTCACATTACG TTTCGCGCGTTCGCGGCGTTCTTCATCGATGCGAAAGCCAAGAGATCCGTTGTT GAAAGTTGTGTTTCGATTTTTATTTCACTGACACGCGCGAGACTGCGAGGCGTTT GTGAAAGACGGGAGGGGCCAAGCCTCTTTCGAGAGGCCTCTCCCAGAGTGCAC GGAGGTGTCGGTCGGGGAGAGAGAGCGCGTCTCCCCCCCGGATGATAAATCGG CAATGATCTCCGCAGTACAGAG | 613 |
| Species | GenBank Accession Number | Nucleotide Identity (%) |
|---|---|---|
| Dictyophora indusiata strain ZS03 | MH464257.1 | 98 |
| Dictyophora echinovolvata strain ASI 32010 | AF324167.2 | 98 |
| Dictyophora echinovolvata strain ASI 32002 | AF324164.2 | 98 |
| Phallus echinovolvatus voucher GDGM 79013 | MN613536.1 | 97 |
| Phallus echinovolvatus strain GDGM 79020 | MN523216.1 | 97 |
| Dictyophora echinovolvata strain ASI 32014 | AF324168.2 | 97 |
| Dictyophora sp. voucher KHTH09070 | MG678511.1 | 97 |
| Dictyophora indusiata f. lutea | HQ414538.1 | 95 |
| Species | GenBank Accession Number | Nucleotide Identity (%) |
|---|---|---|
| Phallus echinovolvatus voucher GDGM 79013 | MN613536.1 | 97 |
| Phallus echinovolvatus strain GDGM 79020 | MN523216.1 | 97 |
| Dictyophora echinovolvata strain ASI 32014 | AF324168.2 | 97 |
| Dictyophora echinovolvata strain ASI 32007 | AF324165.2 | 97 |
| Dictyophora echinovolvata strain ASI 32008 | AF324166.2 | 97 |
| Dictyophora echinovolvata strain ASI 32010 | AF324167.2 | 95 |
| Dictyophora echinovolvata strain ASI 32002 | AF324164.2 | 95 |
| Sample | Total Phenolic Contents (mg GAE/g Extract) | Total Flavonoid Contents (mg CE/g Extract) | Total Polysaccharide Contents (µg GE/g Extract) |
|---|---|---|---|
| PGW | 2.55 ± 0.36 | 0.05 ± 0.02 | 2.22 ± 0.29 |
| CW | 2.05 ± 0.08 | 0.01 ± 0.02 | 1.20 ± 0.14 |
| WW | 1.89 ± 0.17 | 0.02 ± 0.01 | 1.65 ± 0.09 |
| Polyphenol Compounds (mg/g Extract) | PGW | CW | WW |
|---|---|---|---|
| Catechin | 3.481 ± 0.001 | 68.761 ± 0.010 | 2.934 ± 0.010 |
| p-Coumaric acid | 3.887 ± 0.043 | 7.931 ± 0.939 | 4.066 ± 0.079 |
| Rutin | 0.476 ± 0.092 | 2.502 ± 0.008 | 3.290 ± 0.027 |
| Rosmarinic acid | 3.270 ± 0.014 | 0.235 ± 0.009 | 0.178 ± 0.006 |
| Quercetin | 0.754 ± 0.007 | 0.055 ± 0.004 | 0.833 ± 0.013 |
| Naringenin | Nd | 0.516 ± 0.003 | 0.209 ± 0.000 |
| Epigallocatechin gallate (EGCG) | Nd | 0.767 ± 0.004 | Nd |
| Sample | DPPH Scavenging Activity (SC50, mg/mL) | ABTS Scavenging Activity (SC50, mg/mL) | Metal Chelating Activity (MC90, mg/mL) | FRAP Reducing Power (µM Fe2+/g Extract) |
|---|---|---|---|---|
| PGW | 2.51 ± 0.05 * | 8.52 ± 0.09 | 32.18 ± 0.23 * | 6.18 ± 0.08 |
| CW | 1.54 ± 0.02 * | 6.51 ± 0.08 | 5.11 ± 0.45 * | 2.13 ± 0.09 |
| WW | 3.59 ± 0.02 * | 11.81 ± 0.09 | 34.58 ± 0.39 * | 3.00 ± 0.03 |
| EDTA | Nd | Nd | 0.10 ± 0.09 | Nd |
| L-ascorbic acid | 0.26 ± 0.02 | Nd | Nd | Nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, Y.; Linsaenkart, P.; Khantham, C.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; et al. High Efficiency In Vitro Wound Healing of Dictyophora indusiata Extracts via Anti-Inflammatory and Collagen Stimulating (MMP-2 Inhibition) Mechanisms. J. Fungi 2021, 7, 1100. https://doi.org/10.3390/jof7121100
Nazir Y, Linsaenkart P, Khantham C, Chaitep T, Jantrawut P, Chittasupho C, Rachtanapun P, Jantanasakulwong K, Phimolsiripol Y, Sommano SR, et al. High Efficiency In Vitro Wound Healing of Dictyophora indusiata Extracts via Anti-Inflammatory and Collagen Stimulating (MMP-2 Inhibition) Mechanisms. Journal of Fungi. 2021; 7(12):1100. https://doi.org/10.3390/jof7121100
Chicago/Turabian StyleNazir, Yasir, Pichchapa Linsaenkart, Chiranan Khantham, Tanakarn Chaitep, Pensak Jantrawut, Chuda Chittasupho, Pornchai Rachtanapun, Kittisak Jantanasakulwong, Yuthana Phimolsiripol, Sarana Rose Sommano, and et al. 2021. "High Efficiency In Vitro Wound Healing of Dictyophora indusiata Extracts via Anti-Inflammatory and Collagen Stimulating (MMP-2 Inhibition) Mechanisms" Journal of Fungi 7, no. 12: 1100. https://doi.org/10.3390/jof7121100
APA StyleNazir, Y., Linsaenkart, P., Khantham, C., Chaitep, T., Jantrawut, P., Chittasupho, C., Rachtanapun, P., Jantanasakulwong, K., Phimolsiripol, Y., Sommano, S. R., Tocharus, J., Mingmalairak, S., Wongsa, A., Arjin, C., Sringarm, K., Berrada, H., Barba, F. J., & Ruksiriwanich, W. (2021). High Efficiency In Vitro Wound Healing of Dictyophora indusiata Extracts via Anti-Inflammatory and Collagen Stimulating (MMP-2 Inhibition) Mechanisms. Journal of Fungi, 7(12), 1100. https://doi.org/10.3390/jof7121100

















