Metarhizium brunneum (Hypocreales: Clavicipitaceae) and Its Derived Volatile Organic Compounds as Biostimulants of Commercially Valuable Angiosperms and Gymnosperms
Abstract
1. Introduction
2. Materials and Methods
2.1. Metarhizium Production
2.2. Assays to Determine Optimum M. brunneum Dosage
2.3. Assays to Determine Optimum VOC Dosage
2.4. Effects of the Optimized Doses of M. brunneum, VOCs and Hydrogels on Four Commercial Plants
2.5. Statistical Analysis
3. Results
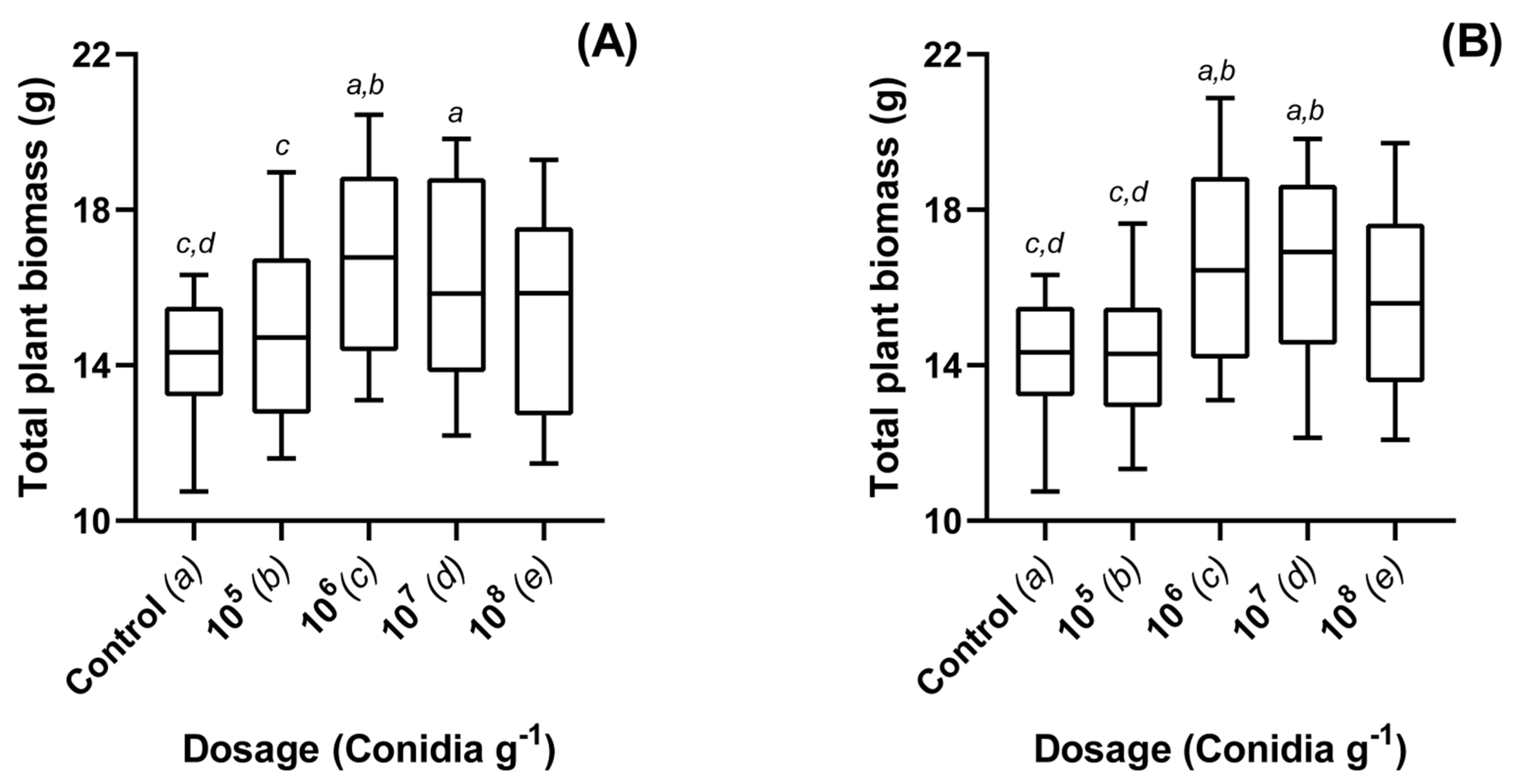
3.1. Dose Dependent Effects of Metarhizium brunneum Applied to Oilseed Rape
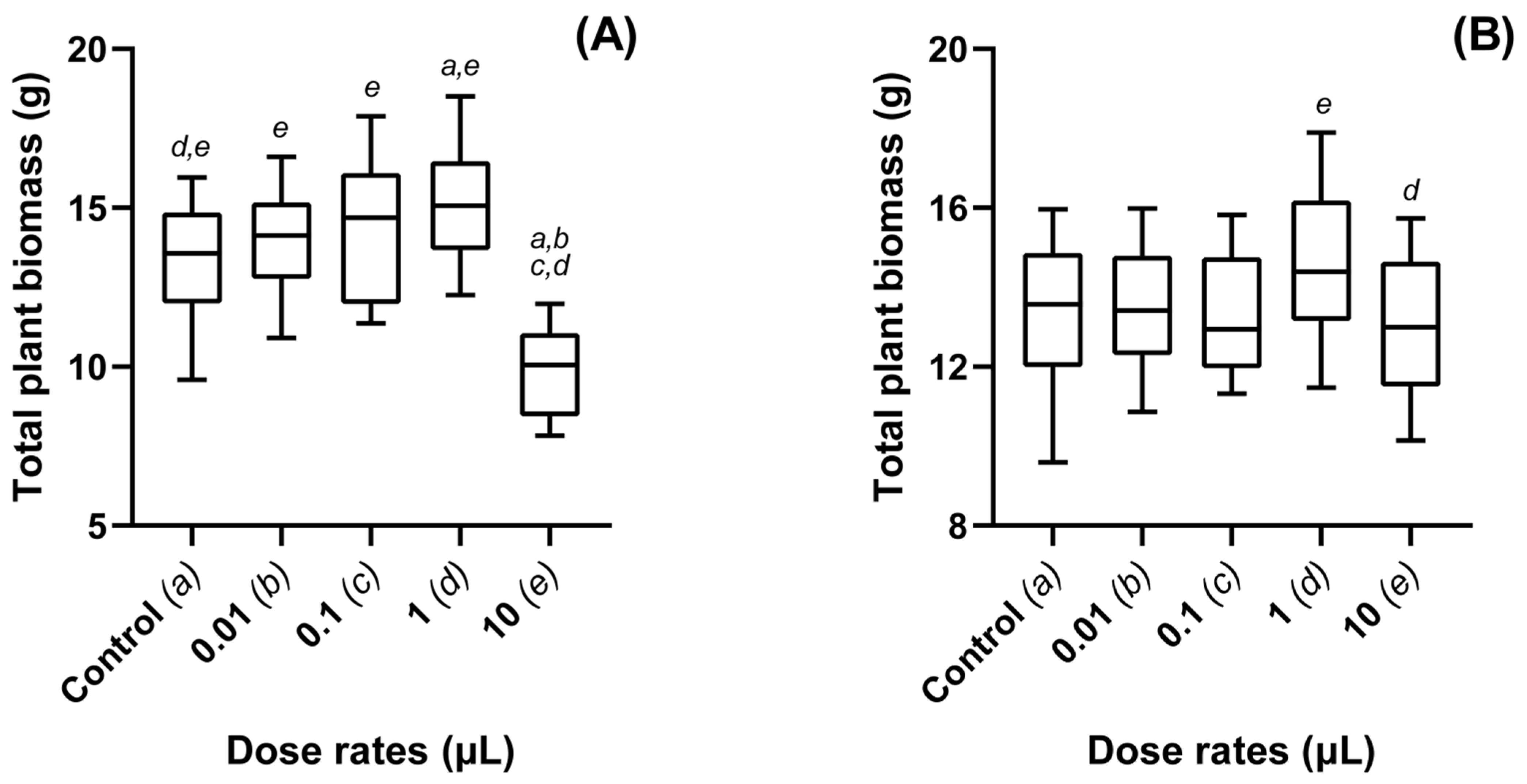
3.2. Dose Dependent Effects of Metarhizium-VOCs Applied to Oilseed Rape
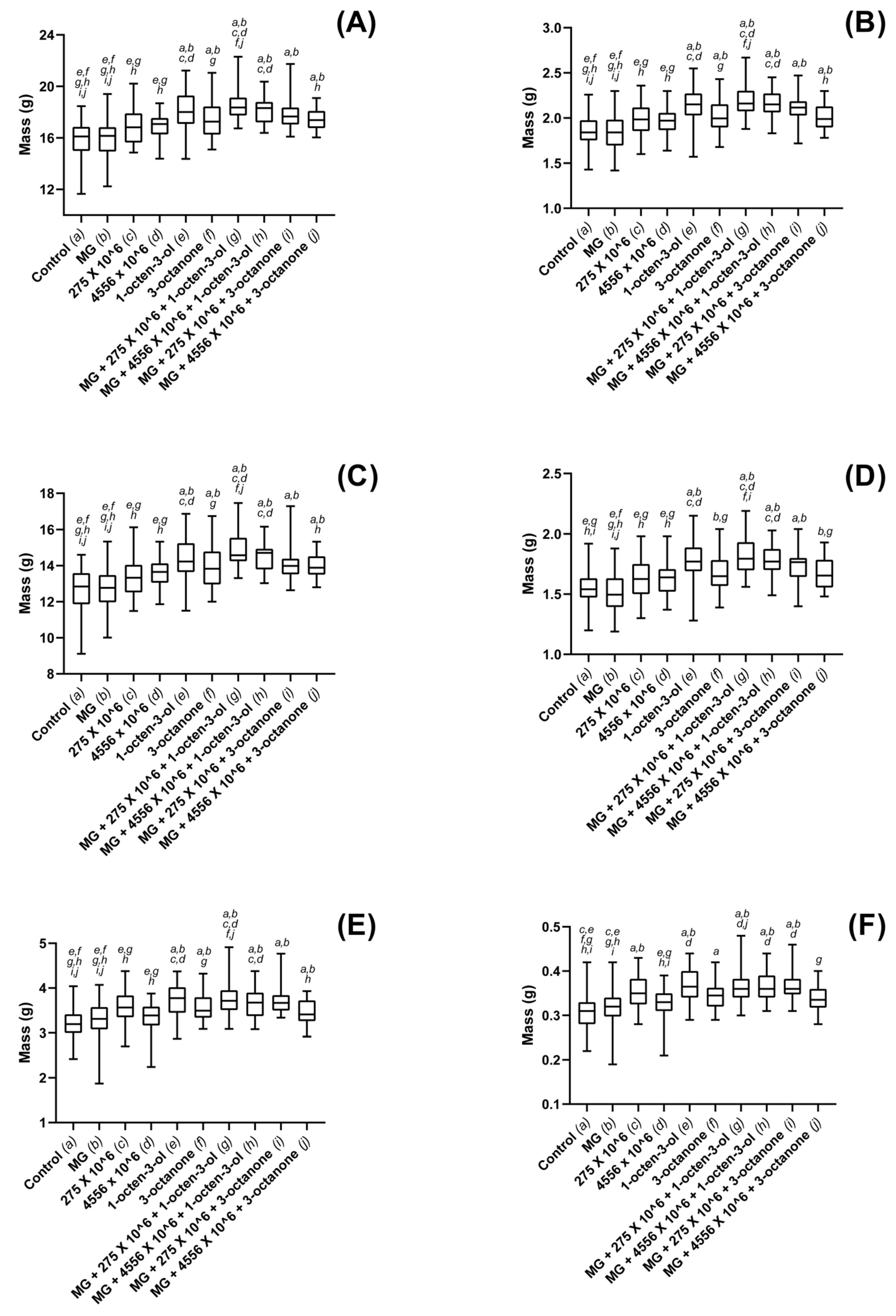
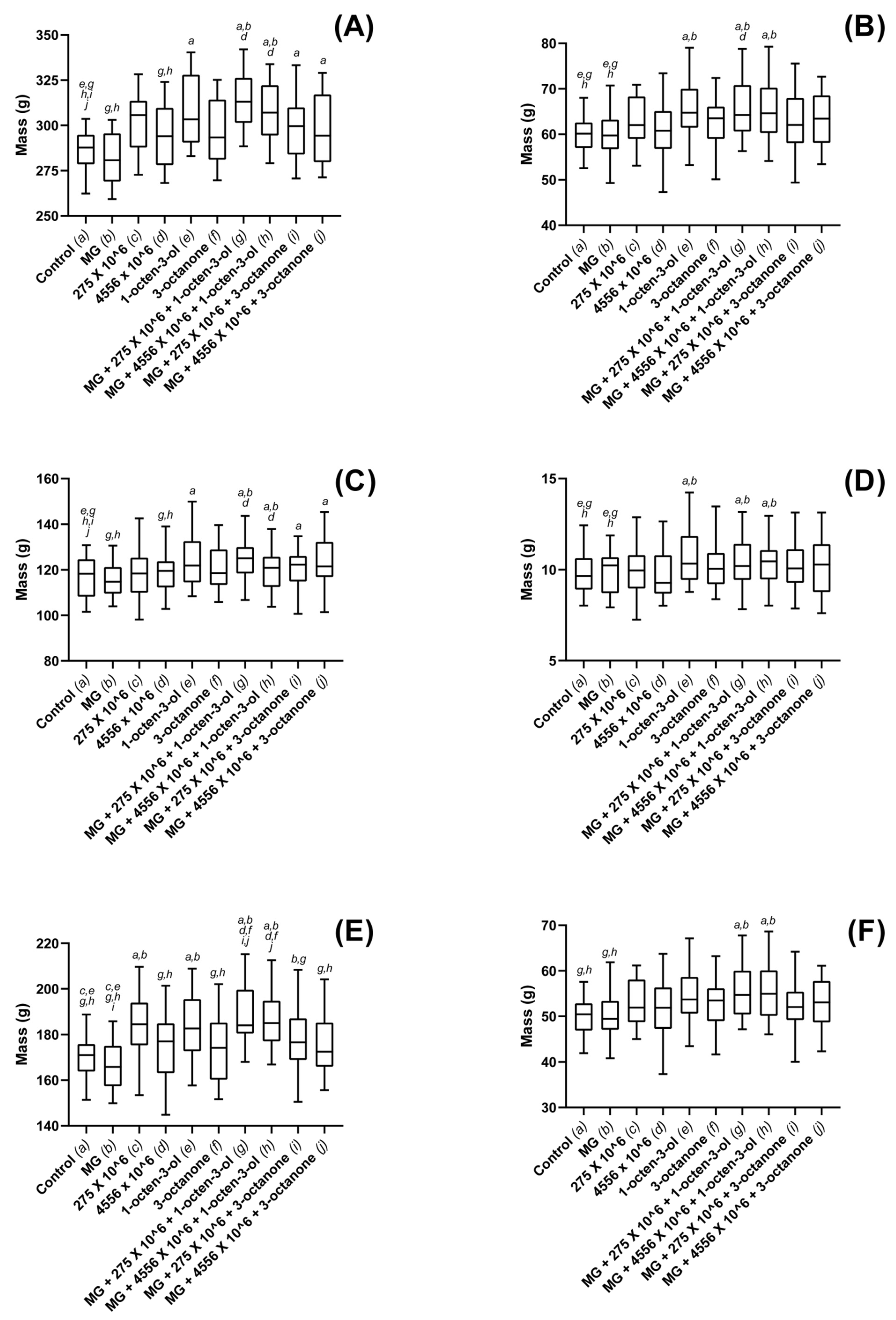
3.3. Biostimulatory Effects of M. brunneum, VOCs and Hydrogel Formulations on Four Commercially Important Plant Species
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scheepmaker, J.W.A.; Butt, T.M. Natural and Released Inoculum Levels of Entomopathogenic Fungal Biocontrol Agents in Soil in Relation to Risk Assessment and in Accordance with EU Regulations. Biocontrol Sci. Technol. 2010, 20, 503–552. [Google Scholar] [CrossRef]
- Faria, M.R.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A Comprehensive List with Worldwide Coverage and International Classification of Formulation Types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Clifton, E.H.; Jaronski, S.T.; Coates, B.S.; Hodgson, E.W.; Gassmann, A.J. Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS ONE 2018, 13, e0194815. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.C.; Sword, G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Elena, G.J.; Beatriz, P.J.; Alejandro, P.; Lecuona, R. Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Adv. Biol. Res. 2011, 5, 22–27. [Google Scholar]
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Hussain, M.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L. (Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392. [Google Scholar] [CrossRef]
- Vidal, S.; Jaber, L.R. Entomopathogenic fungi as endophytes: Plant-endophyte-herbivore interactions and prospects for use in biological control. Curr. Sci. 2015, 109, 46–54. [Google Scholar]
- Lefort, M.C.; McKinnon, A.C.; Nelson, T.L.; Glare, T.R. Natural occurrence of the entomopathogenic fungi Beauveria bassiana as a vertically transmitted endophyte of Pinus radiata and its effect on above- and below-ground insect pests. N. Z. Plant Prot. 2016, 69, 68–77. [Google Scholar] [CrossRef]
- Deaver, N.R.; Hesse, C.; Kuske, C.R.; Porras-Alfaro, A. Presence and distribution of insect-associated and entomopathogenic fungi in a temperate pine forest soil: An integrated approach. Fungal Biol. 2019, 123, 864–874. [Google Scholar] [CrossRef]
- Vega, F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2018, 98, 277–279. [Google Scholar] [CrossRef]
- Rodriguez, R.; White, J., Jr.; Arnold, A.; Redman, R. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Barelli, L.; Moonjely, S.; Behie, W.S.; Bidochka, M.J. Fungi with multifunctional lifestyles: Endophytic insect pathogenic fungi. Plant Mol. Biol. 2016, 90, 657–664. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Fungal entomopathogens as endophytes: Can they promote plant growth? Biocontrol Sci. Technol. 2017, 27, 28–41. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal Endophytes: Beyond Herbivore Management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- St. Leger, R.J.; Wang, J.B. Metarhizium: Jack of all trades, master of many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Kittakoop, P.; Thebtaranonth, Y. Secondary metabolites of Clavicipitalean fungi. In Clavicipitalean Fungi, 1st ed.; White, J.F., Bacon, C.W., Hywel-Jones, N.L., Spatafora, J.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 355–397. [Google Scholar]
- Oller-López, J.L.; Iranzo, M.; Mormeneo, S.; Oliver, E.; Cuerva, J.M.; Oltra, J.E. Bassianolone: An antimicrobial precursor of cephalosporolides E and F from the entomoparasitic fungus Beauveria bassiana. Org. Biomol. Chem. 2005, 3, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Gibson, D.M.; Krasnoff, S.B. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat. Prod. Rep. 2010, 27, 1241–1275. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.M.; Donzelli, B.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef]
- Gómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrophoresis 2009, 30, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic entomopathogenic fungi: A valuable biological control tool against plant pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef]
- Rasool, S.; Vidkjaer, N.H.; Hooshmand, K.; Jensen, B.; Fomsgaard, I.S.; Meyling, N.V. Seed inoculations with entomopathogenic fungi affect aphid populations coinciding with modulation of plant secondary metabolite profiles across plant families. New Phytol. 2021, 229, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lovett, B.; Fang, W.; St. Leger, R.J. Metarhizium robertsii produces indole-3-acetic acid, which promotes root growth in Arabidopsis and enhances virulence to insects. Microbiology 2017, 163, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 35–37. [Google Scholar] [CrossRef]
- Splivallo, R.; Novero, M.; Bertea, C.M.; Bossi, S.; Bonfante, P. Truffle volatiles inhibit growth and induce an oxidative burst in Arabidopsis thaliana. New Phytol. 2007, 175, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Li, Q.; Li, S.; Chen, C.; Chen, Z.; Huang, W. In vitro antimicrobial activities and mechanism of 1-octen-3-ol against food-related bacteria and pathogenic fungi. J. Oleo Sci. 2017, 66, 1041–1049. [Google Scholar] [CrossRef]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Myrta, A.; Bull, J.C.; Butt, T. Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol. Control 2021, 152, 104472. [Google Scholar] [CrossRef]
- Hummadi, E.H.; Dearden, A.; Generalovic, T.; Clunie, B.; Harrott, A.; Cetin, Y.; Demirbek, M.; Khoja, S.; Eastwood, D.; Dudley, E.; et al. Volatile organic compounds of Metarhizium brunneum influence the efficacy of entomopathogenic nematodes in insect control. Biol. Control 2021, 155, 104527. [Google Scholar] [CrossRef]
- Spraker, J.E.; Jewell, K.; Roze, L.V.; Scherf, J.; Ndagano, D.; Beaudry, R.; Linz, J.E.; Allen, C.; Keller, N.P. A Volatile Relationship: Profiling an Inter-Kingdom Dialogue Between Two Plant Pathogens, Ralstonia Solanacearum and Aspergillus Flavus. J. Chem. Ecol. 2014, 40, 502–513. [Google Scholar] [CrossRef]
- Rybakova, D.; Rack-Wetzlinger, U.; Cernava, T.; Schaefer, A.; Schmuck, M.; Berg, G. Aerial Warfare: A Volatile Dialogue between the Plant Pathogen Verticillium longisporum and Its Antagonist Paenibacillus polymyxa. Front. Plant Sci. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, J.; Kubisa, P.; Marechal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- El-Asmar, J.; Jaafar, H.; Bashour, I.; Farran, M.T.; Saoud, I.P. Hydrogel Banding Improves Plant Growth, Survival, and Water Use Efficiency in Two Calcareous Soils. Clean Soil Air Water 2017, 45, E1700251. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable Superabsorbent Hydrogel Increases Water Retention Properties of Growing Media and Plant Growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Dave, A.M.; Vaishnav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled release devices inagriculture. Des. Monomers Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Teodorescu, M.; Lungu, A.; Stanescu, P.O. Preparation and properties of novel slow-release NPK agrochemical formulations based on poly (acrylic acid) hydrogels and liquid fertilizers. Ind. Eng. Chem. Res. 2009, 48, 6527–6534. [Google Scholar] [CrossRef]
- Ansari, M.A.; Butt, T.M. Effects of successive subculturing on stability, virulence, conidial yield, germination and shelf-life of entomopathogenic fungi. J. Appl. Microbiol. 2011, 110, 1460–1469. [Google Scholar] [CrossRef]
- González-Pérez, E.; Ortega-Amaro, M.A.; Bautista, E.; Delgado-Sánchez, P.; Jiménez-Bremont, J.F. The entomopathogenic fungus Metarhizium anisopliae enhances Arabidopsis, tomato, and maize plant growth. Plant Physiol. Biochem. 2022, 176, 34–43. [Google Scholar] [CrossRef]
- Posada, F.; Aime, M.C.; Peterson, S.W.; Rehner, S.A.; Vega, F.E. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol. Res. 2007, 111, 748–757. [Google Scholar] [CrossRef]
- Liang-Dong, G.; Guo-Rui, H.; Yu, W. Seasonal and tissue age influences on endophytic fungi of Pinus tabulaeformis (Pinaceae) in the Dongling Mountains, Beijing. Int. J. Integr. Biol. 2008, 50, 997–1003. [Google Scholar] [CrossRef]
- El-Rehim, H.A.; Hegazy, E.S.A.; El-Mohdy, H.A. Radiation synthesis of hydrogels to enhance sandy soils water retention and increase plant performance. J. Appl. Polym. Sci. 2004, 93, 1360–1371. [Google Scholar] [CrossRef]
- Zohourian, M.M.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Hu, X.; Xiao, G.; Zheng, P.; Shang, Y.; Su, Y.; Zhang, X.; Liu, X.; Zhan, S.; St. Leger, R.J.; Wang, C. Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proc. Natl. Acad. Sci. USA 2014, 111, 16796–16801. [Google Scholar] [CrossRef] [PubMed]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2016, 47, 317–326. [Google Scholar] [CrossRef]
- Yanagawa, A.; Imai, T.; Akino, T.; Toh, Y.; Yoshimura, T. Olfactory cues from pathogenic fungus affect the direction of motion of termites, Coptotermes formosanus. J. Chem. Ecol. 2015, 41, 1118–1126. [Google Scholar] [CrossRef]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Gołębiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef]
- Herrera, J.M.; Pizzolitto, R.P.; Zunino, M.P.; Dambolena, J.S.; Zygadlo, J.A. Effect of fungal volatile organic compounds on a fungus and an insect that damage stored maize. J. Stored Prod. Res. 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Bull, J.C.; Loveridge, E.J.; Butt, T.M. Fungal volatile organic compounds show promise as potent molluscicides. Pest Manag. Sci. 2019, 75, 3392–3404. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sasan, R.K.; Bidochka, M.J. Antagonism of the endophytic insect pathogenic fungus Metarhizium robertsii against the bean plant pathogen Fusarium solani f. sp. phaseoli. Can. J. Plant Pathol. 2013, 35, 288–293. [Google Scholar] [CrossRef]
- Bourdon, P.A.; Zottele, M.; Baxter, I.; Myrta, A.; Midthassel, A.; Wechselberger, K.F.; Khoja, S.; Bull, J.C.; Strasser, H.; Butt, T.M. Fumigation of three major soil pests (Agriotes lineatus, Diabrotica virgifera virgifera, Phyllopertha horticola) with 3-octanone and 1-octen-3-ol enantiomers. Biocontrol Sci. Technol. 2022, 32, 863–876. [Google Scholar] [CrossRef]
- Sánchez-López, Á.M.; Bahaji, A.; De Diego, N.; Baslam, M.; Li, J.; Muñoz, F.J.; Almagro, G.; García-Gómez, P.; Ameztoy, K.; Ricarte-Bermejo, A.; et al. Arabidopsis Responds to Alternaria alternata Volatiles by Triggering Plastid Phosphoglucose Isomerase-Independent Mechanisms. Plant Physiol. 2016, 172, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Bitas, V.; McCartney, N.; Li, N.; Demers, J.; Kim, J.E.; Kim, H.S.; Brown, K.M.; Kang, S. Fusarium oxysporum volatiles enhance plant growth via affecting auxin transport and signaling. Front. Microbiol. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.; Dowd, S.; Paré, P. A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 2019, 58, 568–577. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, M.J.; Kortsinoglou, A.M.; Khoja, S.; Kouvelis, V.N.; Myrta, A.; Midthassel, A.; Loveridge, E.J.; Butt, T.M. Metarhizium brunneum (Hypocreales: Clavicipitaceae) and Its Derived Volatile Organic Compounds as Biostimulants of Commercially Valuable Angiosperms and Gymnosperms. J. Fungi 2022, 8, 1052. https://doi.org/10.3390/jof8101052
Wood MJ, Kortsinoglou AM, Khoja S, Kouvelis VN, Myrta A, Midthassel A, Loveridge EJ, Butt TM. Metarhizium brunneum (Hypocreales: Clavicipitaceae) and Its Derived Volatile Organic Compounds as Biostimulants of Commercially Valuable Angiosperms and Gymnosperms. Journal of Fungi. 2022; 8(10):1052. https://doi.org/10.3390/jof8101052
Chicago/Turabian StyleWood, Martyn J., Alexandra M. Kortsinoglou, Salim Khoja, Vassili N. Kouvelis, Arben Myrta, Audun Midthassel, E. Joel Loveridge, and Tariq M. Butt. 2022. "Metarhizium brunneum (Hypocreales: Clavicipitaceae) and Its Derived Volatile Organic Compounds as Biostimulants of Commercially Valuable Angiosperms and Gymnosperms" Journal of Fungi 8, no. 10: 1052. https://doi.org/10.3390/jof8101052
APA StyleWood, M. J., Kortsinoglou, A. M., Khoja, S., Kouvelis, V. N., Myrta, A., Midthassel, A., Loveridge, E. J., & Butt, T. M. (2022). Metarhizium brunneum (Hypocreales: Clavicipitaceae) and Its Derived Volatile Organic Compounds as Biostimulants of Commercially Valuable Angiosperms and Gymnosperms. Journal of Fungi, 8(10), 1052. https://doi.org/10.3390/jof8101052






