Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Paecilomyces from the Rhizosphere
2.2. Spore Suspension Preparation
2.3. Cell Free Supernatant and Nematode Bioassay
2.4. Analysis of Fungal Densities in Rhizosphere and Soil
2.5. Analysis of Endophytic Colonization of Fungi
2.6. Source and Maintenance of Nematode Culture
2.7. Egg Hatch Inhibition Assay by Cell Free Extract of Fungus
2.8. Influence of Spore Suspensions on the Activity of M. incognita Eggs
2.9. Effect of P. formosus MD12 on Juveniles of Nematode
2.10. Pot Experiment
2.11. Enzyme Extraction
2.12. Peroxidase Activity
2.13. Polyphenoloxidase Activity
2.14. Seed Germination Analysis
2.15. Data Analysis
3. Results
3.1. Characterization of P. formosus MD12 and Density of Fungi in Soil and Rhizosphere Sample
3.2. Endophytic Colonization of P. formosus MD12
3.3. Endophytic Root Colonization of P. formosus MD12 and Other Fungi in Experimental Plants
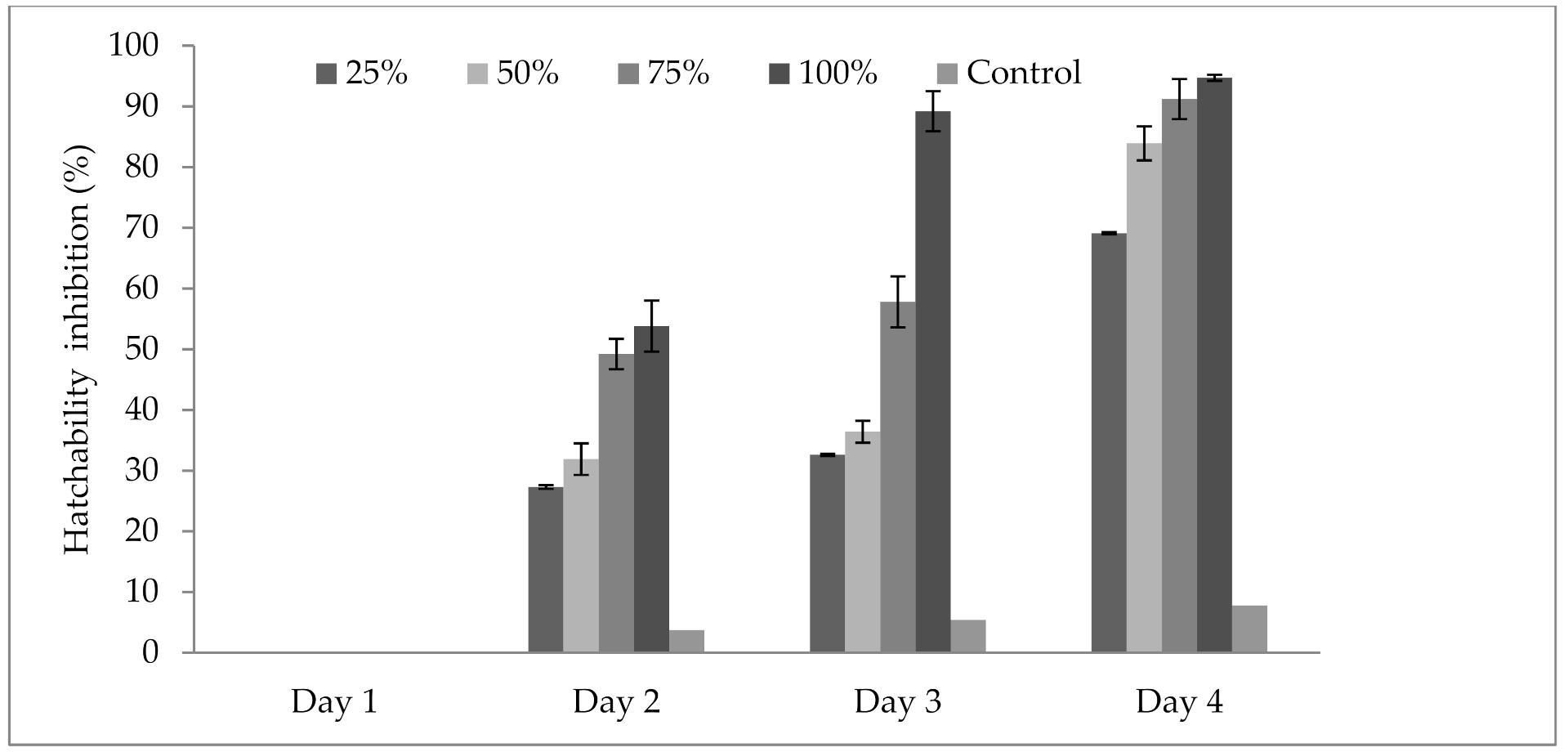
3.4. Influence of P. formosus MD12 Supernatant on Inhibition of Egg Hatching
3.5. Influence of P. formosus MD12 Spores on Egg Hatching
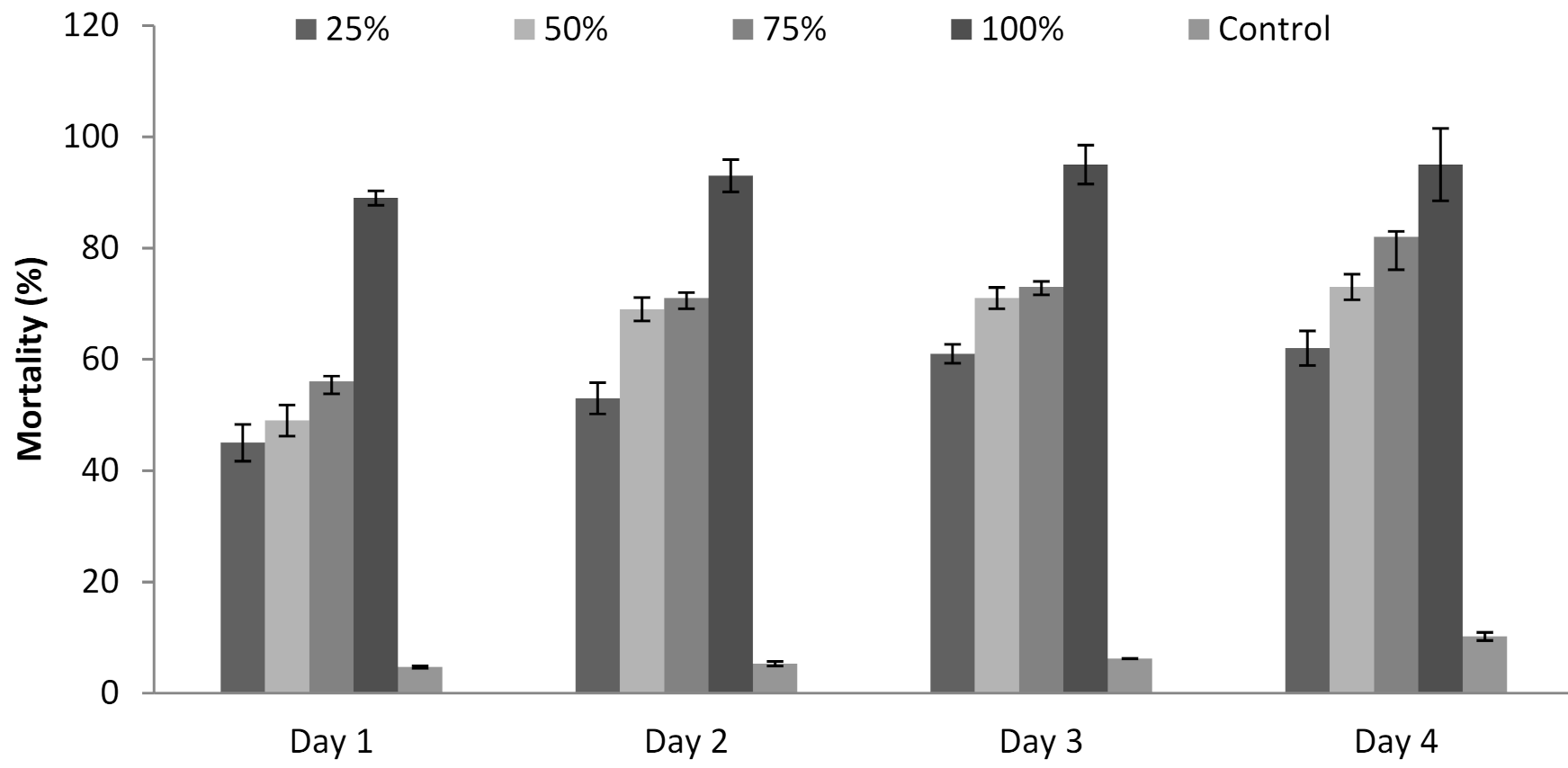
3.6. Influence of P. formosus MD12 Supernatant on Juvenile Mortality
3.7. Effect of Fungal Spore Suspension and Supernatant on the Reduction of Nematode Population in Pot and Plant
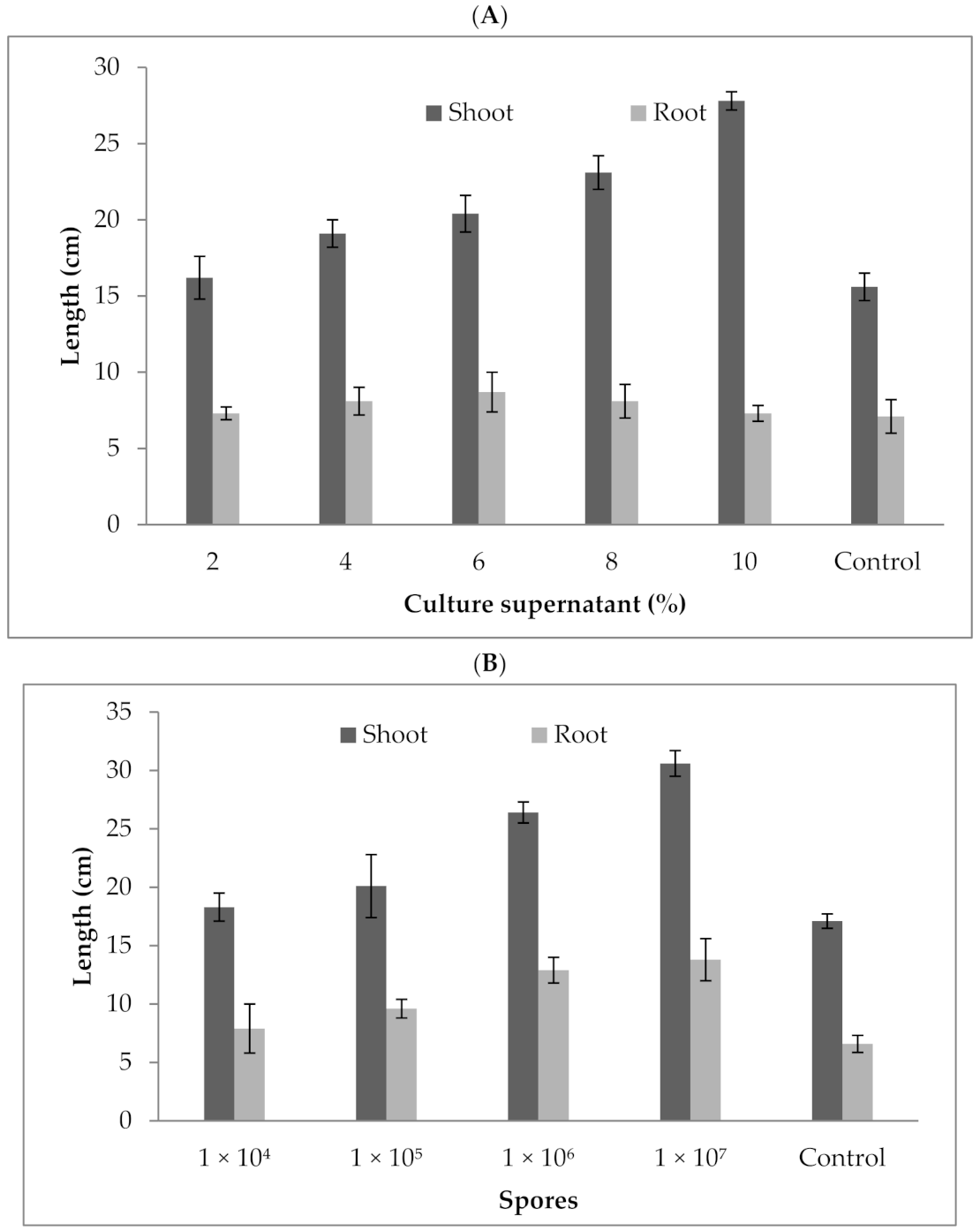
3.8. Effect of P. formosus MD12 Culture Supernatant and Spores on Brinjal Plant Growth
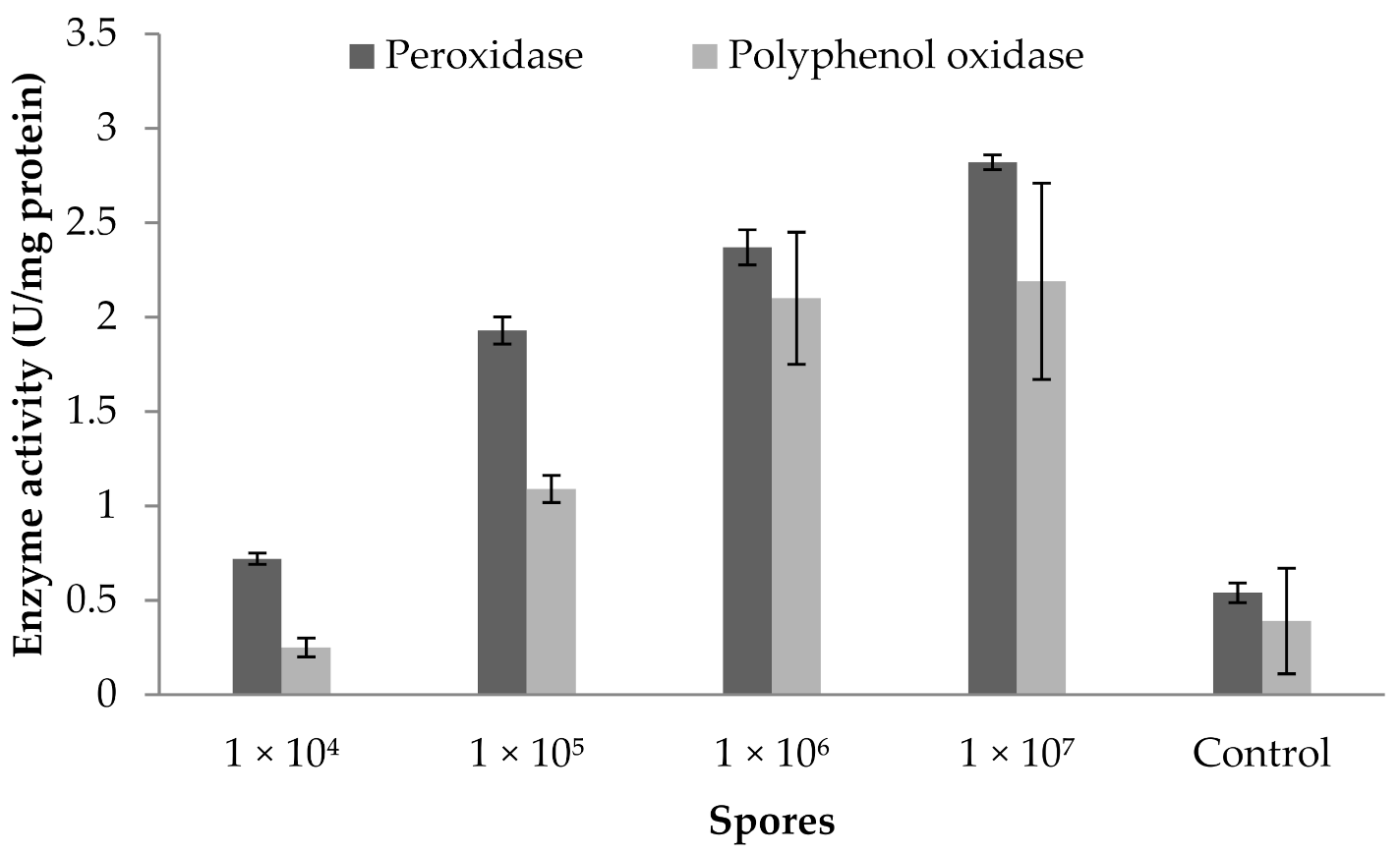
3.9. Influence of Fungal Spores on Peroxidase and Polyphenoloxidase Activity
3.10. Influence of Fungal Spores on Seed Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dijksterhuis, J. Fungal spores: Highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef]
- Van den Brule, T.; Punt, M.; Teertstra, W.; Houbraken, J.; Wösten, H.; Dijksterhuis, J. The most heat-resistant conidia ob-served to date are formed by distinct strains of Paecilomycesvariotii. Environ. Microbiol. 2020, 22, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Brule, T.V.D.; Lee, C.L.S.; Houbraken, J.; Haas, P.-J.; Wösten, H.; Dijksterhuis, J. Conidial heat resistance of various strains of the food spoilage fungus Paecilomycesvariotii correlates with mean spore size, spore shape and size distribution. Food Res. Int. 2020, 137, 109514. [Google Scholar] [CrossRef]
- Moreno-Gavíra, A.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomycesvariotii as a plant-growth promoter in horti-culture. Agronomy 2020, 10, 597. [Google Scholar] [CrossRef]
- Homthong, M.; Kubera, A.; Srihuttagum, M.; Hongtrakul, V. Isolation and characterization of chitinase from soil fungi, Paecilomyces sp. Agric. Nat. Resour. 2016, 50, 232–242. [Google Scholar] [CrossRef][Green Version]
- Mbarek, H.N.; Taidi, B.; Smaoui, T.; Aziz, M.B.; Mansouri, A.; Hajjaj, H. Isolation screening and identification of lig-no-cellulolytic fungi from northern central Morocco. Biotechnol. Agron. Soc. Environ. 2019, 23, 207–217. [Google Scholar]
- Biango-Daniels, M.N.; Snyderb, A.B.; Woroboc, R.W.; Hodge, K.T. Fruit infected with Paecilomycesniveus: Asource of spoil-age inoculum and patulin in apple juice concentrate? Food Control 2019, 97, 81–86. [Google Scholar] [CrossRef]
- Dos Santos, J.L.P.; Samapundo, S.; Biyikli, A.; Van Impe, J.; Akkermans, S.; Höfte, M. Occurrence, distribution and contami-nation levels of heat-resistant moulds throughout the processing of pasteurized high-acid fruit products. Int. J. Food Microbiol. 2018, 281, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberel-lins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomycesformosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Baron, N.C.; Pollo, A.D.S.; Rigobelo, E.C. Purpureocilliumlilacinum and Metarhiziummarquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef] [PubMed]
- Mezeal, I.A.; Mizil, S.N.; Hussin, M.S. Researching biocontrol of Trichoderma viride, Paecilomyceslilacinus in contradiction of effectiveness of fungi insulated as of selected therapeutic herbals. Plant Arch. 2018, 18, 1631–1637. [Google Scholar]
- Nesha, R.; Siddiqui, Z.A. Effects of Paecilomyceslilacinus and Aspergillus niger alone and in combination on the growth, chlorophyll contents and soft rot disease complex of carrot. Sci. Hortic. 2017, 218, 258–264. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.Q.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applica-tions: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Teles, C.; Takahashi, J.A. Paecilomide, a new acetylcholinesterase inhibitor from Paecilomyceslilacinus. Microbiol. Res. 2013, 168, 204–221. [Google Scholar]
- Ham, H.; Kim, S.; Kim, M.; Lee, S.; Hong, S.K.; Ryu, J.; Lee, T. Mycobiota of ground red pepper and their aflatoxigenic po-tential. J. Microbiol. 2016, 54, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Roussos, S.; Zaouia, N.; Salih, G.; Tantaoui-Elaraki, A.; Lamrani, K.; Cheheb, M.; Hassouni, H.; Verh, F.; Perraud-Gaime, I.; Augur, C.; et al. Characterization of filamentous fungi isolated from Moroccan olive and olive cake: Toxinogenic potential of Aspergillus strains. Mol. Nutr. Food Res. 2006, 50, 500–506. [Google Scholar] [CrossRef]
- Alkenz, S.; Sassi, A.A.; Abugnah, Y.S.; Alryani, M.B. Isolation and identification of fungi associated with some Libyan foods. Afr. J. Food Sci. 2015, 9, 406–410. [Google Scholar]
- Akello, J.; Dubois, T.; Gold, C.S.; Coyne, D.; Nakavuma, J.; Paparu, P. Beauveria bassiana (Balsamo) Vuillemin as an endo-phyte in tissue culture banana (Musa spp.). J. Invert. Pathol. 2007, 96, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zawadneak, M.A.C.; Pimentel, I.C.; Robl, D.; Dalzoto, P.; Vicente, V.; Sosa-Gómez, D.R.; Porsani, M.; Cuquel, F.L. PaecilomycesniveusStolk& Samson, 1971 (Ascomycota: Thermoascaceae) as a pathogen of Nasonoviaribisnigri (Mosley, 1841) (Hemiptera, Aphididae) in Brazil. Braz. J. Biol. 2015, 75, 158–162. [Google Scholar] [CrossRef][Green Version]
- Parsa, S.; García-Lemos, A.M.; Castillo, K.; Ortiz, V.; López-Lavalle, L.A.B.; Braun, J.; Vega, F.E. Fungal endophytes in ger-minated seeds of the common bean, Phaseolus vulgaris. Fungal Biol. 2016, 120, 783–790. [Google Scholar] [CrossRef]
- Nisha, M.K.; Kalaiselvi, M. Effect of carbon and nitrogen sources on pectinase by Paecilomycesvariotii. Int. J. Curr. Adv. Res. 2016, 5, 874–877. [Google Scholar]
- Gao, L.; Sun, M.H.; Liu, X.Z.; Che, Y.S. Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycol. Res. 2007, 111, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Chen, S.Y. Nutritional Requirements of the Nematophagous Fungus Hirsutellarhossiliensis. Biocontrol Sci. Technol. 2002, 12, 381–393. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Kumar, A.; Alam, P.; Ahmad, P. Enhancing Nutritional Contents of Lentinussajor-caju Using Residual Biogas Slurry Waste of Detoxified Mahua Cake Mixed with Wheat Straw. Front. Microbiol. 2016, 7, 1529. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Duraipandiyan, V.; Arasu, M.V.; Salem-Bekhit, M.M. Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles 2015, 20, 79–90. [Google Scholar] [CrossRef]
- Marraiki, N.; Vijayaraghavan, P.; Elgorban, A.M.; Dhas, D.D.; Al-Rashed, S.; Yassin, M.T. Low cost feedstock for the produc-tion of endoglucanase in solid state fermentation by Trichoderma hamatum NGL1 using response surface methodology and saccharification efficacy. J. King Saud Univ. Sci. 2020, 32, 1718–1724. [Google Scholar] [CrossRef]
- Jang, J.H.; Moon, K.D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treat-ment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, B.; Wang, Y.; Zhu, X.; Duan, Y.; Chen, L. Screening for nematicidal activities of Beauveria bassiana and associat-ed fungus using culture filtrate. Afr. J. Microbiol. Res. 2013, 7, 974–978. [Google Scholar]
- Khan, A.; Williams, K.L.; Nevalainen, H.K. Infection of plant-parasitic nematodes by Paecilomyceslilacinus and Monacro-sporiumlysipagum. BioControl 2006, 51, 659–678. [Google Scholar] [CrossRef]
- Khan, A.; Williams, K.L.; Nevalainen, H. Effects of Paecilomyceslilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol. Control 2004, 31, 346–352. [Google Scholar] [CrossRef]
- Singh, S.; Mathur, N. In Vitro Studies of Antagonistic Fungi Against Root-knot Nematode, Meloidogyne incognita. Biocontrol Sci. Technol. 2010, 20, 275–285. [Google Scholar] [CrossRef]
- Huang, W.K.; Cui, J.K.; Liu, S.M.; Kong, L.A.; Wu, Q.S.; Peng, H.; He, W.T.; Sun, J.H.; Peng, D.L. Testing various biocontrol agents against the root-knot nematode (Meloidogyne incognita) in cucumber plants identifies a combination of Syncephalas-trumracemosum and Paecilomyceslilacinus as being most effective. Biol. Control 2016, 92, 31–37. [Google Scholar] [CrossRef]
- Jain, R.K.; Mathur, K.N.; Singh, R.V. Estimatimation of Losses due to Plant Parasitic Nematodes on Different Crops in India. Ind. J. Nematol. 2007, 37, 219–221. [Google Scholar]
- D’Errico, G.; Mormile, P.; Malinconico, M.; Bolletti Censi, S.; Lanzuise, S.; Crasto, A.; Woo, S.L.; Marra, R.; Lorito, M.; Vinale, F. Trichoderma spp. and a carob (Ceratonia siliqua) galactomannan to control the root-knot nematode Meloidogyne incognita on tomato plants. Can. J. Plant Pathol. 2021, 43, 267–274. [Google Scholar] [CrossRef]
- Naz, I.; Khan, R.A.A.; Masood, T.; Baig, A.; Siddique, I.; Haq, S. Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biol. Control 2021, 152, 104429. [Google Scholar] [CrossRef]
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop. Prot. 2021, 144, 105573. [Google Scholar] [CrossRef]
- Usman, A.; Siddiqui, M.A. Effect of some fungal strains for the management of root-knot nematode (Meloidogyne in-cognita) on eggplant (Solanum melongena). J. Agricul. Technol. 2012, 8, 213–218. [Google Scholar]
- Spiegel, Y.; McClure, M.A. The Surface Coat of Plant-Parasitic Nematodes: Chemical Composition, Origin, and Biological Role—A Review. J. Nematol. 1995, 27, 127–134. [Google Scholar]
- Hölscher, D.; Fuchser, J.; Knop, K.; Menezes, R.C.; Buerkert, A.; Svatoš, A.; Schubert, U.S.; Schneider, B. High resolution mass spectrometry imaging reveals the occurrence of phenylphenalenone- type compounds in red paracytic stomata and red epidermis tissue of Musa acuminata ssp. zebrine cv. ‘Rowe Red’. Phytochemistry 2015, 116, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Phani, V.; Shivakumara, T.N.; Davies, K.G.; Rao, U. Meloidogyne incognita Fatty Acid- and Retinol- Binding Protein (Mi-FAR-1) Affects Nematode Infection of Plant Roots and the Attachment of Pasteuriapenetrans Endospores. Front. Microbiol. 2017, 8, 2122. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, M.; Haikal, A.M.; Hammad, S.E. Meloidogyne incognita population control and nutritional status and produc-tivity of Thompson seedless grapevines managed with different treatments. PLoS ONE 2020, 15, e0239993. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, M.A.; Rajaselvam, J.; Abdel-Salam, E.M.; Vijayaraghavan, P.; Alatar, A.A.; Biji, G.D. Paecilomyces sp. ZB is a cell factory for the production of gibberellic acid using a cheap substrate in solid state fermentation. Saudi J. Biol. Sci. 2020, 27, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Lokesh, S.; Ravishankar Rai, V.; RudraNaik, V.; Raju, S.G. Induction of systemic resistance and manage-ment of safflower Macrophominaphaseolina root-rot disease by biocontrol agents. Arch. Phytopathol. Plant Prot. 2010, 43, 26–40. [Google Scholar] [CrossRef]



| Fungi (CFU g−1) | ||
|---|---|---|
| Plants | Soil | Rhizosphere |
| Tomato | 6.3 ± 0.13 | 6.72 ± 0.27 |
| Cucumber | 5.8 ± 0.41 | 4.3 ± 0.86 |
| Soybean | 3.7 ± 0.58 | 3.56 ± 1.1 |
| Corn | 2.1 ± 0.32 | 1.92 ± 0.5 |
| Brinjal | 7.68 ± 2.62 | 8.37 ± 2.6 |
| Mustard | 4.59 ± 1.93 | 5.2 ± 0.42 |
| Cow pea | 1.76 ± 0.72 | 0.41 ± 0.02 |
| Onion | 3.2 ± 0.26 | 3.51 ± 0.86 |
| Fungi (CFU g−1) | ||
|---|---|---|
| Plants | P. formosus | Other Fungi |
| Tomato | 0.39 ± 0.0 | 1.9 ± 0.07 |
| Cucumber | 0.34 ± 0.02 | 0.69 ± 0.05 |
| Soybean | 2.34 ± 0.18 | 3.7 ± 0.92 |
| Corn | 2.86 ± 0.72 | 6.2 ± 1.2 |
| Brinjal | 3.867 ± 0.82 | 8.9 ± 1.8 |
| Mustard | 2.28 ± 0.65 | 3.5 ± 1.1 |
| Cow pea | 1.09 ± 0.52 | 5.3 ± 0.2 |
| Onion * Control | 0.21 ± 0.03 0.13 ± 0.02 | 2.6 ± 1.3 0.52 ± 0.27 |
| (a) | |||
|---|---|---|---|
| Spore Suspension (CFU mL−1) | Reduction of Nematode (%) | ||
| Root | Soil | Egg Masses | |
| 1 × 104 | 47 ± 1.9 | 53.1 ± 2.8 | 40.9 ± 3.2 |
| 1 × 105 | 51 ± 2.6 | 60.2 ± 3.3 | 52.7 ± 1.6 |
| 1 × 106 | 58.4 ± 1.1 | 67.2 ± 2.7 | 60.3 ± 2.7 |
| 1 × 107 Control | 60.7 ± 2.2 4.1 ± 0.3 | 71.6 ± 3.3 0.8 ± 0.42 | 63.6 ± 2.4 0 ± 0 |
| (b) | |||
| Culture Supernatant (%) | Reduction of Nematode (%) | ||
| Root | Soil | Egg Masses | |
| 2 | 40 ± 2.1 | 51.1 ± 3.8 | 42.9 ± 4.2 |
| 4 | 43 ± 2.5 | 61.3 ± 2.3 | 56.7 ± 2.7 |
| 6 | 59.2 ± 1.5 | 69.2 ± 2.2 | 68.2 ± 3.1 |
| 8 | 63.8 ± 3.3 | 76.8 ± 3.7 | 69.2 ± 1.9 |
| 10 Control | 72.4 ± 2.2 2 ± 0.1 | 77.9 ± 2.5 0.48 ± 0.16 | 73.2 ± 1.5 4.1 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baazeem, A.; Alorabi, M.; Manikandan, P.; Alotaibi, S.S.; Almanea, A.; Abdel-Hadi, A.; Vijayaraghavan, P.; Raj, S.R.F.; Kim, Y.O.; Kim, H.-J. Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House. J. Fungi 2021, 7, 632. https://doi.org/10.3390/jof7080632
Baazeem A, Alorabi M, Manikandan P, Alotaibi SS, Almanea A, Abdel-Hadi A, Vijayaraghavan P, Raj SRF, Kim YO, Kim H-J. Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House. Journal of Fungi. 2021; 7(8):632. https://doi.org/10.3390/jof7080632
Chicago/Turabian StyleBaazeem, Alaa, Mohammed Alorabi, Palanisamy Manikandan, Saqer S. Alotaibi, Abdulaziz Almanea, Ahmed Abdel-Hadi, Ponnuswamy Vijayaraghavan, Subhanandharaj Russalamma Flanet Raj, Young Ock Kim, and Hak-Jae Kim. 2021. "Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House" Journal of Fungi 7, no. 8: 632. https://doi.org/10.3390/jof7080632
APA StyleBaazeem, A., Alorabi, M., Manikandan, P., Alotaibi, S. S., Almanea, A., Abdel-Hadi, A., Vijayaraghavan, P., Raj, S. R. F., Kim, Y. O., & Kim, H.-J. (2021). Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House. Journal of Fungi, 7(8), 632. https://doi.org/10.3390/jof7080632






