The Plant Salicylic Acid Signalling Pathway Regulates the Infection of a Biotrophic Pathogen in Grasses Associated with an Epichloë Endophyte
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant and Fungal Materials
2.2. Measurement Protocols
2.2.1. Determination of Jasmonic Acid and Salicylic Acid Hormone Concentrations
2.2.2. Disease Investigation
2.2.3. Determination of Plant Growth
2.3. Characterisation of Plant Transcriptomes
2.3.1. Plant RNA Extraction and Library Preparation
2.3.2. Plant Transcriptome Assembly, Annotation, Calculation of Gene Expression Values and Identification of Genes Associated with Plant Defences
2.3.3. Validation of RNA-Seq Data
2.4. Statistical Analyses
3. Results
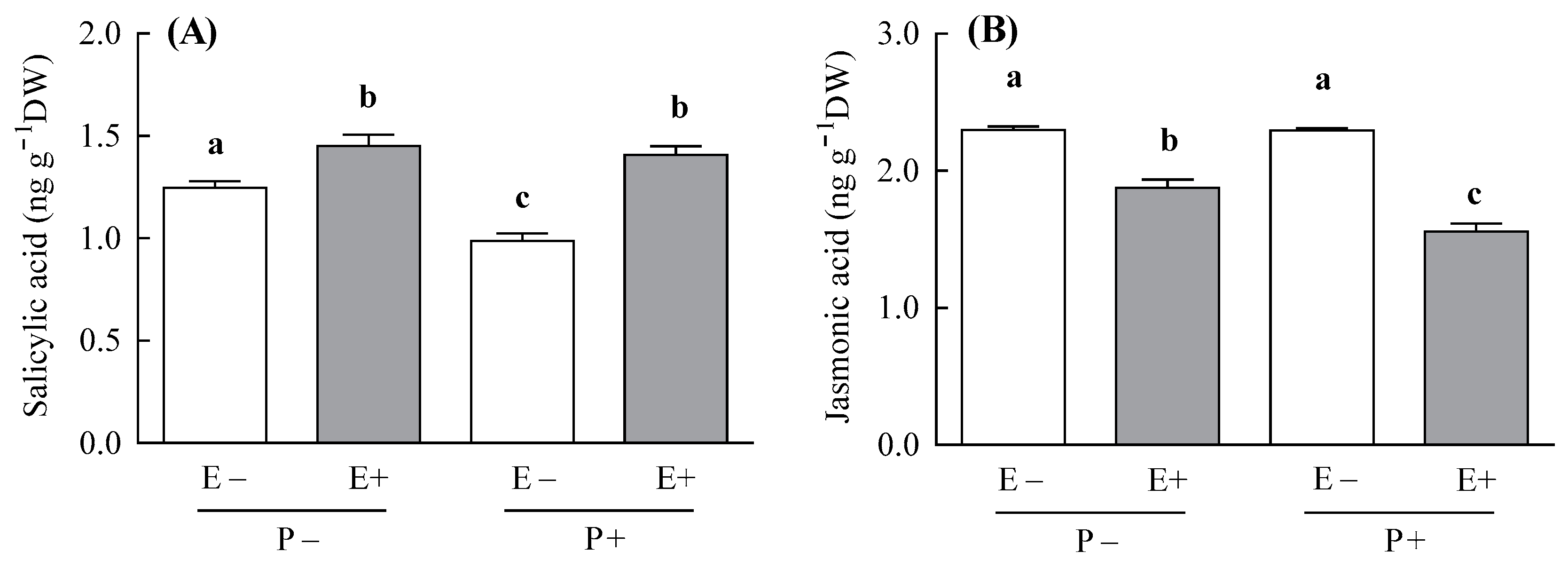
3.1. SA and JA Content
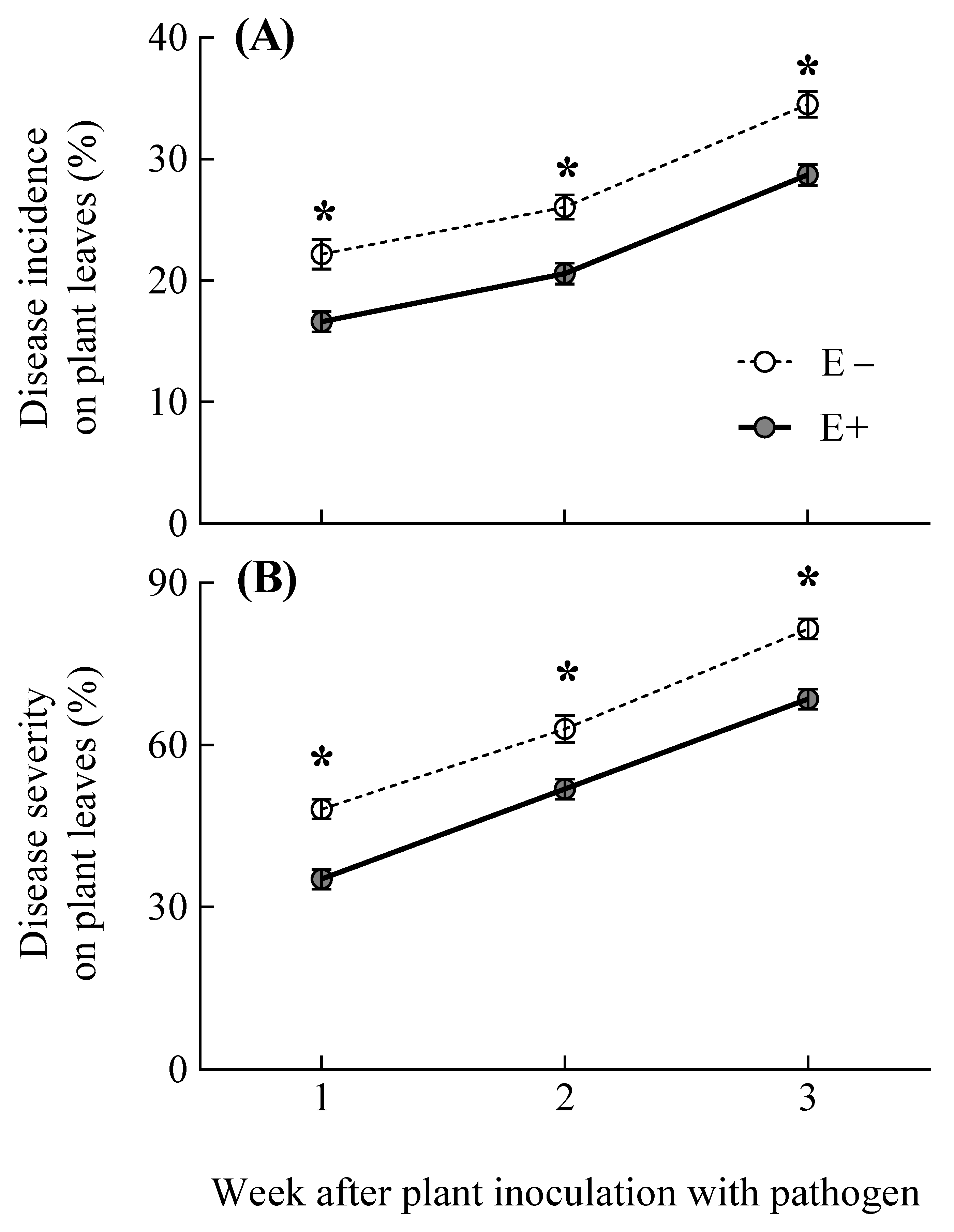
3.2. Pathogen Disease Incidence and Severity on Plants
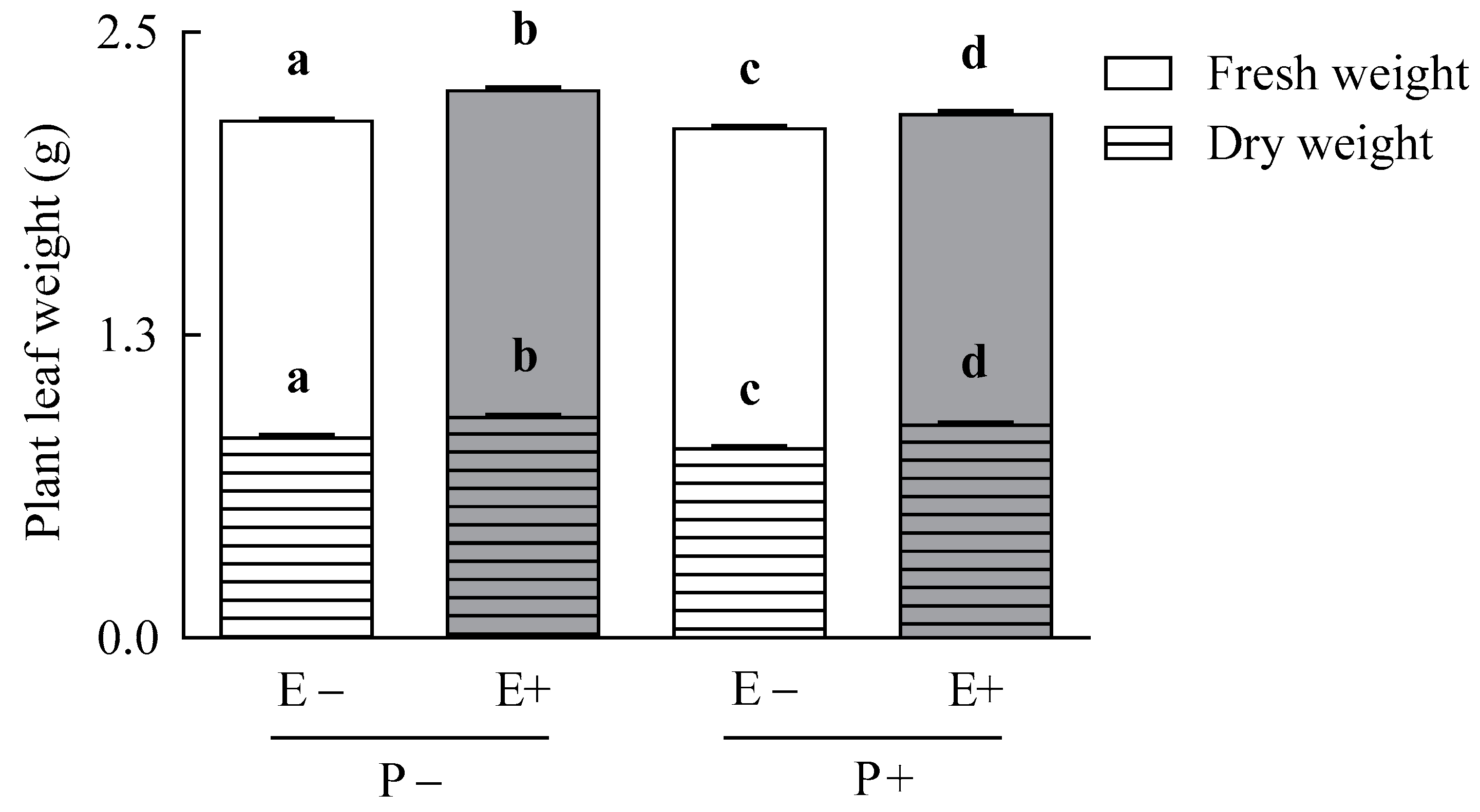
3.3. Plant Growth Parameters
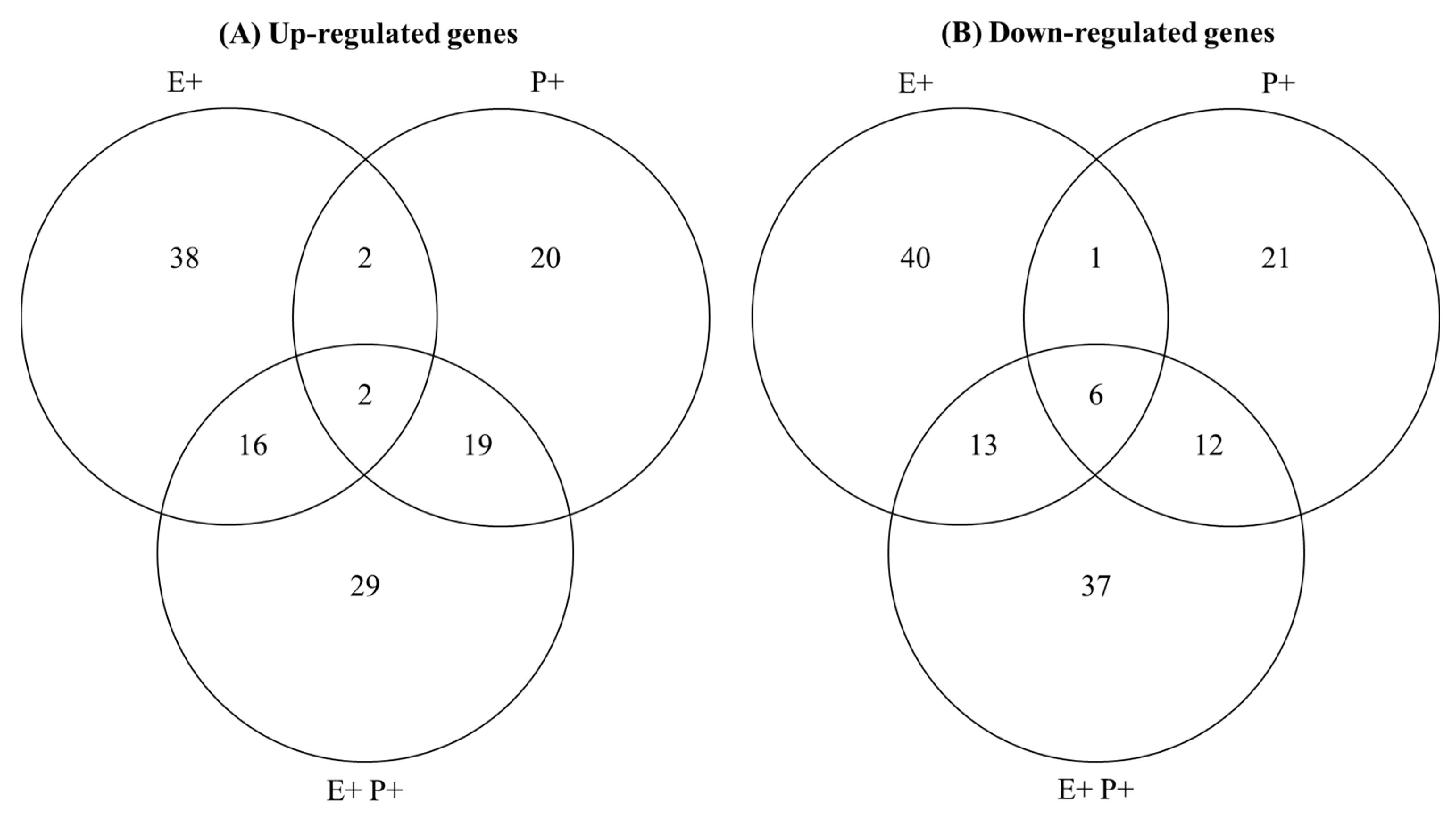
3.4. Characterisation of Plant DEGs Associated with Defence Responses
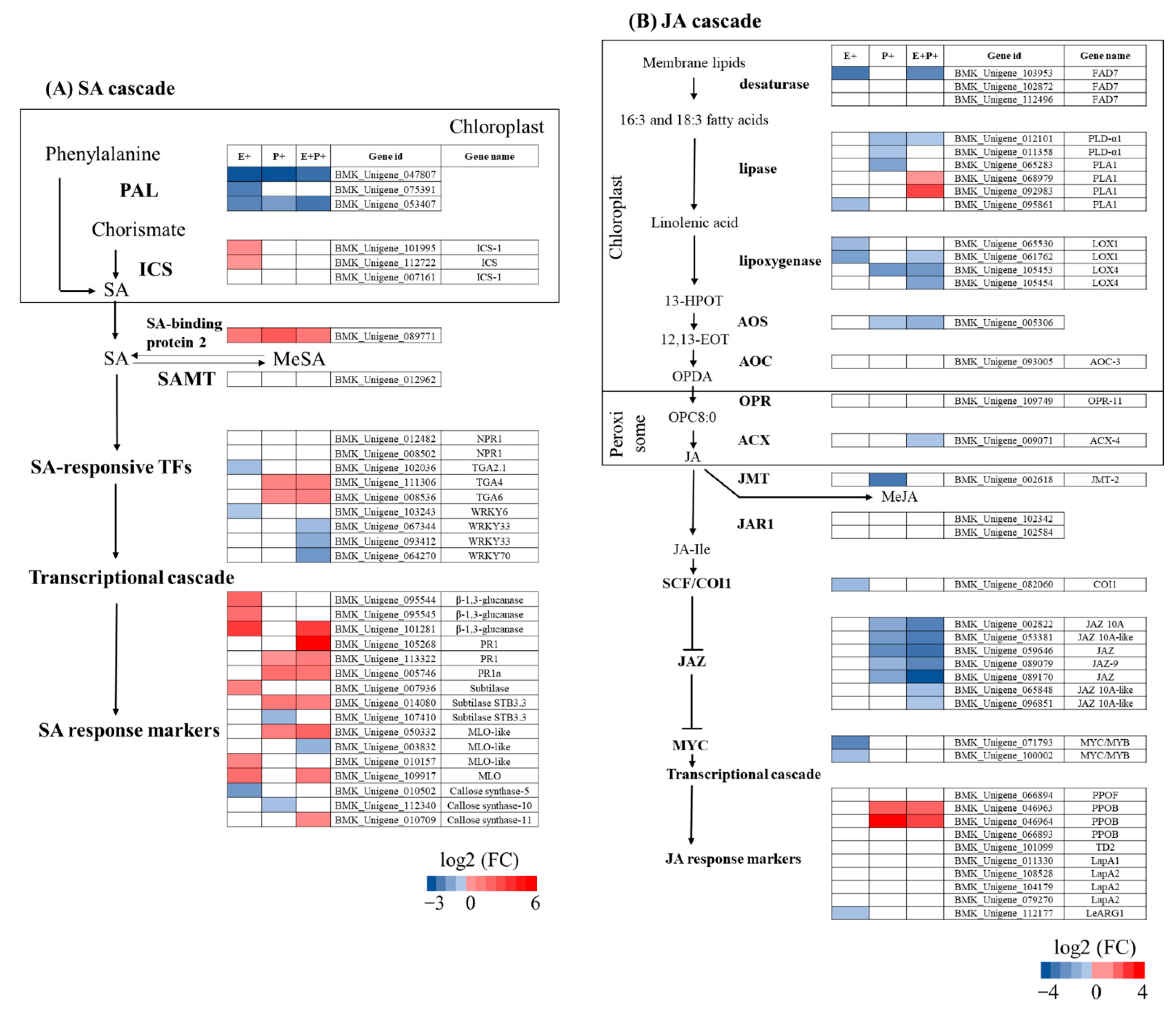
3.5. Expression of Genes Associated with SA and JA Signalling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; López-Ráez, J.A.; Azcón-Aguilar, C.; García-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Bastias, D.A.; Martínez-Ghersa, M.A.; Ballaré, C.L.; Gundel, P.E. Epichloë fungal endophytes and plant defenses: Not just alkaloids. Trends Plant Sci. 2017, 22, 939–948. [Google Scholar] [CrossRef]
- Schardl, C.L.; Florea, S.; Pan, J.; Nagabhyru, P.; Bec, S.; Calie, P.J. The Epichloae: Alkaloid diversity and roles in symbiosis with grasses. Curr. Opin. Plant Biol. 2013, 16, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Panaccione, D.G.; Beaulieu, W.T.; Cook, D. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol. 2014, 28, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.; Day, R.; Zhang, N.; Dupont, P.-Y.; Cox, M.P.; Schardl, C.L.; Minards, N.; Truglio, M.; Moore, N.; Harris, D.R.; et al. Host tissue environment directs activities of an Epichloë endophyte, while it induces systemic hormone and defense responses in its native perennial ryegrass host. Mol. Plant-Microbe Interact. 2017, 30, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.-J.; Qin, T.-Z.; Liu, H.; Wu, M.; Li, J.-J.; Shi, Y.-S.; Gao, Y.-B.; Ren, A.-Z. Endophytic fungi activated similar defense strategies of Achnatherum sibiricum ost to different trophic types of pathogens. Front. Microbiol. 2020, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-J.; Wang, Y.-R.; Li, X.; Zhang, Y.-L. Biosynthesis and regulation of salicylic acid and N-hydroxypipecolic acid in plant immunity. Mol. Plant 2020, 13, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Liu, P.-P.; Cameron, R.K.; Park, S.-W.; Yang, Y.; Kumar, D.; Zhou, F.; Padukkavidana, T.; Gustafsson, C.; Pichersky, E.; et al. Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J. 2008, 56, 445–456. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.-Y.; Huang, L.-J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T.; Simpson, W.R.; Koolaard, J.P.; Nickless, E.M.; Voisey, C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008, 45, 84–93. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.-N.; Zhang, Y.-W.; Li, C.-J.; Zhang, X.-X.; Nan, Z.-B. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef] [Green Version]
- Bonos, S.A.; Wilson, M.M.; Meyer, W.A.; Reed Funk, C. Suppression of red thread in fine fescues through endophyte-mediated resistance. Appl. Turfgrass Sci. 2005, 2, 1–7. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Mirlohi, A.; Sharifnabi, B. Reaction to powdery mildew fungus, Blumeria graminis in endophyte-infected and endophyte-free tall and meadow fescues. Australas. Plant Pathol. 2012, 41, 565–572. [Google Scholar] [CrossRef]
- Pérez, L.I.; Gundel, P.E.; Ghersa, C.M.; Omacini, M. Family issues: Fungal endophyte protects host grass from the closely related pathogen Claviceps purpurea. Fungal Ecol. 2013, 6, 379–386. [Google Scholar] [CrossRef]
- Vignale, M.V.; Astiz-Gassó, M.M.; Novas, M.V.; Iannone, L.J. Epichloid endophytes confer resistance to the smut Ustilago bullata in the wild grass Bromus auleticus (Trin.). Biol. Control 2013, 67, 1–7. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, X.-X.; Christensen, M.J.; Nan, Z.-B.; Li, C.-J. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.-N.; Zhang, X.-X.; Feng, Y.; Christensen, M.J.; Nan, Z.-B. An Epichloë endophyte improves photosynthetic ability and dry matter production of its host Achnatherum inebrians infected by Blumeria graminis under various soil water conditions. Fungal Ecol. 2016, 22, 26–34. [Google Scholar] [CrossRef]
- Green, K.A.; Berry, D.; Feussner, K.; Eaton, C.J.; Ram, A.; Mesarich, C.H.; Solomon, P.; Feussner, I.; Scott, B. Lolium perenne apoplast metabolomics for identification of novel metabolites produced by the symbiotic fungus Epichloë festucae. New Phytol. 2020, 227, 559–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, L.I.; Gundel, P.E.; Zabalgogeazcoa, I.; Omacini, M. An ecological framework for understanding the roles of Epichloë endophytes on plant defenses against fungal diseases. Fungal Biol. Rev. 2020, 34, 115–125. [Google Scholar] [CrossRef]
- Ambrose, K.V.; Tian, Z.; Wang, Y.; Smith, J.; Zylstra, G.; Huang, B.; Belanger, F.C. Functional characterization of salicylate hydroxylase from the fungal endophyte Epichloë festucae. Sci. Rep. 2015, 5, 10939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, P.; Eaton, C.J.; Wargent, J.J.; Fechtner, S.; Solomon, P.; Schmid, J.; Day, R.C.; Scott, B.; Cox, M.P. Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytol. 2015, 208, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Merlos, M.; López-Ráez, J.A.; Martínez-Medina, A.; Ferrol, N.; Azcón, C.; Bonfante, P.; Flors, V.; Pozo, M.J. Defense related phytohormones regulation in arbuscular mycorrhizal symbioses depends on the partner genotypes. J. Chem. Ecol. 2014, 40, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Cosme, M.; Stringlis, I.A.; Yu, K.; de Jonge, R.; van Wees, S.C.M.; Pozo, M.J.; Pieterse, C.M.J.; van der Heijden, M.G.A. Molecular dialogue between arbuscular mycorrhizal fungi and the nonhost plant Arabidopsis thaliana switches from initial detection to antagonism. New Phytol. 2019, 223, 867–881. [Google Scholar] [CrossRef] [Green Version]
- Audenaert, K.; Pattery, T.; Cornelis, P.; Höfte, M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant-Microbe Interact. 2002, 15, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Tjamos, S.E.; Flemetakis, E.; Paplomatas, E.J.; Katinakis, P. Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. Mol. Plant-Microbe Interact. 2005, 18, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.C. Important Poisonous Plants of China Grassland; China Agricultural Press: Beijing, China, 1997. (In Chinese) [Google Scholar]
- Li, C.-J.; Gao, J.-H.; Ma, B. Seven diseases of drunken horse grass (Achnatherum inebrians) in China. Pratacultural Sci. 2003, 51–53. [Google Scholar]
- Perfect, S.E.; Green, J.R. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol. Plant Pathol. 2001, 2, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.J.; Voisey, C.R. The biology of the endophyte/grass partnership. In 6th International Symposium on Fungal Endophytes of Grasses Grasslands and Practice Series; Popay, A.J., Thom, E.R., Eds.; New Zealand Grassland Association: Christchurch, New Zealand, 2007. [Google Scholar]
- Bacon, C.; White, J. Stains, media and procedures for analyzing endophytes. In Biotechnology of Endophytic Fungi of Grasses; Bacon, C.W., White, J., Eds.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Clark, E.M.; White, J.F.; Patterson, R.M. Improved histochemical techniques for the detection of Acremonium coenophialum in tall fescue and methods of in vitro culture of the fungus. J. Microbiol. Methods 1983, 1, 149–155. [Google Scholar] [CrossRef]
- Grossmann, K.; Rosenthal, C.; Kwiatkowski, J. Increases in jasmonic acid caused by indole-3-acetic acid and auxin herbicides in cleavers (Galium aparine). J. Plant Physiol. 2004, 161, 809–814. [Google Scholar] [CrossRef]
- Liu, J.-P.; Lan, X.; Lv, S.; Bao, R.; Yuan, Y.; Wu, S.-Q.; Quan, X.-L. Salicylic acid involved in chilling-induced accumulation of calycosin-7-O-β-d-glucoside in Astragalus membranaceus adventitious roots. Acta Physiol. Plant. 2019, 41, 120. [Google Scholar] [CrossRef]
- Huang, W.; Lamb, D.W.; Niu, Z.; Zhang, Y.-J.; Liu, L.-Y.; Wang, J.-H. Identification of yellow rust in wheat using in-situ spectral reflectance measurements and airborne hyperspectral imaging. Precis. Agric. 2007, 8, 187–197. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Martel, C.; Zhurov, V.; Navarro, M.; Martinez, M.; Cazaux, M.; Auger, P.; Migeon, A.; Santamaria, M.E.; Wybouw, N.; Diaz, I.; et al. Tomato whole genome transcriptional response to Tetranychus urticae identifies divergence of spider mite-induced responses between tomato and Arabidopsis. Mol. Plant-Microbe Interact. 2015, 28, 343–361. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bouwmeester, H.J.; Kappers, I.F. Combined transcriptome and metabolome analysis identifies defence responses in spider mite-infested pepper (Capsicum annuum). J. Exp. Bot. 2020, 71, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.-T.; Ding, Y.-L. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smit, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Sciences: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, N.; Pastor, V.; Pastor-Fernández, J.; Flors, V.; Pozo, M.J.; Sánchez-Bel, P. Role and mechanisms of callose priming in mycorrhiza-induced resistance. J. Exp. Bot. 2020, 71, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, C.; Bucheli, C.S.; Skene, K.G.M.; Robinson, S.P.; Scott, N.S. Chitinase and β-1,3-glucanase in grapevine leaves: A possible defence against powdery mildew infection. Aust. J. Grape Wine Res. 1998, 4, 14–22. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of Phytohormone Pathways by Pathogen Effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [Green Version]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef]
- Thaler, J.S.; Owen, B.; Higgins, V.J. The Role of the Jasmonate Response in Plant Susceptibility to Diverse Pathogens with a Range of Lifestyles. Plant. Physiol. 2004, 135, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemarié, S.; Robert-Seilaniantz, A.; Lariagon, C.; Lemoine, J.; Marnet, N.; Jubault, M.; Manzanares-Dauleux, M.J.; Gravot, A. Both the jasmonic acid and the salicylic acid pathways contribute to resistance to the biotrophic clubroot agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 2015, 56, 2158–2168. [Google Scholar] [PubMed] [Green Version]
- Pagán, I.; García-Arenal, F. Tolerance to plant pathogens: Theory and experimental evidence. Int. J. Mol. Sci. 2018, 19, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastías, D.A.; Gianoli, E.; Gundel, P.E. Fungal endophytes can eliminate the plant growth—defence trade-off. New Phytol. 2021, 230, 2105–2113. [Google Scholar] [CrossRef]
- Iannone, L.J.; Vignale, M.V.; Pinget, A.D.; Re, A.; Mc Cargo, P.D.; Novas, M.V. Seed-transmitted Epichloë sp. endophyte alleviates the negative effects of head smut of grasses (Ustilago bullata) on Bromus auleticus. Fungal Ecol. 2017, 29, 45–51. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Gao, Y.-B.; Card, S.D.; Ren, A.-Z. The advantages of endophyte-infected over uninfected tall fescue in the growth and pathogen resistance are counteracted by elevated CO2. Sci. Rep. 2017, 7, 695. [Google Scholar] [CrossRef]
| Response Variable | Treatment | df | F or t-Value | p-Value | E− | E+ | E− | E+ |
|---|---|---|---|---|---|---|---|---|
| P− | P+ | |||||||
| Salicylic acid (ng g−1 DW) (n = 3–4) | Symbiosis | 1,10 | 53.52 | <0.001 | - | - | - | - |
| Pathogen | 1,10 | 21.78 | <0.001 | |||||
| Symbiosis × Pathogen | 1,10 | 6.49 | 0.028 | |||||
| Jasmonic acid (ng g−1 DW) (n = 3–4) | Symbiosis | 1,10 | 172.56 | <0.001 | - | - | - | - |
| Pathogen | 1,10 | 2.28 | 0.161 | |||||
| Symbiosis × Pathogen | 1,10 | 12.77 | 0.005 | |||||
| Leaf fresh weight (g) (n = 9) | Symbiosis | 1,32 | 110.45 | <0.001 | - | - | - | - |
| Pathogen | 1,32 | 41.78 | <0.001 | |||||
| Symbiosis × Pathogen | 1,32 | 15.10 | <0.001 | |||||
| Leaf dry weight (g) (n = 9) | Symbiosis | 1,32 | 192.53 | <0.001 | - | - | - | - |
| Pathogen | 1,32 | 36.53 | <0.001 | |||||
| Symbiosis × Pathogen | 1,32 | 0.59 | 0.449 | |||||
| Plant height (# plant −1) (n = 9) | Symbiosis | 1,32 | 1.69 | 0.100 | 39.11 ± 0.28a | 39.81 ± 0.23a | 37.38 ± 0.23b | 37.97 ± 0.22b |
| Pathogen | 1,32 | 5.27 | <0.001 | |||||
| Symbiosis × Pathogen | 1,32 | 0.23 | 0.822 | |||||
| Tillers number (# plant −1) (n = 9) | Symbiosis | 1,32 | 1.09 | 0.304 | 10.33 ± 0.33a | 10.88 ± 0.26a | 09.77 ± 0.36b | 09.88 ± 0.30b |
| Pathogen | 1,32 | 5.93 | 0.020 | |||||
| Symbiosis × Pathogen | 1,32 | 0.48 | 0.491 | |||||
| Response Variable | Treatments | df | F | p-Value |
|---|---|---|---|---|
| Disease incidence on plant leaves (%) | Symbiosis | 1,16 | 48.85 | <0.001 |
| Time | 1,32 | 80.48 | <0.001 | |
| Symbiosis ×Time | 1,32 | 0.01 | 0.985 | |
| Disease severity on plant leaves (%) | Symbiosis | 1,16 | 59.25 | <0.001 |
| Time | 1,32 | 144.14 | <0.001 | |
| Symbiosis ×Time | 1,32 | 0.148 | 0.862 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, M.-Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.-B.; Zhang, X.-X. The Plant Salicylic Acid Signalling Pathway Regulates the Infection of a Biotrophic Pathogen in Grasses Associated with an Epichloë Endophyte. J. Fungi 2021, 7, 633. https://doi.org/10.3390/jof7080633
Kou M-Z, Bastías DA, Christensen MJ, Zhong R, Nan Z-B, Zhang X-X. The Plant Salicylic Acid Signalling Pathway Regulates the Infection of a Biotrophic Pathogen in Grasses Associated with an Epichloë Endophyte. Journal of Fungi. 2021; 7(8):633. https://doi.org/10.3390/jof7080633
Chicago/Turabian StyleKou, Ming-Zhu, Daniel A. Bastías, Michael J. Christensen, Rui Zhong, Zhi-Biao Nan, and Xing-Xu Zhang. 2021. "The Plant Salicylic Acid Signalling Pathway Regulates the Infection of a Biotrophic Pathogen in Grasses Associated with an Epichloë Endophyte" Journal of Fungi 7, no. 8: 633. https://doi.org/10.3390/jof7080633
APA StyleKou, M.-Z., Bastías, D. A., Christensen, M. J., Zhong, R., Nan, Z.-B., & Zhang, X.-X. (2021). The Plant Salicylic Acid Signalling Pathway Regulates the Infection of a Biotrophic Pathogen in Grasses Associated with an Epichloë Endophyte. Journal of Fungi, 7(8), 633. https://doi.org/10.3390/jof7080633









