1. Introduction
The world’s population is rapidly growing, with projected global population reaching 9.2 billion people by 2050. This increase, coupled with a rising standard of living, will necessitate an increase of 70% in food production in the next three decades [
1,
2]. Although the green revolution greatly enhanced food production efficiency, pre-harvest pests still reduce crop yield by a mean of 35% becoming the main hurdle in increasing food production per unit of land. These compounding factors make the time-old problem of pest management, perhaps, more meaningful than ever [
1]. Biological control agents are considered at a disadvantage compared to chemical insecticides in terms of replicability, efficiency and speed of kill [
3,
4,
5]. However, an insects’ ability to develop resistance against insecticide [
6], along with concerns of environmental impact and public opinion, push research to abridge known disadvantages of microbial control agents. This improvement can be achieved by scanning for more resilient and pathogenic strains, or by improving the formulations used to apply microbial control agents [
7,
8,
9,
10].
Metarhizium is a genus of soil-borne, anamorphic ascomycete fungi (order: Hypocreales, family: Clavicipitaceae). It may grow endophytically in plant roots, saprophytically on dead matter, or as a pathogen of arthropods. Most entomopathogenic fungi (EPF) do not need to be ingested by the host in order to infect and cause mortality. Instead, conidia attach to the insect integument via non-specific hydrophobic interactions. These interactions are mediated by a rodlet layer on the conidium’s surface, containing hyper-hydrophobic proteins [
11,
12,
13]. These weak bonds are later replaced with more specific protein-mediated interaction [
14]. After active attachment, germination on the host integument occurs, which requires high relative humidity (RH) conditions [
15]. The forming (or lack of forming) of an appressorium is induced via a set of chemical and tactile cues: (A) physical contact; (B) deficiency in nutrients [
16,
17] and (C) epicuticular lipids and cuticular composition [
13,
18]. Once the fungal hyphae penetrated through the cuticle,
Metarhizium colonize the host’s hemocoel. The fungus may employ various secondary metabolites to overcome the host’s response. After host death,
Metarhizium switches to saprophytic growth. Under high RH (over 95%), hyphae will break through the cuticle and form aerial conidia. Alternatively, under low RH, the mycelium might sporulate internally, creating chlamydospores [
19,
20,
21].
EPF are vulnerable to environmental factors such as ultraviolet (UV) radiation and desiccation. Understanding the influence and damage caused by various abiotic stresses, such as desiccation, can help devise novel formulations and application methods to avoid or remedy these detriments [
8,
9]. Fungi require high water activity (aw) for growth and proliferation [
22]. While the calculated threshold for development at 95.5% RH or aw 0.94 for some strains (24), aw of 0.93 seriously delayed and harmed conidial germination, but did not completely halt it for other strains [
15], showing high dependence on high humidity, with strain-specific plasticity. A minimal time of high humidity is required for germination and penetration in order for the infection to be successful [
23]. Hence, subjecting inoculated insects to low RH after this critical period does not negatively affect infection rates. These humidity requirements for the onset of infection, traditionally restricted EPF application to humid or protected environments [
3].
Due to the aforementioned susceptibility to low RH and other environmental factors, formulations are continuously developed and tested to increase shelf-life, improve dispersion during application, improve adhesion to insect cuticle, increase mortality and efficacy and minimize the deleterious effects of sub-optimal conditions [
8,
9,
10]. The hydrophobic nature of Hypocrealean conidia necessitates the use of surfactants [
8,
10,
11] or vegetable- or mineral-derived oils [
8,
11,
24]. Vegetable- or mineral-derived oils are also used as carriers and may disperse conidia far better than water with a surfactant, reducing clumping and improving coverage in the plant after spray application. The low wetting angle between oil and lypophilic surfaces allows for better spread on leaf surface, further improving dispersion, and facilitates in cuticle adhesion [
7,
8,
24]. Furthermore, oils may confer protection of conidia against UV damage [
25], heat-stress [
26,
27], and low RH. Oils were found to retain insecticidal ability of the conidia even at low RH, where aqueous formulations failed to cause mortality [
8,
20,
24,
28], although the mechanisms for both UV and desiccation tolerance are not yet fully understood. Lastly, oils are known insecticides, conferring direct damage to insects, and were found to increase fungus-related mortality, regardless of RH [
20,
29,
30,
31,
32]. In this study we implemented a novel type of formulation for biopesticides based on Pickering emulsion. Pickering emulsion uses nanoparticles as emulsifiers, rather than surfactants. The nanoparticles localize to the oil–water interphase, and may include various inorganic materials, polymers and proteins [
33]. Pickering emulsions are considered superior to conventional emulsions in terms of droplet size control, control of rate of release of encapsulated materials, and stability over time and varying environmental conditions.
In previous trials,
M. brunneum conidia were individually encapsulated in the oil phase of an oil-in-water Pickering emulsion, stabilized by silica (SiO
2) particles. The conidia was applied against third instar
Spodoptera littoralis larvae, and showed elevated conidia-related mortality, while the emulsion itself showed no observable effects on the larvae [
34]. The objective of this study was to identify possible mechanisms which enabled the enhanced pathogenicity of
M. brunneum conidia formulated in a Pickering emulsion.
2. Materials and Methods
2.1. Insect Rearing and Fungal Culturing
Spodoptera littoralis B. (Cotton leafworm) was chosen as a model foliar pest, as it is a polyphagous lepidopteran foliar pest, affecting many crops in the middle-east. S. littoralis insect colony was reared in growth room with 26 ± 2 °C and a photoperiod of 12:12 (light:dark). To synchronize the larvae’s developmental stage, all larvae used were first instar freshly hatched and unfed neonates.
The fungus used in this study was
Metarhizium brunneum isolate 7 (Mb7) constitutively expressing GFP reporter gene [
18]. Fungal conidia were harvested from a grain-based growing technique [
35], as follows: to produce initial inoculum, aseptic conidia from fungal colonies grown on SDA (Difco, France) were harvested. The conidia were suspended in a 0.01% (
v/
v) of Triton-X100 (Merck, Germany) solution and adjusted to a concentration of 106 conidia/mL. One ml of conidia suspension was used to inoculate 50 mL of SDB (Difco, France), incubated at 28 °C with agitation (155 RPM) for 3 days, until micro mycelia were visible in the clear fluid (‘fermentation inoculum’). Organic rice was soaked in water for 45 min and dried for 2 h. The soaked rice was divided into roughly 0.5 kg batches and autoclaved. Each batch was inoculated with 35 mL of liquid fermentation inoculum and incubated at 28 °C for 48 h, for the formation of initial mycelium. Bags were then moved to fermentation at room temperature, until the rice grain were completely covered with conidia.
When the rice-grown mycelia were fully sporulated, the bags were opened in a laminar flow hood to allow a complete drying of the conidia. Conidia were separated from the rice using a set of 2000 and a 600 µm sieves. Harvested conidia were kept in an aseptic 50 mL disposable tube at 4 °C. Before each experiment conidial viability was assessed by overnight germination assay on SDA plates. Conidia were used at >90% germination.
2.2. Formulations
Pickering emulsion was prepared by the Functional Polymers and Nanomaterials laboratory at the agriculture research center at Beit Dagan, as described by Yaakov et al. [
34]. In short, the emulsion was prepared by sonicating water and paraffinic petroleum-derived oil at a ratio of 8:2, respectively, with 2% (
w/
v) (3-Aminopropyl)triethoxysilane (APTES) silanised SiO
2 nanoparticles, to allow emulsification. As control we have used distilled water (DW) with 0.01% (
V/
V) Triton-X100 (from now on will be referred to as aqueous solution). Triton-X100 was used to lower the water’s surface tension to enable incorporation of the hydrophobic conidia into the aqueous solution. Conidial concentration in both formulations was set to 1 mg of conidia per ml of formulation, the concentration was quantified, using an improved Neubauer chamber (Brand, Germany), and averaged at 4.86 × 10
7 (SE ± 3.2 × 10
6) conidia per ml.
2.3. Mortality Bioassay of M. Brunneum Infecting Spodoptera Littoralis Larvae
Ricinus communis (Castor bean) leaves were collected at the onset of every experiment, and surface sterilized by dipping the leaf for 15 s in a 0.4% (V/V) bleach solution, followed by two DW washes for 5 s each. Leaves were then sprayed, using a conical nozzle hand sprayer, with 3 mL of the treatment solution per leaf. Treatments included: (1) Control aqueous solution; (2) Aqueous solution with conidia; (3) Control emulsion; (4) Emulsion with conidia.
Treated leaves were dried completely, cut into squares and embedded in 55 mm Petri plates in 2% agarose. Each plate was populated with 6 freshly hatched, 1st instar larvae. Two RH conditions were conducted for each treatment: (1) plates were sealed with perforated caps and were exposed to the growth chamber’s humidity (from now on referred to as low RH) (
Figure 1A) or (2) unperforated caps ~100% RH (from now on referred to as high RH) (
Figure 1B). Plates were wrapped with Parafilm (Bemis, Shirley, MA, USA). Plates were then incubated in a controlled growth chamber (Percival-scientific, Perry, IA, USA) at 26 °C and 12:12 photoperiod (light:dark), RH and temperature were recorded throughout the experiment using the LOG32TH thermo-hygro data logger (Dostmann electronic, Wertheim, Germany). Chamber’s RH ranged between 62.1% and 67.4% and averaged at 63.4% for all assays.
Mortality was examined daily over a period of five days; a larva was presumed dead only if touching it with a fine brush did not cause any response. Dead larvae were placed on filter paper (Whatman, Maidstone, UK) impregnated with 500 µL DW and incubated at 28 °C for three days, then examined for mycosis. Mortality assays were repeated three times. Two of the consequent repeats included 10 plates per treatment, while the third repeat included eight plates for a total of 28 plates containing roughly, 170 larvae per treatment.
2.4. Conidia Dispersal Assay
Both adaxial and abaxial sides of
Ricinus communis leaves were sprayed with 3 mL of either aqueous solution or emulsion containing Mb7-GFP conidia. The formulated conidia were allowed to dry completely. Nine mm discs were cut from the leaves and placed on a glass slide, adaxial side facing up. The leaf discs were lightly wetted with water and covered with a cover slip. Each disc was photographed using an Olympus IX81 inverted confocal microscope (Olympus, Shinjuku, Japan) at three random locations, at 20× magnification. Number of Conidia in each field was assessed using the ImageJ software (NIH, Stapleton, USA). The experiment was repeated, with freshly made solutions, three times, two repeats included three discs from each treatment, while one repeat included five discs of each treatment, with a total of 11 leaf discs per treatment. To quantify the geographic dispersion pattern of conidia on the leaf surface the following equation was used:
, where S
2 is the variance and
is the mean based on the conidia count of three fields. VMR (variance-to-mean ratio) is an estimate for geographic dispersion pattern of organisms in ecology, by its deviance from the value of 1, the VMR of a Poisson distribution [
36,
37]. When VMR = 1, individuals are randomly dispersed in a two-dimensional space. If VMR is significantly lower than 1, individuals are regularly spaced geographically and if VMR significantly exceeds 1, individuals are clustered.
2.5. Conidia Adhesion Assay
Ricinus communis leaves were sprayed with Mb7-GFP conidia in either aqueous solution or emulsion. The leaves were processed and populated with larvae as described in
Section 2.3. Larvae were left to settle and roam the leaves for approximately 24 h at 26 °C and low RH. Larvae were then collected from the leaves and placed in a 90 mm petri plate. Larvae were anesthetized by placing the larvae-containing plates on ice. For assessing conidial acquisition, larvae were placed on a microscopic slide with a drop of water and flattened using a cover slide. Larvae were then photographed using an inverted confocal microscope at 20× magnification and conidia were counted from the images using the ImageJ software. For assessing active conidia adhesion, unbound conidia were detached based on the protocol of Ment et al. [
38] as follows: collected larvae were washed in 1 mL of tap water for one minute on a MiniMixer tabletop shaker (Benchmark, Lodi, CA, USA) and processed as above. Passive acquisition experiments included four larvae per treatment for three repeats, and five larvae per treatment for the fourth repeat for a total of 17 larvae per treatment. Active adhesion experiments included eight larvae per treatment in three repeats for a total of 24 larvae per treatment.
2.6. Disease Progression via Direct Observation
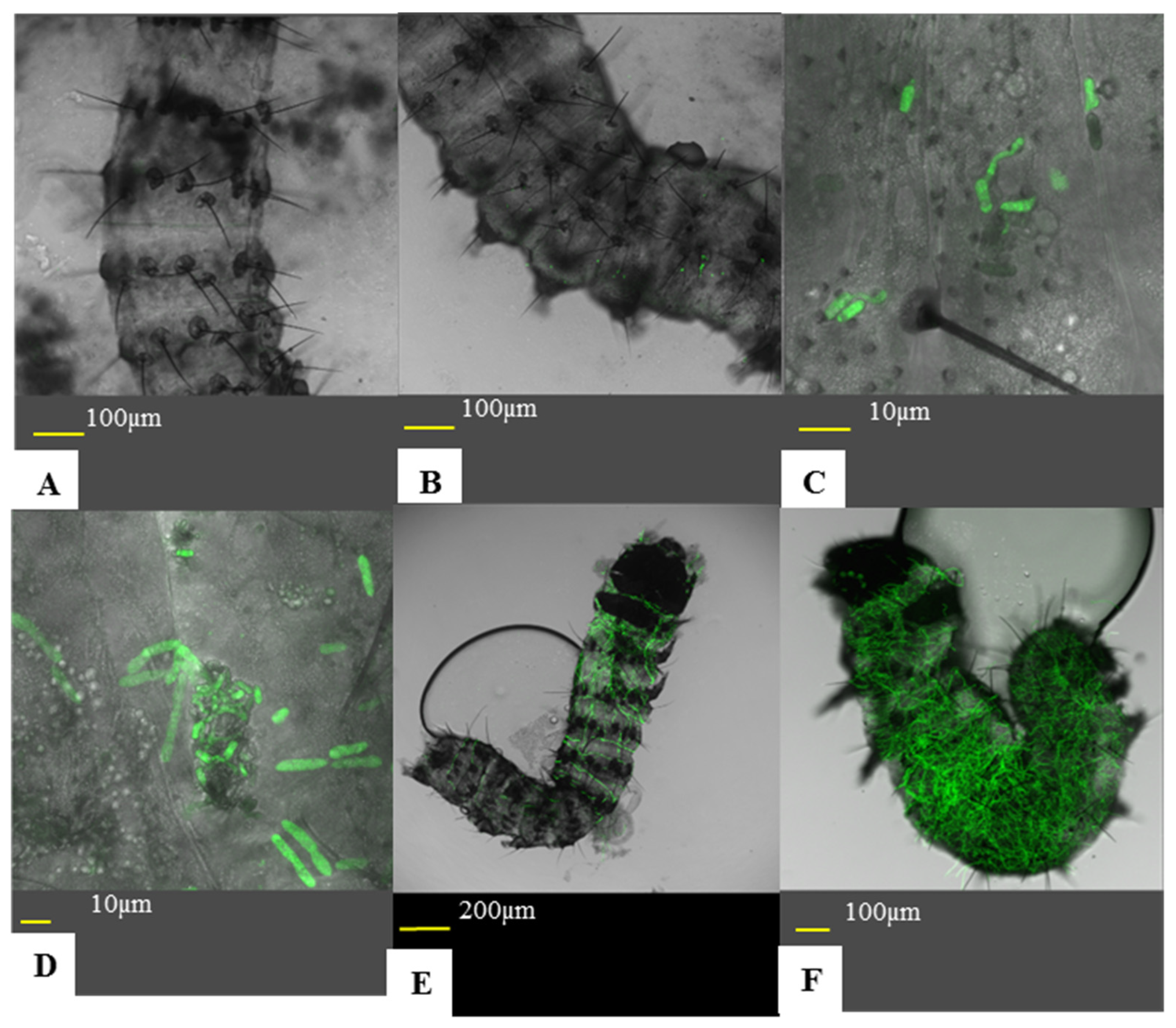
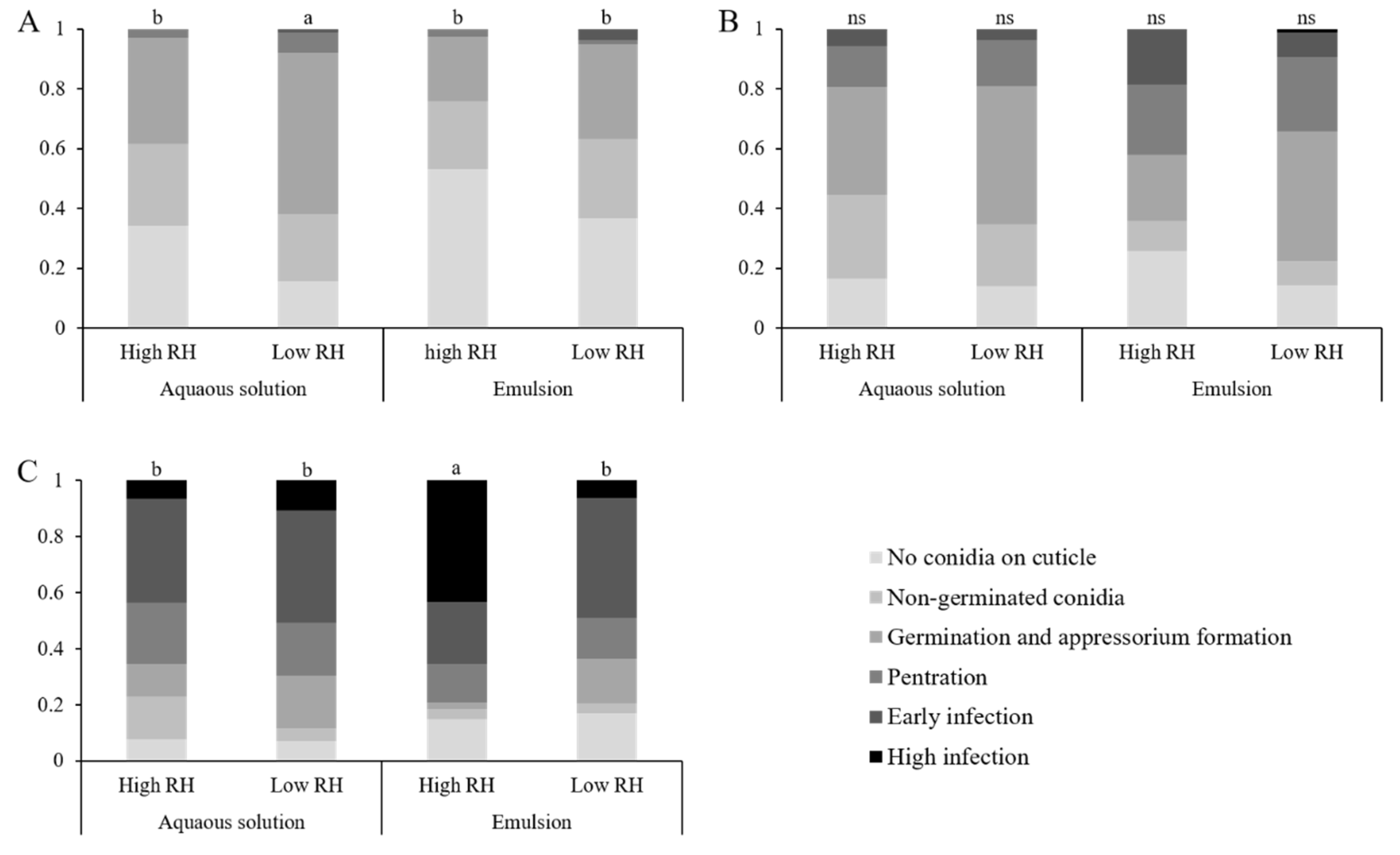
Larvae were introduced to treated leaves, as described in
Section 2.3. Five plates were sacrificed each day over a three-day period. Larvae were fixed to a microscopic slide using a double-sided clear cellulose tape. Larvae were examined using an inverted confocal microscope. Infection was divided in to six statuses (Figure 7): (A) no conidia adhered to the insect cuticle, (B) non-germinated conidia on cuticle, (C) conidial germination and appressoria formation, (D) cuticle penetration, (E) early infection of the hemocoel, (F) high infection of the hemocoel. As this experiment was qualitative, a larva’s infection status was determined by the most advanced fungus found on the larva. The experiment was repeated three times. This experiment was also used to assess sub-lethal effects. For sample sizes for each treatment and time point, see
Table 1.
2.7. Sub-Lethal Effects on Larvae
2.7.1. Larval Growth Rate
After observing and recording disease progression, glued larvae were taken and photographed using a Nikon SMZ645 stereomicroscope (Nikon, Tokyo, Japan) and a Dinoeye edge eyepiece camera (AnMo Electronics, Taipei, Taiwan) to assess larval length. Millimeter paper (Hadar, Jaffa, Israel) was added for scale. Dead larvae were omitted as their time of death was unknown and, therefore, could not represent growth over the given time period. Furthermore, the dead larvae, while being of roughly the same size regardless of treatment or day post-inoculation (DPI), greatly affected variance and significance between treatments. The experiment was repeated three times, alongside the disease progress experiments. For the total sample size for larval length measurement, see
Table 1.
2.7.2. Effects on Larval Feeding
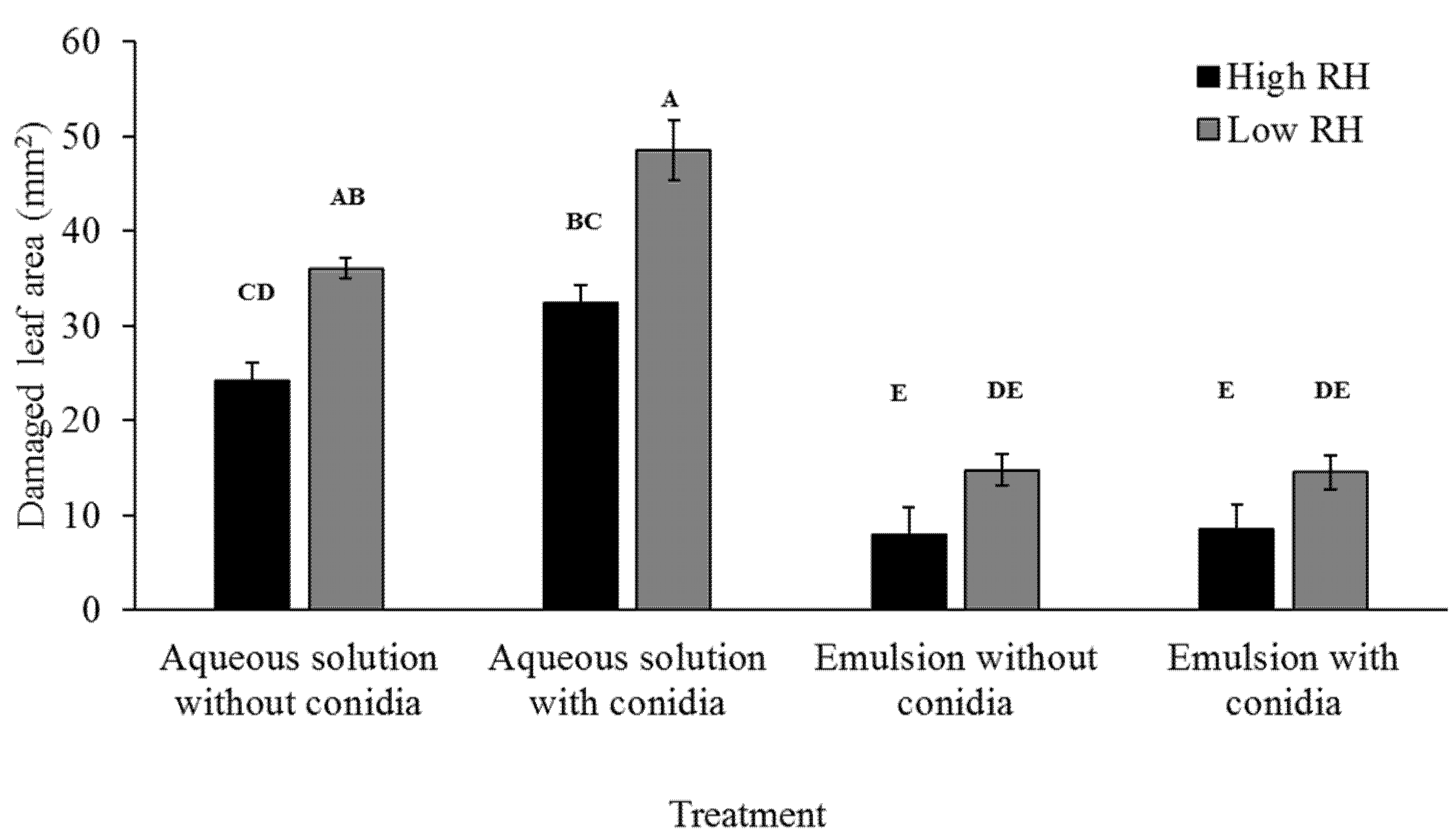
To assess feeding ability by larvae, damaged leaf area was measured. Plates were photographed after larvae were sacrificed with millimeter paper as background for scale (
Figure 2). Damaged leaf area was manually assessed using the ImageJ software. Damaged leaf area was recorded in three repeats. Each repeat included five plates, for a total of 15 plates per treatment.
2.8. Statistical Analysis
All statistical analyses and calculations were performed using the JMP® software, version Pro-15 (SAS institute Inc., Cary, NC, USA). Graphs were formulated using the Excel spreadsheet software (Microsoft, Redmond, WA, USA). The decision rule was fixed for all tests as α = 0.05. All experiments were repeated at least three times. Repeats were counted as random blocks and were inserted in all analyses as random variables and were analyzed using the restricted maximum likelihood (REML) method.
2.8.1. Parametric Analyses
To analyze fifth day mortality (
Section 2.4), larval length and larval feeding area (
Section 2.8) a three-way analysis of variance (ANOVA) in a mixed model was employed, with least squares analysis for RH, presence of conidia and formulation as fixed variables in a full factorial model. Multiple comparisons were conducted with Tukey’s honestly significant difference (HSD) method on the triple interaction. VMR and conidia count in dispersal assays (
Section 2.5) as well as numbers of acquired and adhered conidia (
Section 2.6) were analyzed using a one-way ANOVA in a mixed model, with formulation as a fixed variable and repeats as random variables. Equality of variances was tested for VMR and conidia count in dispersal assays, using Bartlett’s test. As variances were not equal, a Welch’s ANOVA was employed
2.8.2. Transformations and Data Normalization
The number of conidia per microscopic field, observed in the dispersal assay, were log10 transformed. The number of conidia acquired or adhered to larvae were square root transformed. Numbers of dead larvae in the fifth day mortality were weighted against sample size at time zero, to account for setup variability, through variance scaling. For all other analyses, studentized residues were calculated and observations deviating from the mean by more than two standard deviations (SDs) were omitted. In the larval length analysis, dead larvae were omitted, as they did not represent larval growth and largely increased variance.
2.8.3. Non-Parametric Analyses
Differences in mycosis rates were examined using a Fisher’s exact test, with formulation as an explaining factor. The results for each RH were analyzed separately. Disease progression was analyzed using a Wilcoxon rank-sum test, with RH, formulation, RH*formulation combination, and the date of repeat as independent variables. Multiple comparisons were performed by an each-pair Wilcoxon test. A Holm–Bonferroni correction was employed to counter family-wise error rate (FWER).
2.8.4. Synergism via the Bliss Model
Synergism was tested according to Bliss’s model for independent action of two chemical pesticides in a mixture [
39]. According to Bliss’s model, in case there is no interaction between two toxins in an insecticide, insects will die of unknown reasons (natural mortality), or of active ingredient A, or of active ingredient B. This was mathematically formulated as follows: P = p
0 + (1 − p
0) × p
1 + (1 − p
0) × (1 − p
1) × p
2. Where P signifies the expected probability of mortality from the given mixture, p
0 signifies probability of natural mortality, p
1 signifies the probability of mortality from ingredient A, and p
2 signifies the probability of mortality from ingredient B. P was calculated independently for high and low RH. p
0 was the mean percentage of mortality in plates containing control aqueous solution-treated leaves. p
1 was the mean percentage of mortality in plates containing aqueous solution with conidia-treated leaves. p
2 was the mean percentage of mortality in plates containing control emulsion-treated leaves. Mean mortality of the mixture was calculated according to the mortality percentage of individual plates in the emulsion with conidia group, with a 95% confidence interval. If the expected P was lower than the lower confidence interval of the mortality from emulsion with conidia, the mixture was considered synergistic.