Japanese Traditional Miso and Koji Making
Abstract
:1. Introduction
2. Miso Varieties and Its Culinary Scene
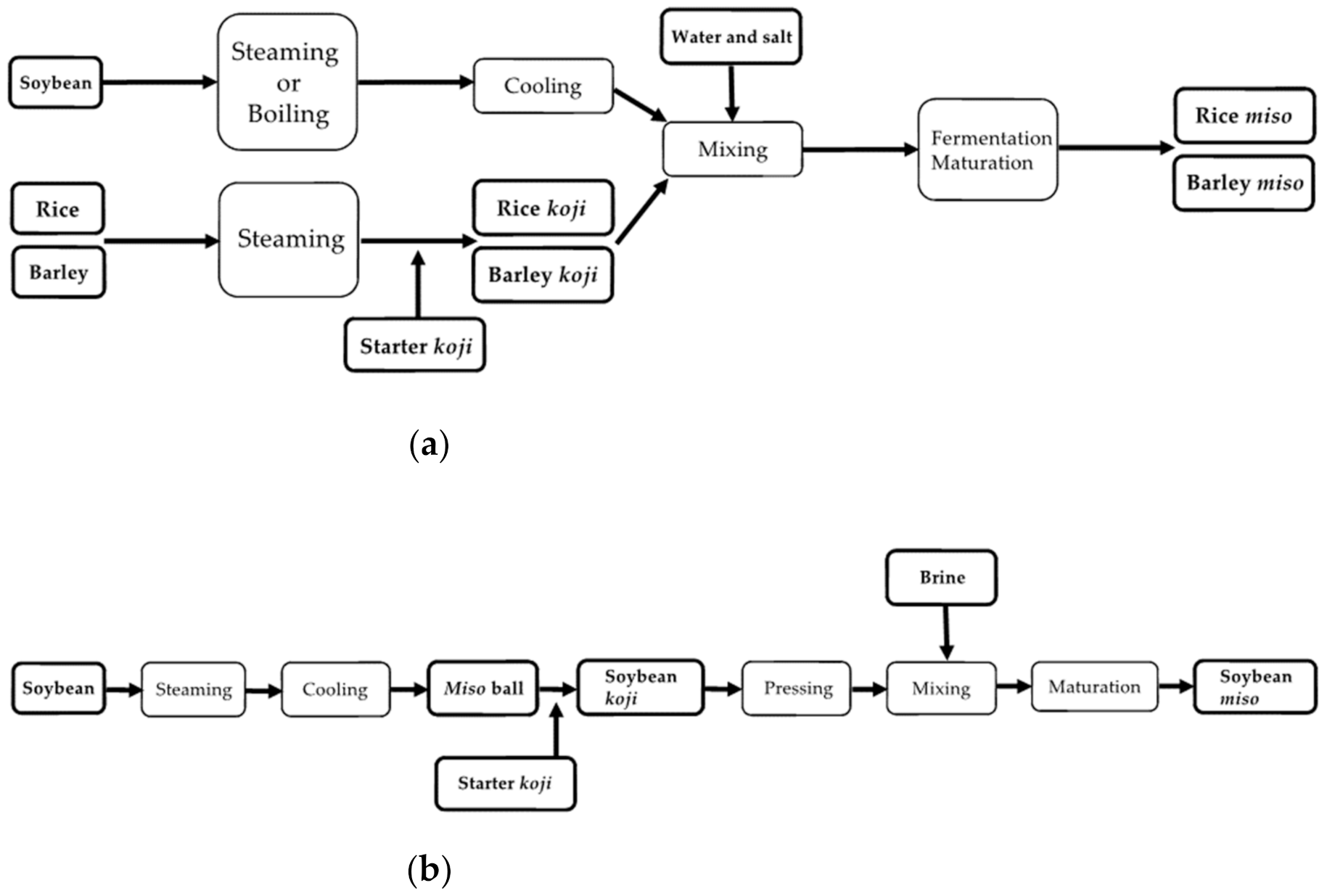
3. Process of Miso Making
3.1. Rice Miso
- (1)
- Ensure uniform fermentation and maturation across all parts of the tank;
- (2)
- Provide aeration to facilitate the yeast’s aerobic fermentation and growth (yeast does not grow under anaerobic conditions, although it generates alcohol that affects the flavor);
- (3)
- Release fermentation heat.
3.2. Barley Miso
3.3. Soybean Miso
4. Filamentous Fungi, Koji Mold, in Japanese Fermented Soybean Paste
4.1. Brewer’s Koji Mold
4.1.1. Koji Mold for Sake, Miso and Soy Sauce
4.1.2. Starter Koji for Miso Making
- (1)
- A broader spectrum of protease activities;
- (2)
- About 8% higher amylase activities and 20% higher protease activities compared to brewer’s strains;
- (3)
- Correlations among amylase activities and among protease activities but not between amylases and proteases [11].
4.1.3. Safety of Koji Mold
4.2. Koji Making and Enzyme Production
4.2.1. Amount of Starter Koji
4.2.2. Blending Different Koji Starters
4.2.3. Effects of Additives
4.2.4. Conditions for Koji Making
- Temperature Transition
- Ambient Humidity
- Duration of Koji Making
4.3. Enzymes in Koji
5. Koji Making (Seikiku) [20]
5.1. Rice Koji
5.1.1. Roles of Koji Making
- Growth and elaboration of fungal hyphae around and into the ingredients by solid-state culture;
- production of amylases, (neutral) proteases and other enzymes important for miso fermentation and maturation (hypha extension into the ingredients (hazekomi) enhances this process);
- growth of salt-tolerant yeast and lactic acid bacteria that are essential for maturation and production of precursors of aromatic components in miso (the growth of koji mold facilitates this process); and
- elimination of ingredient odors.
5.1.2. Growth Conditions of Koji Mold in Koji Making Process
5.1.3. Optimal Koji Making Conditions for Enzyme Production
5.1.4. Koji Making Methods
5.1.5. Quality of Koji
- Contains enzymatic activities required for the type of miso to be manufactured;
- Sufficient depth of hazekomi with minimal coloration and brilliant color;
- Aromatic without foul odor from bacterial contamination;
- Fluffy and soft texture;
- Minimal sporulation and coloration with high amylase activities for white or yellow miso through shorter culture time; and
- High protease activities through slightly longer culture for red miso.
5.2. Barley Koji
5.3. Soybean Koji
6. Koji Enzymes Involved in Miso Making
6.1. Acidic Endopeptidases
6.2. Neutral Endopeptidases
6.3. Alkaline Endopeptidases
6.4. Exopeptidases
6.5. Pro-Xaa Peptidases
6.6. Glutaminase
7. Z. rouxii and T. halophilus in Miso Making
8. Changes in Miso Components during Fermentation and Maturation
8.1. Macronutrients
8.2. Organic Acids
8.3. Color and Aromatic Compounds
9. Nutritional Function of Miso
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamamoto, Y.; Tanaka, H. An Introductory Guide to Miso and Shoyu, 5th ed.; The Japan Food Journal Co., Ltd.: Tokyo, Japan, 2013; pp. 3–6. (In Japanese) [Google Scholar]
- Kusumoto, K.; Rai, A.K. Miso, the traditional fermented soybean paste of Japan. In Fermented Foods, Part II: Technological Interventions; Ray, R.C., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 122–134. [Google Scholar]
- Shurtleff, W.; Aoyagi, A. The Book of Miso, 2nd ed.; Soyinfo Center: Lafayette, CA, USA, 2018; pp. 30–44. [Google Scholar]
- Food Labeling Standards of Japan [in Japanese]. Available online: https://www.caa.go.jp/policies/policy/food_labeling/food_labeling_act/pdf/food_labeling_cms101_201009_4.pdf (accessed on 7 May 2021).
- Announcement by the Japanese Ministry of Agriculture, Forestry and Fisheries. Washoku, the Traditional Dietary Culture of the Japanese, Has Been Registered on UNESCO’s Intangible Cultural Heritage List [in Japanese]. Available online: https://www.maff.go.jp/j/keikaku/syokubunka/ich/ (accessed on 24 June 2021).
- Miso/Soy Sauce. JETRO. 2020. Available online: https://www.jetro.go.jp/en/trends/foods/ingredients/misoshoyu.html (accessed on 7 July 2021).
- Kitagawa, M. Ever-changing Miso: Past History and Future Prospects of Miso. J. Brew. Soc. Jpn. 2021, 116, 211–219. (In Japanese) [Google Scholar]
- Zenkoku Miso Gijutsukai. New Handbook of Miso Technology; Zenkoku Miso Gijutsukai: Tokyo, Japan, 2006; pp. 21–85. (In Japanese) [Google Scholar]
- Ohyama, T.; Takahashi, Y.; Joh, T.; Whitaker, A.C.; Nishiwaki, T.; Morobashi, K.; Watanabe, S.; Shimojo, S. Traditional and Modern Japanese Soy Foods; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 91–104. [Google Scholar]
- Narahara, H. Koji mold and koji (part 2): Handling of koji mold in koji making [in Japanese]. J. Brew. Soc. Jpn. 1994, 89, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Narahara, H. Starter koji and koji in miso (4). Miso Sci. Tech. 1999, 47, 13–21. (In Japanese) [Google Scholar]
- Tanaka, K.; Kushiro, M.; Manabe, M. A review of studies and measures to improve the mycotoxicological safety of traditional Japanese mold-fermented foods. Mycotoxin Res. 2006, 22, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, K.; Nogata, Y.; Ohta, A. Directed deletions in the aflatoxin biosynthesis gene homolog cluster of Aspergillus oryzae. Curr. Genet. 2000, 37, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Lee, Y.-H.; Hayashi, R.; Suzuki, Y.; Yamada, O.; Sakamoto, K.; Gotoh, K.; Akita, O. Molecular analysis of an inactive aflatoxin biosynthesis gene cluster in Aspergillus oryzae RIB strains. Appl. Environ. Microbiol. 2006, 72, 484–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akao, T.; Sano, M.; Yamada, O.; Akeno, T.; Fujii, K.; Goto, K.; Ohashi-Kunihiro., S.; Takase, K.; Yasukawa-Watanabe, M.; Yamaguchi, K.; et al. Analysis of expressed sequence tags from the fungus Aspergillus oryzae cultured under different conditions. DNA Res. 2007, 14, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Narahara, H.; Iwata, M. Studies on the koji making process for rice koji: (1) factors affecting the growth of koji mold on steamed rice. Miso Sci. Tech. 1983, 31, 127–133. (In Japanese) [Google Scholar]
- Shiota, H.; Sakurai, Y. Protease production in rice koji (part 2). J. Brew. Soc. Jpn. 1963, 58, 392. (In Japanese) [Google Scholar] [CrossRef]
- Narahara, H. Water activity and growth in koji. J. Brew. Soc. Jpn. 1988, 83, 729–733. (In Japanese) [Google Scholar] [CrossRef]
- Abe, K.; Gomi, K.; Hasegawa, F.; Machida, M. Impact of Aspergillus oryzae genomics on industrial production of metabolites. Mycopathologia 2006, 162, 143–153. [Google Scholar] [CrossRef]
- Yoshii, H. Koji making. In Kaitei Jozogaku; Nojiro, K., Kozaki, M., Yoshii, H., Koizumi, T., Eds.; Kodansha Scientific: Tokyo, Japan, 1997; pp. 165–170. (In Japanese) [Google Scholar]
- Murakami, H. Aspergillus oryzae group (V). J. Brew. Soc. Jpn. 1971, 66, 1042–1045. (In Japanese) [Google Scholar] [CrossRef]
- Machida, M. Progress of Aspergillus oryzae genomics. Adv. Appl. Microbiol. 2002, 51, 81–106. [Google Scholar]
- Sugawara, E.; Hashimoto, S.; Sakurai, Y.; Kobayashi, A. Formation by yeast of the HEMF (4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone) aroma component in miso with aging. Biosci. Biotechnol. Biochem. 1994, 58, 1134–1135. [Google Scholar] [CrossRef]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.; Arima, T.; Akita, O.; Kashiwagi, Y. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, I.; Imai, S. Enzymatic activities of rice koji and the quality of miso. J. Brew. Soc. Jpn. 1977, 72, 565–569. (In Japanese) [Google Scholar]
- Gomi, K.; Arikawa, K.; Kamiya, N.; Kitamoto, K.; Kumagai, C. Cloning and nucleotide sequence of the acid protease-encoding gene (pepA) from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 1993, 57, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Ogura, K.; Hamamoto, T.; Kobayashi, Y. Molecular cloning and sequence analysis of a gene encoding an aspartic proteinase from Aspergillus oryzae. Adv. Exp. Med. Biol. 1995, 362, 577–580. [Google Scholar]
- Nunokawa, Y. Fermentation of Seishu Moromi from an enzymological point of view. J. Brew. Soc. Jpn. 1981, 76, 6–11. (In Japanese) [Google Scholar] [CrossRef]
- Yamagata, Y. Proteolytic enzymes of A. oryzae. Kagaku Seibutsu 2016, 54, 109–116. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Sitanggang, N.V.; Kato, N.; Inoue, J.; Murakami, T.; Watanabe, T.; Iguchi, T.; Okazaki, Y. Beneficial effects of protease preparations derived from Aspergillus on the colonic luminal environment in rats consuming a high-fat diet. Biomed. Rep. 2015, 3, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Ushiyama, E.; Ukaji, Y. Amylases and proteases in soy sauce taste (6th report); characterization of alkaline, neutral, acid protease fractions. Chomi Kagaku 1959, 7, 1–7. (In Japanese) [Google Scholar]
- Harayama, F.; Yasuhira, H. Comparison of hydrolytic action on soybean protein by the genus Aspergillus and Rhizopus. J. Brew. Soc. Jpn. 1988, 83, 828–833. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.R.; Furukawa, M.; Yamashita, K.; Kanasugi, Y.; Kawabata, C.; Hirano, K.; Ando, K.; Ichishima, E. Aorsin, a novel serine proteinase with trypsin-like specificity at acidic pH. Biochem. J. 2003, 371, 541–548. [Google Scholar] [CrossRef]
- Nakadai, T.; Nasuno, S.; Iguchi, N. Purification and properties of neutral proteinase I from Aspergillus oryzae. Agric. Biol. Chem. 1973, 37, 2695–2701. [Google Scholar] [CrossRef]
- Fushimi, N.; Ee, C.E.; Nakajima, T.; Ichishima, E. Aspzincin, a family of metalloendopeptidases with a new zinc-binding motif. Identification of new zinc-binding sites (His(128), His(132), and Asp(164)) and three catalytically crucial residues (Glu(129), Asp(143), and Tyr(106)) of deuterolysin from Aspergillus oryzae by site-directed mutagenesis. J. Biol. Chem. 1999, 274, 24195–24201. [Google Scholar]
- Maeda, H.; Katase, T.; Sakai, D.; Takeuchi, M.; Kusumoto, K.; Amano, H.; Ishida, H.; Abe, K.; Yamagata, Y. A novel non-thermostable deuterolysin from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2006, 80, 1813–1819. [Google Scholar] [CrossRef] [Green Version]
- Nakadai, T.; Nasuno, S.; Iguchi, N. Purification and properties of neutral proteinase II from Aspergillus oryzae. Agric. Biol. Chem. 1973, 37, 2703–2708. [Google Scholar] [CrossRef]
- Morihara, K.; Tsuzuki, H. Comparison of the specificities of various serine proteinases from microorganisms. Arch. Biochem. Biophys. 1969, 129, 620–634. [Google Scholar] [CrossRef]
- Nakadai, T.; Nasuno, S.; Iguchi, N. Purification and properties of alkaline proteinase from Aspergillus oryzae. Agric. Biol. Chem. 1973, 37, 2685–2694. [Google Scholar] [CrossRef]
- MEROPS the Peptidase Database. Available online: https://www.ebi.ac.uk/merops/ (accessed on 7 May 2021).
- Blinkovsky, A.M.; Byun, T.; Brown, K.M.; Golightly, E.J. Purification, characterization, and heterologous expression in Fusarium venenatum of a novel serine carboxypeptidase from Aspergillus oryzae. Appl. Environ. Microbiol. 1999, 65, 3298–3303. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Ichishima, E. A 155K acid carboxypeptidase O from Aspergillus oryzae. Agric. Biol. Chem. 1986, 50, 633–638. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ushijima, T.; Ichishima, E. A new acid carboxypeptidase, O-1, from Aspergillus oryzae. Curr. Microbiol. 1982, 7, 19–23. [Google Scholar] [CrossRef]
- Morita, H.; Okamoto, A.; Yamagata, Y.; Kusumoto, K.; Koide, Y.; Ishida, H.; Takeuchi, M. Heterologous expression and characterization of CpI, OcpA, and novel serine-type carboxypeptidase OcpB from Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2009, 85, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, H.; Kuriyama, K.; Akiyama, N.; Okamoto, A.; Yamagata, Y.; Kusumoto, K.; Koide, Y.; Ishida, H.; Takeuchi, M. Molecular cloning of ocpO encoding carboxypeptidase O of Aspergillus oryzae IAM2640. Biosci. Biotechnol. Biochem. 2010, 74, 1000–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita-Morita, M.; Tada, S.; Suzuki, S.; Hattori, R.; Marui, J.; Furukawa, I.; Yamagata, Y.; Amano, H.; Ishida, H.; Takeuchi, M.; et al. Overexpression and characterization of an extracellular leucine aminopeptidase from Aspergillus oryzae. Curr. Microbiol. 2011, 62, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Blinkovsky, A.M.; Byun, T.; Brown, K.M.; Golightly, E.J.; Klotz, A.V. A non-specific aminopeptidase from Aspergillus. Biochim. Biophys. Acta 2000, 1480, 171–181. [Google Scholar] [CrossRef]
- Maeda, H.; Sakai, D.; Kobayashi, T.; Morita, H.; Okamoto, A.; Takeuchi, M.; Kusumoto, K.; Amano, H.; Ishida, H.; Yamagata, Y. Three extracellular dipeptidyl peptidases found in Aspergillus oryzae show varying substrate specificities. Appl. Microbiol. Biotechnol. 2016, 100, 4947–4958. [Google Scholar] [CrossRef]
- Tachi, H.; Ito, H.; Ichishima, E. An X-prolyl dipeptidyl-aminopeptidase from Aspergillus oryzae. Phytochemistry 1992, 31, 3707–3709. [Google Scholar] [CrossRef]
- Eugster, P.J.; Salamin, K.; Grouzmann, E.; Monod, M. Production and characterization of two major Aspergillus oryzae secreted prolyl endopeptidases able to efficiently digest proline-rich peptides of gliadin. Microbiology 2015, 161, 2277–2288. [Google Scholar] [CrossRef]
- Salamin, K.; Eugster, P.J.; Jousson, O.; Waridel, P.; Grouzmann, E.; Monod, M. AoS28D, a proline-Xaa carboxypeptidase secreted by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2017, 101, 4129–4137. [Google Scholar] [CrossRef]
- Ito, H. Taste of miso. J. Brew. Soc. Jpn. 1980, 75, 881–884. (In Japanese) [Google Scholar]
- Ito, K.; Hanya, Y.; Koyama, Y. Purification and characterization of a glutaminase enzyme accounting for the majority of glutaminase activity in Aspergillus sojae under solid-state culture. Appl. Microbiol. Biotechnol. 2013, 97, 8581–8590. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Matsushima, K.; Koyama, Y. Gene cloning, purification, and characterization of a novel peptidoglutaminase-asparaginase from Aspergillus sojae. Appl. Environ. Microbiol. 2012, 78, 5182–5188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, M.D.; Williams, A.M.; Wallbanks, S. The phylogeny of Aerococcus and Pediococcus as determined by 16S rRNA sequence analysis: Description of Tetragenococcus gen. nov. FEMS Microbiol. Lett. 1990, 58, 255–262. [Google Scholar] [CrossRef]
- Kanbe, C.; Uchida, K. Oxygen consumption by Pediococcus halophilus. Agric. Biol. Chem. 1985, 49, 2931–2937. [Google Scholar]
- Uchida, K. Multiplicity in soy pediococci carbohydrate fermentation and its application for analysis of their flora. J. Gen. Appl. Microbiol. 1982, 28, 215–223. [Google Scholar] [CrossRef]
- Uchida, K. Diversity and ecology of salt tolerant lactic acid bacteria: Tetragenococcus halophilus in soy sauce fermentation. Japan. J. Lact. Acid Bact. 2000, 11, 60–65. [Google Scholar] [CrossRef]
- Standard Tables of Food Composition in Japan—2015—(Seventh Revised Edition). Available online: https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/sdetail01/sdetail01/1385122.htm (accessed on 7 May 2021).
- Mochizuki, T.; Ouchi, I.; Matsumoto, K. Amino acids in miso. J. Brew. Soc. Jpn. 1968, 63, 378–381. (In Japanese) [Google Scholar]
- Honma, N. Aroma and aroma components of miso (2). J. Brew. Soc. Jpn. 1987, 82, 548–553. (In Japanese) [Google Scholar]
- Sugawara, E.; Saiga, S.; Kobayashi, A. Relationship between aroma components and sensory evaluation of miso. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 1098–1104. (In Japanese) [Google Scholar] [CrossRef]
- Sugawara, E. Change in aroma components of miso with aging. Nippon Shokuhin Kogyo Gakkaishi 1991, 38, 1093–1097. (In Japanese) [Google Scholar] [CrossRef]
- Sugawara, E.; Yonekura, Y. Comparison of aroma components in five types of miso. Nippon Shokuhin Kogyo Gakkaishi 1998, 45, 323–329. (In Japanese) [Google Scholar] [CrossRef]
- Kumazawa, K.; Kaneko, S.; Nishimura, O. Identification and characterization of volatile components causing the characteristic flavor in miso (Japanese fermented soybean paste) and heat-processed miso products. J. Agric. Food Chem. 2013, 61, 11968–11973. [Google Scholar] [CrossRef]
- Reports of Central Miso Research Institute [in Japanese]. Available online: https://www.miso.jp/chumiken_hokoku.html (accessed on 7 July 2021).
- Kitagawa, M.; Ito, K.; Yamada, M.; Koike, S.; Yamamoto, T.; Uehara, Y. Long term intake of miso soup unaffected blood pressure in subjects with normal or stage I hypertension-double blind comparative interventional trial. Jpn Pharm. Ther. 2016, 44, 1601–1612. (In Japanese) [Google Scholar]
- Kondo, H.; Sakuyama, T.H.; Yamakawa, S.; Kitagawa, M.; Yamada, M.; Itou, S.; Yamamoto, T.; Uehara, Y. Long-term intake of miso soup decreases nighttime blood pressure in subjects with high-normal blood pressure or stage I hypertension. Hypertens Res. 2019, 42, 1757–1767. [Google Scholar] [CrossRef] [Green Version]
- Ito, K. Review of the health benefits of habitual consumption of miso soup: Focus on the effects on sympathetic nerve activity, blood pressure, and heart rate. Env. Health Prev Med. 2020, 25, 1–9. [Google Scholar] [CrossRef]
- Katagiri, R.; Sawada, N.; Goto, A.; Yamaji, T.; Iwasaki, M.; Noda, M.; Iso, H.; Tsugane, S. Association of soy and fermented soy product intake with total and cause specific mortality: Prospective cohort study. BMJ 2020, 368, m34. [Google Scholar] [CrossRef] [Green Version]
- Okada, E.; Saito, A.; Takimoto, H. Association between the portion sizes of traditional Japanese seasonings—Soy sauce and miso—And blood pressure: Cross-sectional study using national health and nutrition survey, 2012–2016 data. Nutrients 2018, 10, 1865. [Google Scholar] [CrossRef] [Green Version]
| Nutrients | Rice Miso, Sweet | Rice Miso, Light Yellow | Rice Miso, Red | Barley Miso | Soybean Miso |
|---|---|---|---|---|---|
| Water (g) | 42.6 | 45.4 | 45.7 | 44.0 | 44.9 |
| Protein (g) | 9.7 | 12.5 | 13.1 | 9.7 | 17.2 |
| Lipid (g) | 3.0 | 6.0 | 5.5 | 4.3 | 10.5 |
| Carbohydrate (g) | 37.9 | 21.9 | 21.1 | 30.0 | 14.5 |
| Dietary fiber, total (g) | 5.6 | 4.9 | 4.1 | 6.3 | 6.5 |
| Salt equivalents (g) | 6.1 | 12.4 | 13.0 | 10.7 | 10.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusumoto, K.-I.; Yamagata, Y.; Tazawa, R.; Kitagawa, M.; Kato, T.; Isobe, K.; Kashiwagi, Y. Japanese Traditional Miso and Koji Making. J. Fungi 2021, 7, 579. https://doi.org/10.3390/jof7070579
Kusumoto K-I, Yamagata Y, Tazawa R, Kitagawa M, Kato T, Isobe K, Kashiwagi Y. Japanese Traditional Miso and Koji Making. Journal of Fungi. 2021; 7(7):579. https://doi.org/10.3390/jof7070579
Chicago/Turabian StyleKusumoto, Ken-Ichi, Youhei Yamagata, Rina Tazawa, Manabu Kitagawa, Taeko Kato, Kenji Isobe, and Yutaka Kashiwagi. 2021. "Japanese Traditional Miso and Koji Making" Journal of Fungi 7, no. 7: 579. https://doi.org/10.3390/jof7070579
APA StyleKusumoto, K.-I., Yamagata, Y., Tazawa, R., Kitagawa, M., Kato, T., Isobe, K., & Kashiwagi, Y. (2021). Japanese Traditional Miso and Koji Making. Journal of Fungi, 7(7), 579. https://doi.org/10.3390/jof7070579





