Comparative Study of Secreted Proteins, Enzymatic Activities of Wood Degradation and Stilbene Metabolization in Grapevine Botryosphaeria Dieback Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. Extracellular Protein Extraction
2.3. Enzymatic Activity of Wood Degradation
2.4. MS-Based Proteomic Analysis
2.4.1. Sample Preparation
2.4.2. NanoLC-MS/MS Analysis
2.4.3. Protein Identification
2.5. Kinetic Monitoring of Stilbene Metabolization
2.5.1. Chemicals
2.5.2. Preparation of Solutions
2.5.3. HPLC-MS Analysis
3. Results
3.1. Extracellular Laccase and Mn Peroxidase Activities of N. parvum and D. seriata
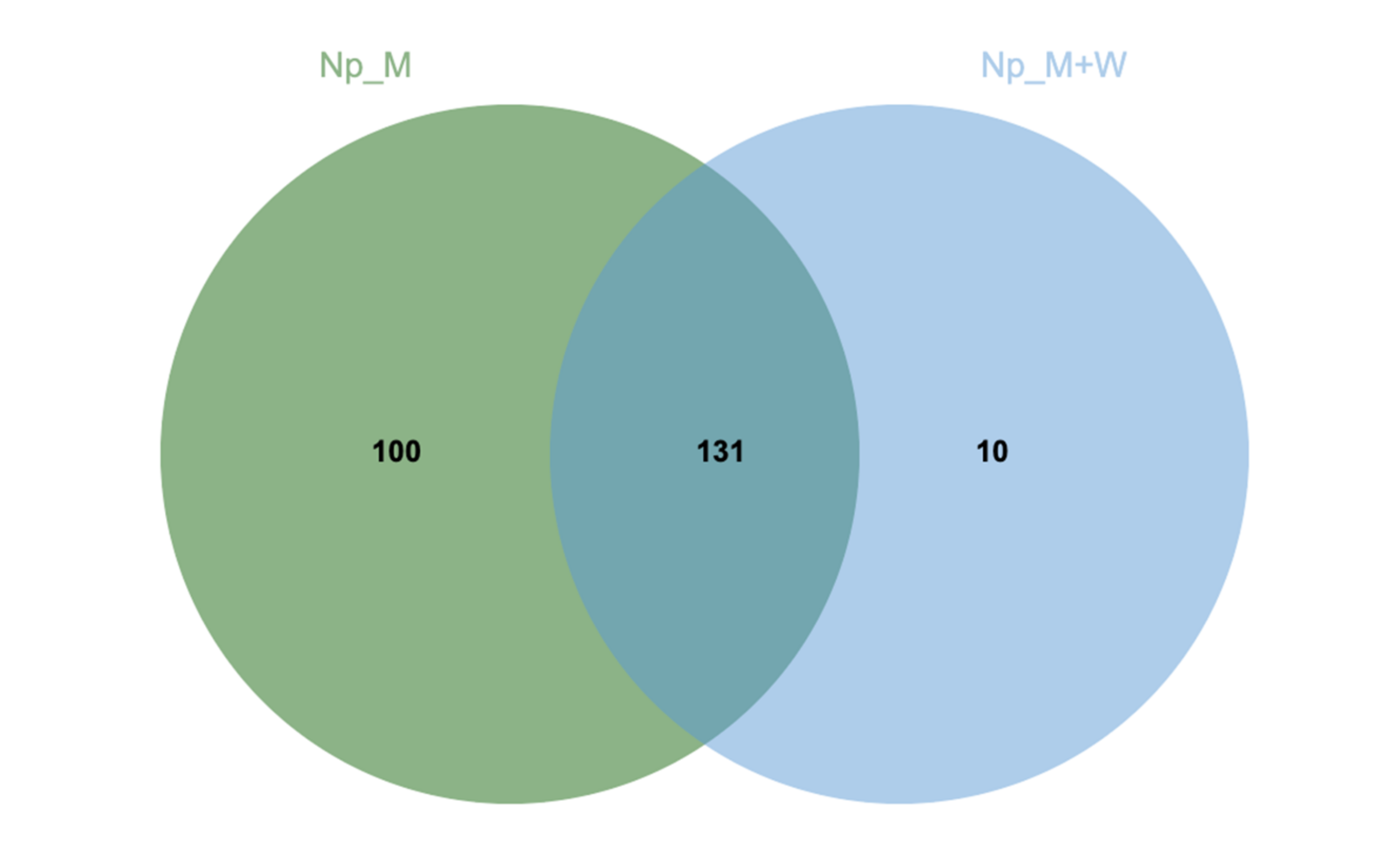
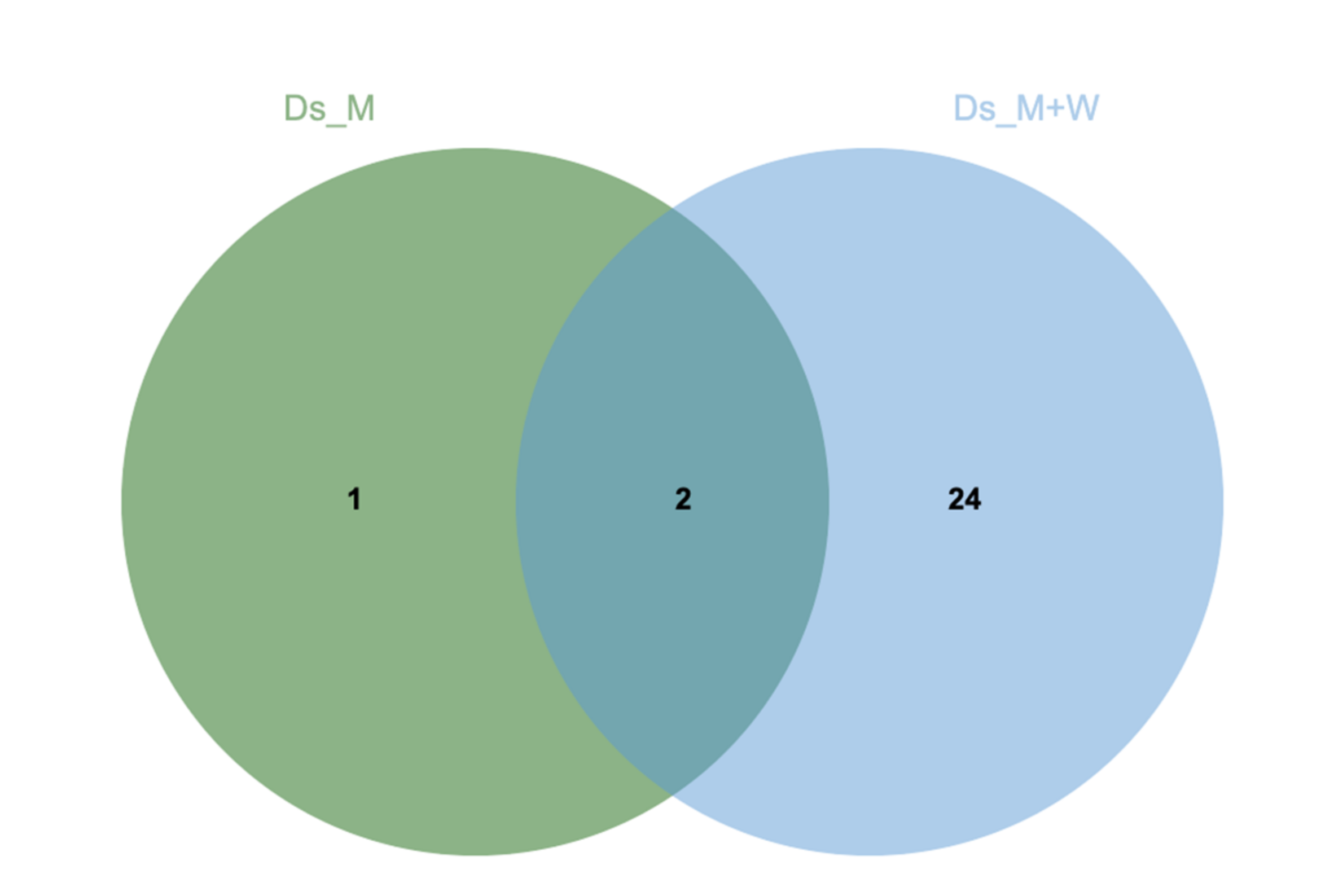
3.2. N. parvum and D. seriata Extracellular Protein Content Investigation
3.3. Kinetics of Stilbene Metabolization by Botryosphaeriaceae
3.4. Kinetics of Stilbene Metabolization by Commercial Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baránek, M.; Armengol, J.; Holleinová, V.; Pečenka, J.; Calzarano, F.; Peňázová, E.; Vachůn, M.; Eichmeier, A. Incidence of symptoms and fungal pathogens associated with grapevine trunk diseases in Czech vineyards: First example from a north-eastern European grape-growing region. Phytopathol. Mediterr. 2018, 57, 449–458. [Google Scholar] [CrossRef]
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Calzarano, F.; Di Marco, S. Further Evidence That Calcium, Magnesium and Seaweed Mixtures Reduce Grapevine Leaf Stripe Symptoms and Increase Grape Yields. Phytopathol. Mediterr. 2018, 57, 459–471. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R. The Status of Botryosphaeriaceae Species Infecting Grapevines. Phytopathol. Mediterr. 2011, 50, 5–45. [Google Scholar] [CrossRef]
- Ahimera, N.; Driever, G.F.; Michailides, T.J. Relationships Among Propagule Numbers of Botryosphaeria dothidea, Latent Infections, and Severity of Panicle and Shoot Blight in Pistachio Orchards. Plant Dis. 2003, 87, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larignon, P.; Fulchic, R.; Cere, L.; Dubos, B. Observation on Black Dead Arm in French Vineyards. Phytopathol. Mediterr. 2001, 40, 336–342. [Google Scholar] [CrossRef]
- Fischer, M. Biodiversity and Geographic Distribution of Basidiomycetes Causing Esca-Associated White Rot in Grapevine: A Worldwide Perspective. Phytopathol. Mediterr. 2006, 45, 30–42. [Google Scholar] [CrossRef]
- Hallenn, F.; Fourie, P.H.; Crous, P.W. A Review of Black Foot Disease of Grapevine. Phytopathol. Mediterr. 2006, 45, 55–67. [Google Scholar] [CrossRef]
- Niekerk, J.M.; Fourie, P.H.; Hallenn, F.; Crous, P. Botryosphaeria spp. as Grapevine Trunk Disease Pathogens. Phytopathol. Mediterr. 2006, 45, 43–54. [Google Scholar] [CrossRef]
- Larignon, P. Maladies Cryptogamiques Du Bois de La Vigne: Symptomatologie et Agents Pathogènes. Available online: http://www.vignevin.com (accessed on 1 December 2020).
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black Measles) and Brown Wood-Streaking: Two Old and Elusive Diseases of Grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, C.; Ramírez-Suero, M.; Magninrobert, M.; Larignon, P.; Chong, J.; Mansour, E.A.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2012, 62, 243–265. [Google Scholar] [CrossRef] [Green Version]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabacchi, R.; Fkyerat, A.; Poliart, C.; Dubin, G.-M. Phytotoxins from Fungi of Esca of Grapevine. Phytopathol. Mediterr. 2000, 39, 151–161. [Google Scholar] [CrossRef]
- Amborabé, B.-E.; Fleurat-Lessard, P.; Bonmort, J.; Roustan, J.-P.; Roblin, G. Effects of eutypine, a toxin from Eutypa lata, on plant cell plasma membrane: Possible subsequent implication in disease development. Plant Physiol. Biochem. 2001, 39, 51–58. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins Produced by Fungi Associated with Grapevine Trunk Diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renaud, J.-M.; Tsoupras, G.; Tabacchi, R. Biologically Active Natural Acetylenic Compounds from Eutypa lata (Pers: F.) TUL. Helvetica Chim. Acta 1989, 72, 929–932. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Andolfi, A.; Bruno, G. Two Naphthalenone Pentaketides from Liquid Cultures of Phaeoacremo-nium Aleophilum, a Fungus Associated with Esca of Grapevine. Phytopathol. Mediterranea. 2000, 39, 162–168. [Google Scholar]
- Bruno, G.L.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: V. Changes in the chemical and biological profile of xylem sap from diseased cv. Sangiovese vines. Physiol. Mol. Plant Pathol. 2007, 71, 210–229. [Google Scholar] [CrossRef]
- Sparapano, L.; Bruno, G.; Graniti, A. Effects on Plants of Metabolites Produced in Culture by Phaeoacremonium Chlamydo-sporum, P. Aleophilum and Fomitiporia Punctata. Phytopathol. Mediterr. 2000, 39, 169–177. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on Fungal Phytotoxins and Their Role in Grapevine Trunk Diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef]
- Bénard-Gellon, M.; Farine, S.; Goddard, M.L.; Schmitt, M.; Stempien, E.; Pensec, F.; Laloue, H.; Mazet-Kieffer, F.; Fontaine, F.; Larignon, P.; et al. Toxicity of extracellular proteins from Diplodia seriata and Neofusicoccum parvum involved in grapevine Botryosphaeria dieback. Protoplasma 2014, 252, 679–687. [Google Scholar] [CrossRef]
- Elghazali, B.; Gas, G. Biodegradation des lignocelluloses de vigne (Vitis vinifera cv. Cabernet Sauvignon) par Eutypa lata (PERS. FR.) TUL. J. Grapevine Res. 1992, 31, 95–103. [Google Scholar] [CrossRef]
- Schmid, C.S.; Wolf, G.A.; Lorenz, D. Production of Extracellular Hydrolytic Enzymes by the Grapevine Dieback Fungus Eutypa Lata. J. Plant Dis. 1999, 106, 1–11. [Google Scholar]
- Rolshausen, P.E.; Greve, L.C.; Labavitch, J.M.; Mahoney, N.E.; Molyneux, R.J.; Gubler, W.D. Pathogenesis of Eutypa lata in Grapevine: Identification of Virulence Factors and Biochemical Characterization of Cordon Dieback. Phytopathology 2008, 98, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, A.C.; Saraiva, M.; Correia, A.; Alves, A. Botryosphaeriales fungi produce extracellular enzymes with biotechnological potential. Can. J. Microbiol. 2014, 60, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cruz, A.; Amrine, K.C.H.; Blanco-Ulate, B.; Lawrence, D.P.; Travadon, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Distinctive expansion of gene families associated with plant cell wall degradation, secondary metabolism, and nutrient uptake in the genomes of grapevine trunk pathogens. BMC Genom. 2015, 16, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Langcake, P.; Cornford, C.; Pryce, R. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry 1979, 18, 1025–1027. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, A.K.; Viret, O. δ-Viniferin, a Resveratrol Dehydrodimer: One of the Major Stilbenes Synthesized by Stressed Grapevine Leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef] [PubMed]
- Amalfitano, C.; Evidente, A.; Surico, G.; Tegli, S.; Bertelli, E.; Mugnai, L. Phenols and Stilbene Polyphenols in the Wood of Esca-Diseased Grapevines. Phytopathol. Mediterr. 2000, 39, 178–183. [Google Scholar]
- Calzarano, F.; D’Agostino, V.; Pepe, A.; Osti, F.; Della Pelle, F.; De Rosso, M.; Flamini, R.; Di Marco, S. Patterns of Phytoalexins in the Grapevine Leaf Stripe Disease (Esca Complex)/Grapevine Pathosystem. Phytopathol. Mediterr. 2016, 55, 410–426. [Google Scholar] [CrossRef]
- Martin, N.; Vesentini, D.; Rego, C.; Monteiro, S.; Oliveira, H.; Ferreira, R.B. Phaeomoniella Chlamydospora Infection Induces Changes in Phenolic Compounds Content in Vitis Vinifera. Phytopathol. Mediterr. 2009, 48, 101–116. [Google Scholar] [CrossRef]
- Felgueiras, M.L.; Lima, M.R.M.; Dias, A.C.P.; Gil, A.M.; Graça, G.; Rodrigues, J.E.A.; Barros, A. NMR Metabolomics of Esca Disease-Affected Vitis Vinifera Cv. Alvarinho Leaves. J. Exp. Bot. 2010, 61, 4033–4042. [Google Scholar] [CrossRef] [Green Version]
- Amalfitano, C.; Agrelli, D.; Arrigo, A.; Mugnai, L.; Surico, G.; Evidente, A. Stilbene polyphenols in the brown red wood of Vitis vinifera cv. Sangiovese affected by “esca proper”. Phytopathol. Mediterr. 2011, 50, 224–235. [Google Scholar] [CrossRef]
- Labois, C.; Wilhelm, K.; Laloue, H.; Tarnus, C.; Bertsch, C.; Goddard, M.-L.; Chong, J. Wood Metabolomic Responses of Wild and Cultivated Grapevine to Infection with Neofusicoccum parvum, a Trunk Disease Pathogen. Metabolities 2020, 10, 232. [Google Scholar] [CrossRef]
- Letousey, P.; Baillieul, F.; Perrot, G.; Rabenoelina, F.; Boulay, M.; Vaillant-Gaveau, N.; Clément, C.; Fontaine, F. Early Events Prior to Visual Symptoms in the Apoplectic Form of Grapevine Esca Disease. Phytopathology 2010, 100, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Stempien, E.; Goddard, M.-L.; Leva, Y.; Bénard-Gellon, M.; Laloue, H.; Farine, S.; Kieffer-Mazet, F.; Tarnus, C.; Bertsch, C.; Chong, J. Secreted proteins produced by fungi associated with Botryosphaeria dieback trigger distinct defense responses in Vitis vinifera and Vitis rupestris cells. Protoplasma 2017, 255, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Stempien, E.; Goddard, M.-L.; Wilhelm, K.; Tarnus, C.; Bertsch, C.; Chong, J. Grapevine Botryosphaeria dieback fungi have specific aggressiveness factor repertory involved in wood decay and stilbene metabolization. PLoS ONE 2017, 12, e0188766. [Google Scholar] [CrossRef]
- Van Niekerk, J.M.; Crous, P.W.; Groenewald, J.Z.; Fourie, P.H.; Halleen, F. DNA Phylogeny, Morphology and Pathogenicity of Botryosphaeria Species on Grapevines. Mycologia 2004, 96, 781–798. [Google Scholar] [CrossRef]
- Robert-Siegwald, G.; Vallet, J.; Mansour, E.A.; Xu, J.; Rey, P.; Bertsch, C.; Rego, C.; Larignon, P.; Fontaine, F.; Lebrun, M.-H. Draft Genome Sequence of Diplodia seriata F98.1, a Fungal Species Involved in Grapevine Trunk Diseases. Genome Announc. 2017, 5, e00061-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, Y.; Gelhaye, E.; Dumarçay, S.; Gérardin, P.; Harvengt, L.; Buée, M. Selection and validation of enzymatic activities as functional markers in wood biotechnology and fungal ecology. J. Microbiol. Methods 2013, 92, 157–163. [Google Scholar] [CrossRef]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error bars in experimental biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carapito, C.; Burel, A.; Guterl, P.; Walter, A.; Varrier, F.; Bertile, F.; Van Dorsselaer, A. MSDA, a proteomics software suite for in-depth Mass Spectrometry Data Analysis using grid computing. Proteomics 2014, 14, 1014–1019. [Google Scholar] [CrossRef]
- Bouyssié, D.; Hesse, A.-M.; Mouton-Barbosa, E.; Rompais, M.; Macron, C.; Carapito, C.; De Peredo, A.G.; Couté, Y.; Dupierris, V.; Burel, A.; et al. Proline: An efficient and user-friendly software suite for large-scale proteomics. Bioinformatics 2020, 36, 3148–3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lu, J.; Xu, X.; Hu, R.; Pan, Y. pH-switched HRP-catalyzed dimerization of resveratrol: A selective biomimetic synthesis. Green Chem. 2012, 14, 3281–3284. [Google Scholar] [CrossRef]
- Matsuura, B.S.; Keylor, M.; Li, B.; Lin, Y.; Allison, S.; Pratt, D.A.; Stephenson, C.R.J. A Scalable Biomimetic Synthesis of Resveratrol Dimers and Systematic Evaluation of their Antioxidant Activities. Angew. Chem. Int. Ed. 2015, 54, 3754–3757. [Google Scholar] [CrossRef] [Green Version]
- Ohyama, M.; Tanaka, T.; Iinuma, M. Five resveratrol oligomers from roots of Sophora leachiana. Phytochemistry 1995, 38, 733–740. [Google Scholar] [CrossRef]
- Nagel, J.; Wingfield, M.; Slippers, B. Abundant Secreted Hydrolytic Enzymes and Secondary Metabolite Gene Clusters in Genomes of the Botryosphaeriaceae Reflect Their Role as Important Plant Pathogens. bioRxiv 2021. [Google Scholar] [CrossRef]
- De Queiroz, C.B.; Santana, M.F. Prediction of the secretomes of endophytic and nonendophytic fungi reveals similarities in host plant infection and colonization strategies. Mycologia 2020, 112, 491–503. [Google Scholar] [CrossRef]
- Massonnet, M.; Morales-Cruz, A.; Figueroa-Balderas, R.; Lawrence, D.P.; Baumgartner, K.; Cantu, D. Condition-dependent co-regulation of genomic clusters of virulence factors in the grapevine trunk pathogen Neofusicoccum parvum. Mol. Plant Pathol. 2018, 19, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, L.; Guo, Y.-X.; Dong, Y.-S.; Zhang, D.-J.; Xiu, Z.-L. Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2007, 75, 763–768. [Google Scholar] [CrossRef]
- Jin, S.; Luo, M.; Wang, W.; Zhao, C.; Gu, C.-B.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J.; Guan, Y. Biotransformation of polydatin to resveratrol in Polygonum cuspidatum roots by highly immobilized edible Aspergillus niger and Yeast. Bioresour. Technol. 2013, 136, 766–770. [Google Scholar] [CrossRef]
- Chen, M.; Li, D.; Gao, Z.; Zhang, C. Enzymatic transformation of polydatin to resveratrol by piceid-β-d-glucosidase from Aspergillus oryzae. Bioprocess Biosyst. Eng. 2014, 37, 1411–1416. [Google Scholar] [CrossRef]
- Milton, R.; Giroud, F.; Thumser, A.E.; Minteer, S.; Slade, R.C.T. Hydrogen peroxide produced by glucose oxidase affects the performance of laccase cathodes in glucose/oxygen fuel cells: FAD-dependent glucose dehydrogenase as a replacement. Phys. Chem. Chem. Phys. 2013, 15, 19371–19379. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H.; Kouzi, S.A.; Hamann, M.T. Dimerization of Resveratrol by the Grapevine PathogenBotrytis cinerea. J. Nat. Prod. 2000, 63, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Breuil, A.-C.; Adrian, M.; Pirio, N.; Meunier, P.; Bessis, R.; Jeandet, P. Metabolism of stilbene phytoalexins by Botrytis cinerea: 1. Characterization of a resveratrol dehydrodimer. Tetrahedron Lett. 1998, 39, 537–540. [Google Scholar] [CrossRef]
- Pezet, R. Purification and Characterization of a 32-KDa Laccase-like Stilbene Oxidase Produced by Botrytis Cinerea Pers.:Fr. FEMS Microbiol. Ecol. 1998, 167, 203–208. [Google Scholar] [CrossRef]
- Beneventi, E.; Conte, S.; Cramarossa, M.R.; Riva, S.; Forti, L. Chemo-enzymatic synthesis of new resveratrol-related dimers containing the benzo[b]furan framework and evaluation of their radical scavenger activities. Tetrahedron 2015, 71, 3052–3058. [Google Scholar] [CrossRef] [Green Version]
- Gindro, K.; Schnee, S.; Righi, D.; Marcourt, L.; Ebrahimi, S.N.; Massana-Codina, J.; Voinesco, F.; Michellod, E.; Wolfender, J.-L.; Queiroz, E.F. Generation of Antifungal Stilbenes Using the Enzymatic Secretome of Botrytis cinerea. J. Nat. Prod. 2017, 80, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Pezet, R.; Gindro, K.; Viret, O.; Spring, J.-L. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol. Mol. Plant Pathol. 2004, 65, 297–303. [Google Scholar] [CrossRef]
- Khattab, I.M.; Sahi, V.P.; Baltenweck, R.; Maia-Grondard, A.; Hugueney, P.; Bieler, E.; Dürrenberger, M.; Riemann, M.; Nick, P. Ancestral chemotypes of cultivated grapevine with resistance to Botryosphaeriaceae-related dieback allocate metabolism towards bioactive stilbenes. New Phytol. 2021, 229, 1133–1146. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labois, C.; Stempien, E.; Schneider, J.; Schaeffer-Reiss, C.; Bertsch, C.; Goddard, M.-L.; Chong, J. Comparative Study of Secreted Proteins, Enzymatic Activities of Wood Degradation and Stilbene Metabolization in Grapevine Botryosphaeria Dieback Fungi. J. Fungi 2021, 7, 568. https://doi.org/10.3390/jof7070568
Labois C, Stempien E, Schneider J, Schaeffer-Reiss C, Bertsch C, Goddard M-L, Chong J. Comparative Study of Secreted Proteins, Enzymatic Activities of Wood Degradation and Stilbene Metabolization in Grapevine Botryosphaeria Dieback Fungi. Journal of Fungi. 2021; 7(7):568. https://doi.org/10.3390/jof7070568
Chicago/Turabian StyleLabois, Clément, Elodie Stempien, Justine Schneider, Christine Schaeffer-Reiss, Christophe Bertsch, Mary-Lorène Goddard, and Julie Chong. 2021. "Comparative Study of Secreted Proteins, Enzymatic Activities of Wood Degradation and Stilbene Metabolization in Grapevine Botryosphaeria Dieback Fungi" Journal of Fungi 7, no. 7: 568. https://doi.org/10.3390/jof7070568
APA StyleLabois, C., Stempien, E., Schneider, J., Schaeffer-Reiss, C., Bertsch, C., Goddard, M.-L., & Chong, J. (2021). Comparative Study of Secreted Proteins, Enzymatic Activities of Wood Degradation and Stilbene Metabolization in Grapevine Botryosphaeria Dieback Fungi. Journal of Fungi, 7(7), 568. https://doi.org/10.3390/jof7070568






