Membrane Traffic in Aspergillus oryzae and Related Filamentous Fungi
Abstract
1. Introduction
2. Secretory Pathway
2.1. Historical Studies of Secretory Proteins in A. oryzae
2.2. Molecular Machinery of Secretory Pathway
2.2.1. N-Glycosylation
2.2.2. ER, Golgi and Spitzenkörper
2.2.3. mRNA Localization
2.3. Unconventional Protein Secretion
2.4. Secretion of Metabolites
3. Endocytic Pathway
3.1. Historical Studies of Endocytic Pathway in A. oryzae
3.1.1. Existence of Endocytosis in A. oryzae
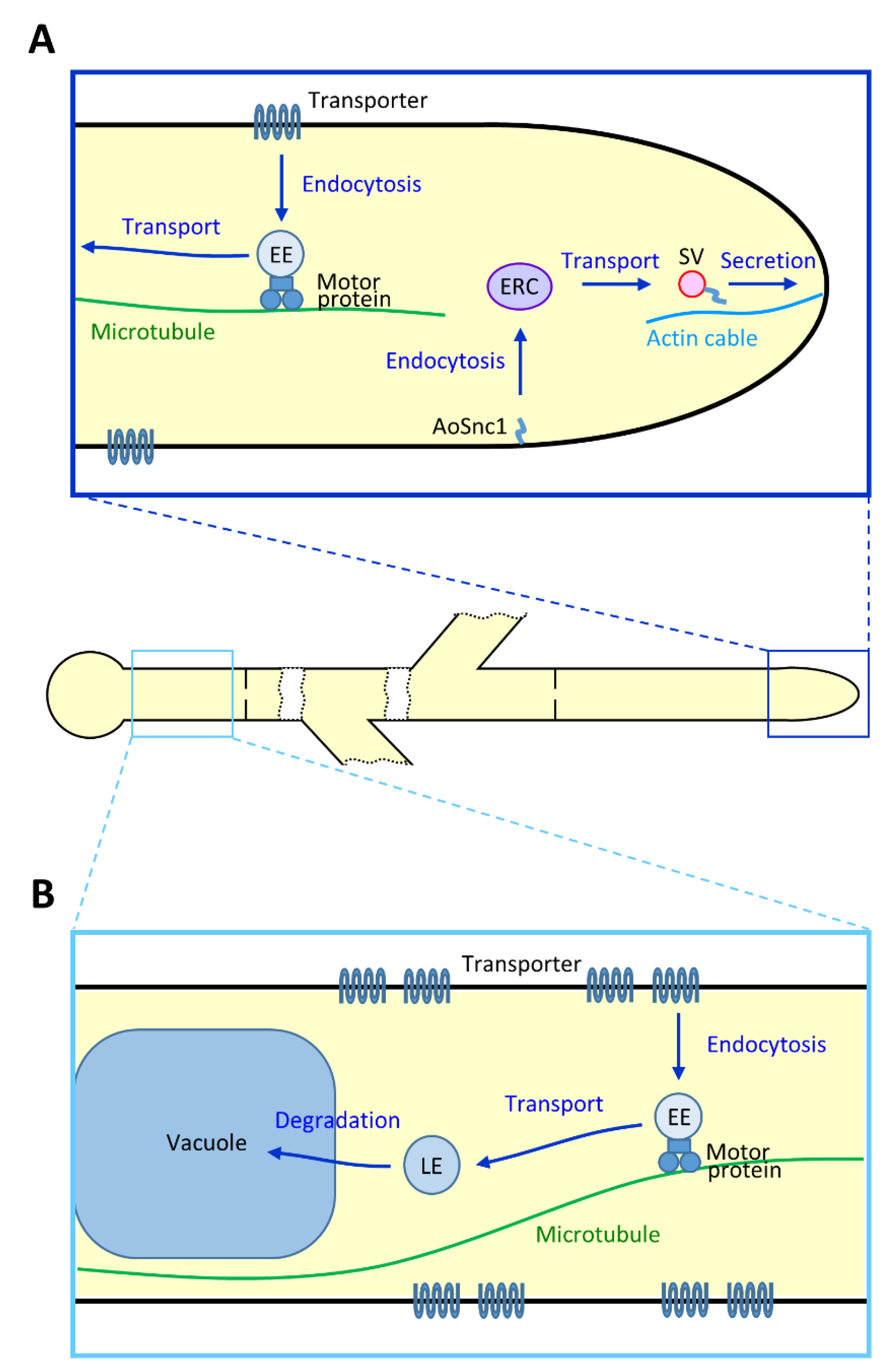
3.1.2. Endocytic Recycling at the Hyphal Tip Region
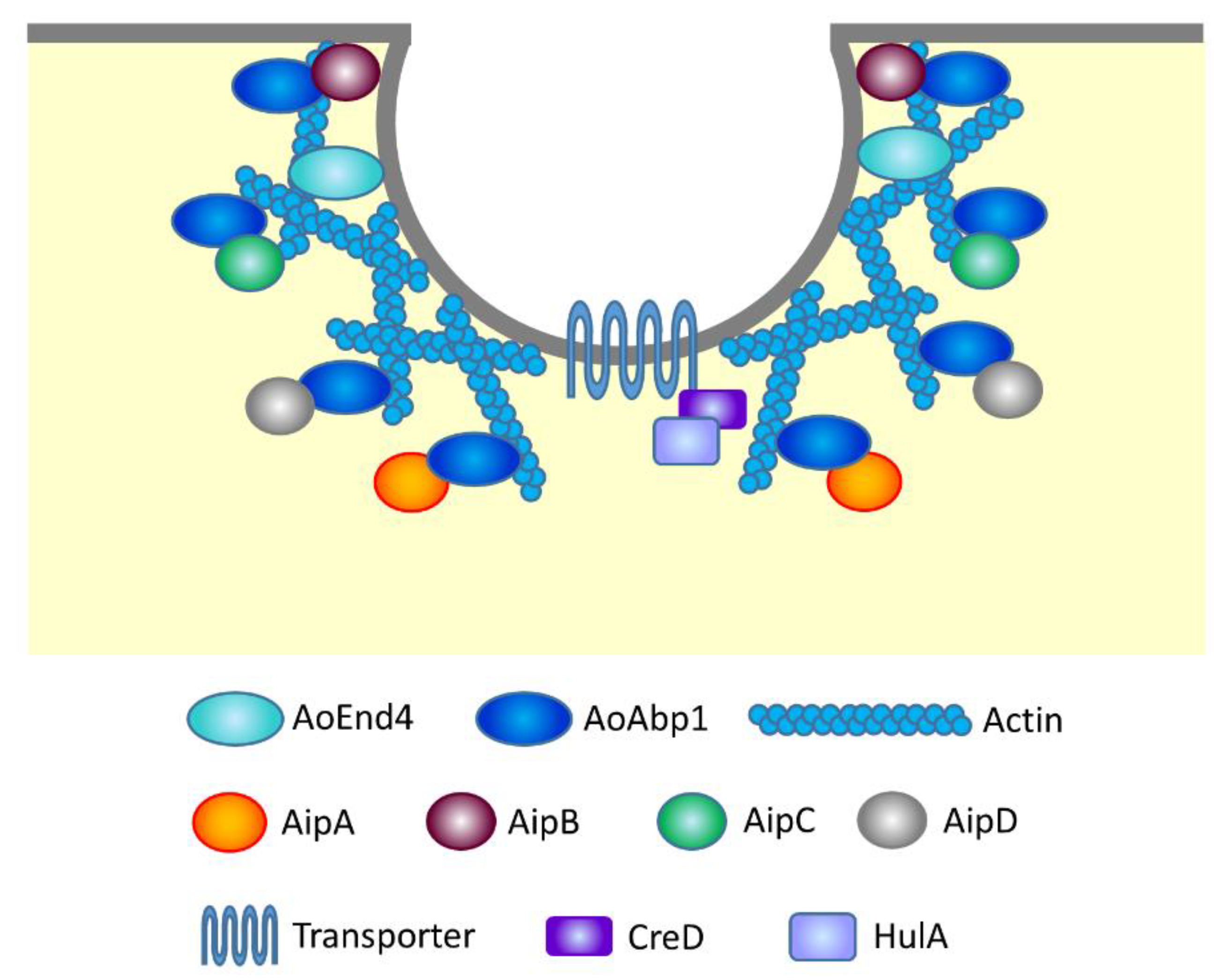
3.1.3. Other Molecular Mechanisms in Endocytosis
3.1.4. Molecular Machinery Related to Endocytic Organelles
3.2. Dynamics of Endocytic Organelles
3.2.1. Early Endosome
3.2.2. Vacuole
4. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitamoto, K. Cell biology of the Koji mold Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2015, 79, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Ichishima, E. Development of enzyme technology for Aspergillus oryzae, A. sojae, and A. luchuensis, the national microorganisms of Japan. Biosci. Biotechnol. Biochem. 2016, 80, 1681–1692. [Google Scholar] [CrossRef]
- Kitagaki, H. Medical application of substances derived from non-pathogenic fungi Aspergillus oryzae and A. luchuensis-containing Koji. J. Fungi 2021, 7, 243. [Google Scholar] [CrossRef]
- Declaration. Available online: https://www.jozo.or.jp/gakkai/wp-content/uploads/sites/4/2020/02/koujikinnituite2.pdf (accessed on 1 June 2021).
- Shoji, J.Y.; Kikuma, T.; Kitamoto, K. Vesicle trafficking, organelle functions, and unconventional secretion in fungal physiology and pathogenicity. Curr. Opin. Microbiol. 2014, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef]
- Jin, F.J.; Hu, S.; Wang, B.T.; Jin, L. Advances in genetic engineering technology and its application in the industrial fungus Aspergillus oryzae. Front. Microbiol. 2021, 12, 644404. [Google Scholar] [CrossRef]
- Higuchi, Y. Membrane traffic related to endosome dynamics and protein secretion in filamentous fungi. Biosci. Biotechnol. Biochem. 2021, 85, 1038–1045. [Google Scholar] [CrossRef]
- Gomi, K. Regulatory mechanisms for amylolytic gene expression in the koji mold Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2019, 83, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.H.; de Montmollin, R. Crystallization of the α-amylase of Aspergillus oryzae. Nature 1951, 168, 606–607. [Google Scholar] [CrossRef]
- Akabori, S.; Ikenaka, T.; Hagihara, B. Isolation of crystalline taka-amylase A from “Takadiastase Sankyo”. J. Biochem. 1954, 41, 577–582. [Google Scholar] [CrossRef]
- Tonomura, K.; Futai, F.; Tanabe, O. Binding of α-amylase to the cell wall of Aspergillus oryzae. Biochim. Biophys. Acta 1963, 78, 802–805. [Google Scholar] [CrossRef]
- Tonomura, K.; Tanabe, O. Localization of cell-bound α-amylase in Aspergillus oryzae demonstrated by fluorescent-antibody technique. J. Bacteriol. 1964, 87, 226–227. [Google Scholar] [CrossRef]
- Zhang, S.; Sato, H.; Ichinose, S.; Tanaka, M.; Miyazawa, K.; Yoshimi, A.; Abe, K.; Shintani, T.; Gomi, K. Cell wall α-1,3-glucan prevents α-amylase adsorption onto fungal cell in submerged culture of Aspergillus oryzae. J. Biosci. Bioeng. 2017, 124, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Santerre Henriksen, A.L.; Carlsen, M.; de Bang, H.; Nielsen, J. Kinetics of α-amylase secretion in Aspergillus oryzae. Biotechnol. Bioeng. 1999, 65, 76–82. [Google Scholar] [CrossRef]
- Tada, S.; Iimura, Y.; Gomi, K.; Takahashi, K.; Hara, S.; Yoshizawa, K. Cloning and nucleotide sequence of the genomic Taka-amylase A gene of Aspergillus oryzae. Agric. Biol. Chem. 1989, 53, 593–599. [Google Scholar] [CrossRef][Green Version]
- Nemoto, T.; Maruyama, J.I.; Kitamoto, K. Contribution ratios of amyA, amyB, amyC genes to high-level α-amylase expression in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2012, 76, 1477–1483. [Google Scholar] [CrossRef]
- Takahashi, K. The amino acid sequence of ribonuclease T1. J. Biol. Chem. 1965, 240, 4117–4119. [Google Scholar] [CrossRef]
- Nakahama, T.; Nakanishi, Y.; Viscomi, A.R.; Takaya, K.; Kitamoto, K.; Ottonello, S.; Arioka, M. Distinct enzymatic and cellular characteristics of two secretory phospholipases A2 in the filamentous fungus Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 318–331. [Google Scholar] [CrossRef]
- Nakagawara, C.; Arioka, M. Distinct enzymatic and cellular characteristics of two phospholipases A1 in Aspergillus oryzae. Biochem. Biophys. Res. Commun. 2019, 518, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Arikawa, K.; Kamiya, N.; Kitamoto, K.; Kumagai, C. Cloning and nucleotide sequence of the acid protease-encoding gene (pepA) from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 1993, 57, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Maruyama, J.; Kitamoto, K. Disruption of ten protease genes in the filamentous fungus Aspergillus oryzae highly improves production of heterologous proteins. Appl. Microbiol. Biotechnol. 2011, 89, 747–759. [Google Scholar] [CrossRef]
- Machida, M.; Yamada, O.; Gomi, K. Genomics of Aspergillus oryzae: Learning from the history of Koji mold and exploration of its future. DNA Res. 2008, 15, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Kakizono, D.; Yamada, O.; Iefuji, H.; Akita, O.; Iwashita, K. Proteomic analysis of extracellular proteins from Aspergillus oryzae grown under submerged and solid-state culture conditions. Appl. Environ. Microbiol. 2006, 72, 3448–3457. [Google Scholar] [CrossRef]
- Hata, Y.; Ishida, H.; Ichikawa, E.; Kawato, A.; Suginami, K.; Imayasu, S. Nucleotide sequence of an alternative glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Gene 1998, 207, 127–134. [Google Scholar] [CrossRef]
- Ishida, H.; Hata, Y.; Kawato, A.; Abe, Y.; Suginami, K.; Imayasu, S. Identification of functional elements that regulate the glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Curr. Genet. 2000, 37, 373–379. [Google Scholar] [CrossRef]
- Hata, Y.; Tsuchiya, K.; Kitamoto, K.; Gomi, K.; Kumagai, C.; Tamura, G.; Hara, S. Nucleotide sequence and expression of the glucoamylase-encoding gene (glaA) from Aspergillus oryzae. Gene 1991, 108, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Kitamoto, K.; Gomi, K.; Kumagai, C.; Tamura, G. Functional elements of the promoter region of the Aspergillus oryzae glaA gene encoding glucoamylase. Curr. Genet. 1992, 22, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, X.; Zhang, H.; Feng, W.; Liu, Y.; Zhang, F.; Linhardt, R.J. A comparative secretome analysis of industrial Aspergillus oryzae and its spontaneous mutant ZJGS-LZ-21. Int. J. Food Microbiol. 2017, 248, 1–9. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, L.L.; Yao, Y.; Cao, Y.; Pan, Z.H.; Kong, D.H. Extracellular proteome analysis and flavor formation during soy sauce fermentation. Front. Microbiol. 2018, 9, 1872. [Google Scholar] [CrossRef]
- Benoit-Gelber, I.; Gruntjes, T.; Vinck, A.; van Veluw, J.G.; Wösten, H.A.B.; Boeren, S.; Vervoort, J.J.M.; de Vries, R.P. Mixed colonies of Aspergillus niger and Aspergillus oryzae cooperatively degrading wheat bran. Fungal Genet. Biol. 2017, 102, 31–37. [Google Scholar] [CrossRef]
- Goto, M. Protein O-glycosylation in fungi: Diverse structures and multiple functions. Biosci. Biotechnol. Biochem. 2007, 71, 1415–1427. [Google Scholar] [CrossRef]
- Deshpande, N.; Wilkins, M.R.; Packer, N.; Nevalainen, H. Protein glycosylation pathways in filamentous fungi. Glycobiology 2008, 18, 626–637. [Google Scholar] [CrossRef]
- Watanabe, T.; Totani, K.; Matsuo, I.; Maruyama, J.; Kitamoto, K.; Ito, Y. Genetic analysis of glucosidase II beta-subunit in trimming of high-mannose-type glycans. Glycobiology 2009, 19, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Matsuo, I.; Maruyama, J.; Kitamoto, K.; Ito, Y. Identification and characterization of an intracellular lectin, calnexin, from Aspergillus oryzae using N-glycan-conjugated beads. Biosci. Biotechnol. Biochem. 2007, 71, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kato, Y.; Asada, Y.; Nakajima, T. Filamentous fungus Aspergillus oryzae has two types of alpha-1,2-mannosidases, one of which is a microsomal enzyme that removes a single mannose residue from Man9GlcNAc2. Glycoconj. J. 2000, 17, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Yamaguchi, M.; Yahara, A.; Yoshiuchi, K.; Fujita, H.; Yamada, O.; Akita, O.; Ohmachi, T.; Asada, Y.; Yoshida, T. Cloning and expression of 1,2-alpha-mannosidase gene (fmanIB) from filamentous fungus Aspergillus oryzae: In vivo visualization of the FmanIBp-GFP fusion protein. Biosci. Biotechnol. Biochem. 2006, 70, 471–479. [Google Scholar] [CrossRef]
- Akao, T.; Yahara, A.; Sakamoto, K.; Yamada, O.; Akita, O.; Yoshida, T. Lack of endoplasmic reticulum 1,2-α-mannosidase activity that trims N-glycan Man9GlcNAc2 to Man8GlcNAc2 isomer B in a manE gene disruptant of Aspergillus oryzae. J. Biosci. Bioeng. 2012, 113, 438–441. [Google Scholar] [CrossRef]
- Kasajima, Y.; Yamaguchi, M.; Hirai, N.; Ohmachi, T.; Yoshida, T. In vivo expression of UDP-N-acetylglucosamine: Alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I (GnT-1) in Aspergillus oryzae and effects on the sugar chain of alpha-amylase. Biosci. Biotechnol. Biochem. 2006, 70, 2662–2668. [Google Scholar] [CrossRef][Green Version]
- Huynh, H.H.; Morita, N.; Sakamoto, T.; Katayama, T.; Miyakawa, T.; Tanokura, M.; Chiba, Y.; Shinkura, R.; Maruyama, J.I. Functional production of human antibody by the filamentous fungus Aspergillus oryzae. Fungal Biol. Biotechnol. 2020, 7, 7. [Google Scholar] [CrossRef]
- Li, Q.; Higuchi, Y.; Tanabe, K.; Katakura, Y.; Takegawa, K. Secretory production of N-glycan-deleted glycoprotein in Aspergillus oryzae. J. Biosci. Bioeng. 2020, 129, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Masai, K.; Maruyama, J.; Nakajima, H.; Kitamoto, K. In vivo visualization of the distribution of a secretory protein in Aspergillus oryzae hyphae using the RntA-EGFP fusion protein. Biosci. Biotechnol. Biochem. 2003, 67, 455–459. [Google Scholar] [CrossRef][Green Version]
- Kimura, S.; Maruyama, J.; Watanabe, T.; Ito, Y.; Arioka, M.; Kitamoto, K. In vivo imaging of endoplasmic reticulum and distribution of mutant α-amylase in Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 1044–1054. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Ishikawa, E.; Shoji, J.Y.; Nakano, H.; Kitamoto, K. Septum-directed secretion in the filamentous fungus Aspergillus oryzae. Mol. Microbiol. 2011, 81, 40–55. [Google Scholar] [CrossRef]
- Read, N.D. Exocytosis and growth do not occur only at hyphal tips. Mol. Microbiol. 2011, 81, 4–7. [Google Scholar] [CrossRef]
- Veerana, M.; Mitra, S.; Ki, S.H.; Kim, S.M.; Choi, E.H.; Lee, T.; Park, G. Plasma-mediated enhancement of enzyme secretion in Aspergillus oryzae. Microb. Biotechnol. 2021, 14, 262–276. [Google Scholar] [CrossRef]
- Steinberg, G.; Peñalva, M.A.; Riquelme, M.; Wösten, H.A.; Harris, S.D. Cell biology of hyphal growth. Microbiol. Spectr. 2017, 5, FUNK-0034-2016. [Google Scholar] [CrossRef]
- Schuster, M.; Martin-Urdiroz, M.; Higuchi, Y.; Hacker, C.; Kilaru, S.; Gurr, S.J.; Steinberg, G. Co-delivery of cell-wall-forming enzymes in the same vesicle for coordinated fungal cell wall formation. Nat. Microbiol. 2016, 1, 16149. [Google Scholar] [CrossRef]
- Maruyama, J.; Kikuchi, S.; Kitamoto, K. Differential distribution of the endoplasmic reticulum network as visualized by the BipA-EGFP fusion protein in hyphal compartments across the septum of the filamentous fungus, Aspergillus oryzae. Fungal Genet. Biol. 2006, 43, 642–654. [Google Scholar] [CrossRef]
- Hoang, H.D.; Maruyama, J.; Kitamoto, K. Modulating endoplasmic reticulum-Golgi cargo receptors for improving secretion of carrier-fused heterologous proteins in the filamentous fungus Aspergillus oryzae. Appl. Environ. Microbiol. 2015, 81, 533–543. [Google Scholar] [CrossRef]
- Liu, L.; Feizi, A.; Österlund, T.; Hjort, C.; Nielsen, J. Genome-scale analysis of the high-efficient protein secretion system of Aspergillus oryzae. BMC Syst. Biol. 2014, 8, 73. [Google Scholar] [CrossRef]
- Rothman, J.E.; Warren, G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 1994, 4, 220–233. [Google Scholar] [CrossRef]
- Kuratsu, M.; Taura, A.; Shoji, J.Y.; Kikuchi, S.; Arioka, M.; Kitamoto, K. Systematic analysis of SNARE localization in the filamentous fungus Aspergillus oryzae. Fungal Genet. Biol. 2007, 44, 1310–1323. [Google Scholar] [CrossRef]
- Higuchi, Y.; Takegawa, K. Single-molecule FISH reveals subcellular localization of α-amylase and actin mRNAs in the filamentous fungus Aspergillus oryzae. Front. Microbiol. 2020, 11, 578862. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; van den Bogaard, P.; Rifkin, S.A.; van Oudenaarden, A.; Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Method. 2008, 5, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, V. Unconventional protein secretion: An evolving mechanism. EMBO J. 2013, 32, 1660–1664. [Google Scholar] [CrossRef]
- Zhang, M.; Schekman, R. Cell biology. Unconventional secretion, unconventional solutions. Science 2013, 340, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.M.; Anjard, C.; Stefan, C.; Loomis, W.F.; Malhotra, V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 2010, 188, 527–536. [Google Scholar] [CrossRef]
- Curwin, A.J.; Brouwers, N.; Alonso, Y.; Adell, M.; Teis, D.; Turacchio, G.; Parashuraman, S.; Ronchi, P.; Malhotra, V. ESCRT-III drives the final stages of CUPS maturation for unconventional protein secretion. eLife 2016, 5, e16299. [Google Scholar] [CrossRef]
- Cruz-Garcia, D.; Brouwers, N.; Malhotra, V.; Curwin, A.J. Reactive oxygen species triggers unconventional secretion of antioxidants and Acb1. J. Cell Biol. 2020, 219, e201905028. [Google Scholar] [CrossRef]
- Reindl, M.; Hänsch, S.; Weidtkamp-Peters, S.; Schipper, K. A potential lock-type mechanism for unconventional secretion in fungi. Int. J. Mol. Sci. 2019, 20, 460. [Google Scholar] [CrossRef]
- Stock, J.; Sarkari, P.; Kreibich, S.; Brefort, T.; Feldbrügge, M.; Schipper, K. Applying unconventional secretion of the endochitinase Cts1 to export heterologous proteins in Ustilago maydis. J. Biotechnol. 2012, 161, 80–91. [Google Scholar] [CrossRef]
- Hussnaetter, K.P.; Philipp, M.; Müntjes, K.; Feldbrügge, M.; Schipper, K. Controlling unconventional secretion for production of heterologous proteins in Ustilago maydis through transcriptional regulation and chemical inhibition of the kinase Don3. J. Fungi 2021, 7, 179. [Google Scholar] [CrossRef]
- Kwon, H.S.; Kawaguchi, K.; Kikuma, T.; Takegawa, K.; Kitamoto, K.; Higuchi, Y. Analysis of an acyl-CoA binding protein in Aspergillus oryzae that undergoes unconventional secretion. Biochem. Biophys. Res. Commun. 2017, 493, 481–486. [Google Scholar] [CrossRef]
- Burggraaf, A.M.; Punt, P.J.; Ram, A.F. The unconventional secretion of PepN is independent of a functional autophagy machinery in the filamentous fungus Aspergillus niger. FEMS Microbiol. Lett. 2016, 363, fnw152. [Google Scholar] [CrossRef]
- Dimou, S.; Martzoukou, O.; Dionysopoulou, M.; Bouris, V.; Amillis, S.; Diallinas, G. Translocation of nutrient transporters to cell membrane via Golgi bypass in Aspergillus nidulans. EMBO Rep. 2020, 21, e49929. [Google Scholar] [CrossRef]
- Dimou, S.; Diallinas, G. Life and death of fungal transporters under the challenge of polarity. Int. J. Mol. Sci. 2020, 21, 5376. [Google Scholar] [CrossRef]
- Chanda, A.; Roze, L.V.; Kang, S.; Artymovich, K.A.; Hicks, G.R.; Raikhel, N.V.; Calvo, A.M.; Linz, J.E. A key role for vesicles in fungal secondary metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 19533–19538. [Google Scholar] [CrossRef]
- Keller, N.P. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 2015, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. From miso, saké and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006, 23, 1046–1062. [Google Scholar] [CrossRef]
- Terabayashi, Y.; Sano, M.; Yamane, N.; Marui, J.; Tamano, K.; Sagara, J.; Dohmoto, M.; Oda, K.; Ohshima, E.; Tachibana, K.; et al. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 953–961. [Google Scholar] [CrossRef]
- Arakawa, G.Y.; Kudo, H.; Yanase, A.; Eguchi, Y.; Kodama, H.; Ogawa, M.; Koyama, Y.; Shindo, H.; Hosaka, M.; Tokuoka, M. A unique Zn(II)2-Cys6-type protein, KpeA, is involved in secondary metabolism and conidiation in Aspergillus oryzae. Fungal Genet. Biol. 2019, 127, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Marui, J.; Yamane, N.; Ohashi-Kunihiro, S.; Ando, T.; Terabayashi, Y.; Sano, M.; Ohashi, S.; Ohshima, E.; Tachibana, K.; Higa, Y.; et al. Kojic acid biosynthesis in Aspergillus oryzae is regulated by a Zn(II)(2)Cys(6) transcriptional activator and induced by kojic acid at the transcriptional level. J. Biosci. Bioeng. 2011, 112, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Yoshie, T.; Wakai, S.; Asai-Nakashima, N.; Okazaki, F.; Ogino, C.; Hisada, H.; Tsutsumi, H.; Hata, Y.; Kondo, A. Aspergillus oryzae-based cell factory for direct kojic acid production from cellulose. Microb. Cell Fact. 2014, 13, 71. [Google Scholar] [CrossRef]
- Zhang, S.; Ban, A.; Ebara, N.; Mizutani, O.; Tanaka, M.; Shintani, T.; Gomi, K. Self-excising Cre/mutant lox marker recycling system for multiple gene integrations and consecutive gene deletions in Aspergillus oryzae. J. Biosci. Bioeng. 2017, 123, 403–411. [Google Scholar] [CrossRef]
- Oda, K.; Kobayashi, A.; Ohashi, S.; Sano, M. Aspergillus oryzae laeA regulates kojic acid synthesis genes. Biosci. Biotechnol. Biochem. 2011, 75, 1832–1834. [Google Scholar] [CrossRef]
- Sano, M. Aspergillus oryzae nrtA affects kojic acid production. Biosci. Biotechnol. Biochem. 2016, 80, 1776–1780. [Google Scholar] [CrossRef]
- Futagami, T.; Mori, K.; Yamashita, A.; Wada, S.; Kajiwara, Y.; Takashita, H.; Omori, T.; Takegawa, K.; Tashiro, K.; Kuhara, S.; et al. Genome sequence of the white koji mold Aspergillus kawachii IFO 4308, used for brewing the Japanese distilled spirit shochu. Eukaryot. Cell 2011, 10, 1586–1587. [Google Scholar] [CrossRef]
- Yamada, O.; Machida, M.; Hosoyama, A.; Goto, M.; Takahashi, T.; Futagami, T.; Yamagata, Y.; Takeuchi, M.; Kobayashi, T.; Koike, H.; et al. Genome sequence of Aspergillus luchuensis NBRC 4314. DNA Res. 2016, 23, 507–515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Futagami, T.; Mori, K.; Wada, S.; Ida, H.; Kajiwara, Y.; Takashita, H.; Tashiro, K.; Yamada, O.; Omori, T.; Kuhara, S.; et al. Transcriptomic analysis of temperature responses of Aspergillus kawachii during barley koji production. Appl. Environ. Microbiol. 2015, 81, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, C.; Izumitsu, K.; Onoue, M.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. Mitochondrial citrate transporters CtpA and YhmA are required for extracellular citric acid accumulation and contribute to cytosolic acetyl coenzyme A generation in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2019, 85, e03136-18. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, C.; Nakamura, E.; Mori, K.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. LaeA controls citric acid production through regulation of the citrate exporter-encoding cexA gene in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2020, 86, e01950-19. [Google Scholar] [CrossRef]
- Nakamura, E.; Kadooka, C.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. Citrate exporter enhances both extracellular and intracellular citric acid accumulation in the koji fungi Aspergillus luchuensis mut. kawachii and Aspergillus oryzae. J. Biosci. Bioeng. 2021, 131, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ghosh, R.N.; Maxfield, F.R. Endocytosis. Physiol. Rev. 1997, 77, 759–803. [Google Scholar] [CrossRef]
- Read, N.D.; Kalkman, E.R. Does endocytosis occur in fungal hyphae? Fungal Genet. Biol. 2003, 39, 199–203. [Google Scholar] [CrossRef]
- Vida, T.A.; Emr, S.D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 1995, 128, 779–792. [Google Scholar] [CrossRef]
- Peñalva, M.A. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 2005, 42, 963–975. [Google Scholar] [CrossRef]
- Higuchi, Y.; Nakahama, T.; Shoji, J.Y.; Arioka, M.; Kitamoto, K. Visualization of the endocytic pathway in the filamentous fungus Aspergillus oryzae using an EGFP-fused plasma membrane protein. Biochem. Biophys. Res. Commun. 2006, 340, 784–791. [Google Scholar] [CrossRef]
- Higuchi, Y.; Shoji, J.Y.; Arioka, M.; Kitamoto, K. Endocytosis is crucial for cell polarity and apical membrane recycling in the filamentous fungus Aspergillus oryzae. Eukaryot. Cell 2009, 8, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Arioka, M.; Kitamoto, K. Endocytic recycling at the tip region in the filamentous fungus Aspergillus oryzae. Commun. Integr. Biol. 2009, 2, 327–328. [Google Scholar] [CrossRef]
- Shaw, B.D.; Chung, D.W.; Wang, C.L.; Quintanilla, L.A.; Upadhyay, S. A role for endocytic recycling in hyphal growth. Fungal Biol. 2011, 115, 541–546. [Google Scholar] [CrossRef]
- Schultzhaus, Z.; Yan, H.; Shaw, B.D. Aspergillus nidulans flippase DnfA is cargo of the endocytic collar and plays complementary roles in growth and phosphatidylserine asymmetry with another flippase, DnfB. Mol. Microbiol. 2015, 97, 18–32. [Google Scholar] [CrossRef]
- Hernández-González, M.; Bravo-Plaza, I.; Pinar, M.; de Los Ríos, V.; Arst, H.N., Jr.; Peñalva, M.A. Endocytic recycling via the TGN underlies the polarized hyphal mode of life. PLoS Genet. 2018, 14, e1007291. [Google Scholar] [CrossRef]
- Berepiki, A.; Lichius, A.; Read, N.D. Actin organization and dynamics in filamentous fungi. Nat. Rev. Microbiol. 2011, 9, 876–887. [Google Scholar] [CrossRef]
- Higuchi, Y.; Arioka, M.; Kitamoto, K. Functional analysis of the putative AAA ATPase AipA localizing at the endocytic sites in the filamentous fungus Aspergillus oryzae. FEMS Microbiol. Lett. 2011, 320, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Higuchi, Y.; Kikuma, T.; Arioka, M.; Kitamoto, K. Functional analysis of Abp1p-interacting proteins involved in endocytosis of the MCC component in Aspergillus oryzae. Fungal Genet. Biol. 2013, 56, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, T.; Tanaka, M.; Ichikawa, T.; Matsuura, Y.; Hasegawa-Shiro, S.; Shintani, T.; Gomi, K. Endocytosis of a maltose permease is induced when amylolytic enzyme production is repressed in Aspergillus oryzae. Fungal Genet. Biol. 2015, 82, 136–144. [Google Scholar] [CrossRef]
- Tanaka, M.; Hiramoto, T.; Tada, H.; Shintani, T.; Gomi, K. Improved α-amylase production by dephosphorylation mutation of CreD, an arrestin-like protein required for glucose-induced endocytosis of maltose permease and carbon catabolite derepression in Aspergillus oryzae. Appl. Environ. Microbiol. 2017, 83, e00592-17. [Google Scholar] [CrossRef]
- Togo, Y.; Higuchi, Y.; Katakura, Y.; Takegawa, K. Early endosome motility mediates α-amylase production and cell differentiation in Aspergillus oryzae. Sci. Rep. 2017, 7, 15757. [Google Scholar] [CrossRef] [PubMed]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef]
- Abenza, J.F.; Galindo, A.; Pinar, M.; Pantazopoulou, A.; de los Ríos, V.; Peñalva, M.A. Endosomal maturation by Rab conversion in Aspergillus nidulans is coupled to dynein-mediated basipetal movement. Mol. Biol. Cell. 2012, 23, 1889–1901. [Google Scholar] [CrossRef]
- Higuchi, Y.; Ashwin, P.; Roger, Y.; Steinberg, G. Early endosome motility spatially organizes polysome distribution. J. Cell Biol. 2014, 204, 343–357. [Google Scholar] [CrossRef]
- Bankaitis, V.A.; Johnson, L.M.; Emr, S.D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc. Natl. Acad. Sci. USA 1986, 83, 9075–9079. [Google Scholar] [CrossRef]
- Rothman, J.H.; Stevens, T.H. Protein sorting in yeast: Mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell 1986, 47, 1041–1051. [Google Scholar] [CrossRef]
- Robinson, J.S.; Klionsky, D.J.; Banta, L.M.; Emr, S.D. Protein sorting in Saccharomyces cerevisiae: Isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell Biol. 1988, 8, 4936–4948. [Google Scholar] [CrossRef] [PubMed]
- Ohneda, M.; Arioka, M.; Kitamoto, K. Isolation and characterization of Aspergillus oryzae vacuolar protein sorting mutants. Appl. Environ. Microbiol. 2005, 71, 4856–4861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tatsumi, A.; Kikuma, T.; Arioka, M.; Kitamoto, K. Aovps24, a homologue of VPS24, is requir for vacuolar formation which could maintain proper growth and development in the filamentous fungus Aspergillus oryzae. Biochem. Biophys. Res. Commun. 2006, 347, 970–978. [Google Scholar] [CrossRef]
- Tatsumi, A.; Shoji, J.Y.; Kikuma, T.; Arioka, M.; Kitamoto, K. Aggregation of endosomal-vacuolar compartments in the Aovps24-deleted strain in the filamentous fungus Aspergillus oryzae. Biochem. Biophys. Res. Commun. 2007, 362, 474–479. [Google Scholar] [CrossRef]
- Egan, M.J.; McClintock, M.A.; Reck-Peterson, S.L. Microtubule-based transport in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 637–645. [Google Scholar] [CrossRef]
- Steinberg, G. Endocytosis and early endosome motility in filamentous fungi. Curr. Opin. Microbiol. 2014, 20, 10–18. [Google Scholar] [CrossRef]
- Higuchi, Y.; Steinberg, G. Early endosome motility in filamentous fungi: How and why they move. Fungal Biol. Rev. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Wedlich-Söldner, R.; Straube, A.; Friedrich, M.W.; Steinberg, G. A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis. EMBO J. 2002, 21, 2946–2957. [Google Scholar] [CrossRef]
- Lenz, J.H.; Schuchardt, I.; Straube, A.; Steinberg, G. A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J. 2006, 25, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Kilaru, S.; Ashwin, P.; Lin, C.; Severs, N.J.; Steinberg, G. Controlled and stochastic retention concentrates dynein at microtubule ends to keep endosomes on track. EMBO J. 2011, 30, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Lipowsky, R.; Assmann, M.A.; Lenz, P.; Steinberg, G. Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc. Natl. Acad. Sci. USA 2011, 108, 3618–3623. [Google Scholar] [CrossRef]
- Schuster, M.; Kilaru, S.; Fink, G.; Collemare, J.; Roger, Y.; Steinberg, G. Kinesin-3 and dynein cooperate in long-range retrograde endosome motility along a nonuniform microtubule array. Mol. Biol. Cell. 2011, 22, 3645–3657. [Google Scholar] [CrossRef]
- Bielska, E.; Schuster, M.; Roger, Y.; Berepiki, A.; Soanes, D.M.; Talbot, N.J.; Steinberg, G. Hook is an adapter that coordinates kinesin-3 and dynein cargo attachment on early endosomes. J. Cell Biol. 2014, 204, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, X.; Xiang, X. FHIP and FTS proteins are critical for dynein-mediated transport of early endosomes in Aspergillus. Mol. Biol. Cell. 2014, 25, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, R.; Arst, H.N., Jr.; Peñalva, M.A.; Xiang, X. HookA is a novel dynein-early endosome linker critical for cargo movement in vivo. J. Cell Biol. 2014, 204, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, S.C.; Schuster, M.; Bielska, E.; Dagdas, G.; Kilaru, S.; Meadows, B.R.; Schrader, M.; Steinberg, G. Peroxisomes, lipid droplets, and endoplasmic reticulum "hitchhike" on motile early endosomes. J. Cell Biol. 2015, 211, 945–954. [Google Scholar] [CrossRef]
- Bielska, E.; Higuchi, Y.; Schuster, M.; Steinberg, N.; Kilaru, S.; Talbot, N.J.; Steinberg, G. Long-distance endosome trafficking drives fungal effector production during plant infection. Nat. Commun. 2014, 5, 5097. [Google Scholar] [CrossRef]
- Hernández-González, M.; Pantazopoulou, A.; Spanoudakis, D.; Seegers, C.L.C.; Peñalva, M.A. Genetic dissection of the secretory route followed by a fungal extracellular glycosyl hydrolase. Mol. Microbiol. 2018, 109, 781–800. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Herman, P.K.; Emr, S.D. The fungal vacuole: Composition, function, and biogenesis. Microbiol. Rev. 1990, 54, 266–292. [Google Scholar] [CrossRef]
- Ohneda, M.; Arioka, M.; Nakajima, H.; Kitamoto, K. Visualization of vacuoles in Aspergillus oryzae by expression of CPY-EGFP. Fungal Genet. Biol. 2002, 37, 29–38. [Google Scholar] [CrossRef]
- Shoji, J.Y.; Arioka, M.; Kitamoto, K. Vacuolar Membrane Dynamics in the Filamentous Fungus Aspergillus oryzae. Eukaryot Cell. 2006, 5, 411–421. [Google Scholar] [CrossRef]
- Ashford, A.E. Dynamic pleiomorphic vacuole systems: Are they endosomes and transport compartments in fungal hyphae? Adv. Bot. Res. 1998, 28, 119–159. [Google Scholar]
- Klionsky, D.J.; Ohsumi, Y. Vacuolar import of proteins and organelles from the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999, 15, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Shoji, J.Y.; Kikuma, T.; Arioka, M.; Kitamoto, K. Macroautophagy-mediated degradation of whole nuclei in the filamentous fungus Aspergillus oryzae. PLoS ONE 2010, 5, e15650. [Google Scholar] [CrossRef] [PubMed]
- Kikuma, T.; Mitani, T.; Kohara, T.; Maruyama, J.I.; Kitamoto, K. Carbon and nitrogen depletion-induced nucleophagy and selective autophagic sequestration of a whole nucleus in multinucleate cells of the filamentous fungus Aspergillus oryzae. J. Gen. Appl. Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shoji, J.Y.; Arioka, M.; Kitamoto, K. Possible involvement of pleiomorphic vacuolar networks in nutrient recycling in filamentous fungi. Autophagy 2006, 2, 226–227. [Google Scholar] [CrossRef]
- Kikuma, T.; Arioka, M.; Kitamoto, K. Autophagy during conidiation and conidial germination in filamentous fungi. Autophagy 2007, 3, 128–129. [Google Scholar] [CrossRef]
- Tadokoro, T.; Kikuma, T.; Kitamoto, K. Functional analysis of AoAtg11 in selective autophagy in the filamentous fungus Aspergillus oryzae. Fungal Biol. 2015, 119, 560–567. [Google Scholar] [CrossRef]
- Kikuma, T.; Tadokoro, T.; Maruyama, J.I.; Kitamoto, K. AoAtg26, a putative sterol glucosyltransferase, is required for autophagic degradation of peroxisomes, mitochondria, and nuclei in the filamentous fungus Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2017, 81, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Kikuma, T.; Higuchi, Y.; Takegawa, K.; Kitamoto, K. Subcellular localization of acyl-CoA binding protein in Aspergillus oryzae is regulated by autophagy machinery. Biochem. Biophys. Res. Commun. 2016, 480, 8–12. [Google Scholar] [CrossRef]
- Van den Brink, H.J.; Petersen, S.G.; Rahbek-Nielsen, H.; Hellmuth, K.; Harboe, M. Increased production of chymosin by glycosylation. J. Biotechnol. 2006, 125, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Kołaczkowski, B.M.; Schaller, K.S.; Sørensen, T.H.; Peters, G.H.J.; Jensen, K.; Krogh, K.B.R.M.; Westh, P. Removal of N-linked glycans in cellobiohydrolase Cel7A from Trichoderma reesei reveals higher activity and binding affinity on crystalline cellulose. Biotechnol. Biofuels 2020, 13, 136. [Google Scholar] [CrossRef]
- Tegelaar, M.; Bleichrodt, R.J.; Nitsche, B.; Ram, A.F.J.; Wösten, H.A.B. Subpopulations of hyphae secrete proteins or resist heat stress in Aspergillus oryzae colonies. Environ. Microbiol. 2020, 22, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Maruyama, J.; Kitamoto, K. Aspergillus oryzae AoSO is a novel component of stress granules upon heat stress in filamentous fungi. PLoS ONE 2013, 8, e72209. [Google Scholar] [CrossRef]
- Herr, A.; Fischer, R. Improvement of Aspergillus nidulans penicillin production by targeting AcvA to peroxisomes. Metab. Eng. 2014, 25, 131–139. [Google Scholar] [CrossRef]
- Oikawa, H. Reconstitution of biosynthetic machinery of fungal natural products in heterologous hosts. Biosci. Biotechnol. Biochem. 2020, 84, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Tanaka, Y.; Okabe, T.; Nakamura, H.; Fujii, W.; Kitamoto, K.; Maruyama, J. Development of a genome editing technique using the CRISPR/Cas9 system in the industrial filamentous fungus Aspergillus oryzae. Biotechnol. Lett. 2016, 38, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Nakamura, H.; Zhang, Y.; Pascal, A.; Fujii, W.; Maruyama, J.I. Forced recycling of an AMA1-based genome-editing plasmid allows for efficient multiple gene deletion/integration in the industrial filamentous fungus Aspergillus oryzae. Appl. Environ. Microbiol. 2019, 85, e01896-18. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, Y. Membrane Traffic in Aspergillus oryzae and Related Filamentous Fungi. J. Fungi 2021, 7, 534. https://doi.org/10.3390/jof7070534
Higuchi Y. Membrane Traffic in Aspergillus oryzae and Related Filamentous Fungi. Journal of Fungi. 2021; 7(7):534. https://doi.org/10.3390/jof7070534
Chicago/Turabian StyleHiguchi, Yujiro. 2021. "Membrane Traffic in Aspergillus oryzae and Related Filamentous Fungi" Journal of Fungi 7, no. 7: 534. https://doi.org/10.3390/jof7070534
APA StyleHiguchi, Y. (2021). Membrane Traffic in Aspergillus oryzae and Related Filamentous Fungi. Journal of Fungi, 7(7), 534. https://doi.org/10.3390/jof7070534





