Higher Number of EBI3 Cells in Mucosal Chronic Hyperplastic Candidiasis May Serve to Regulate IL-17-Producing Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results
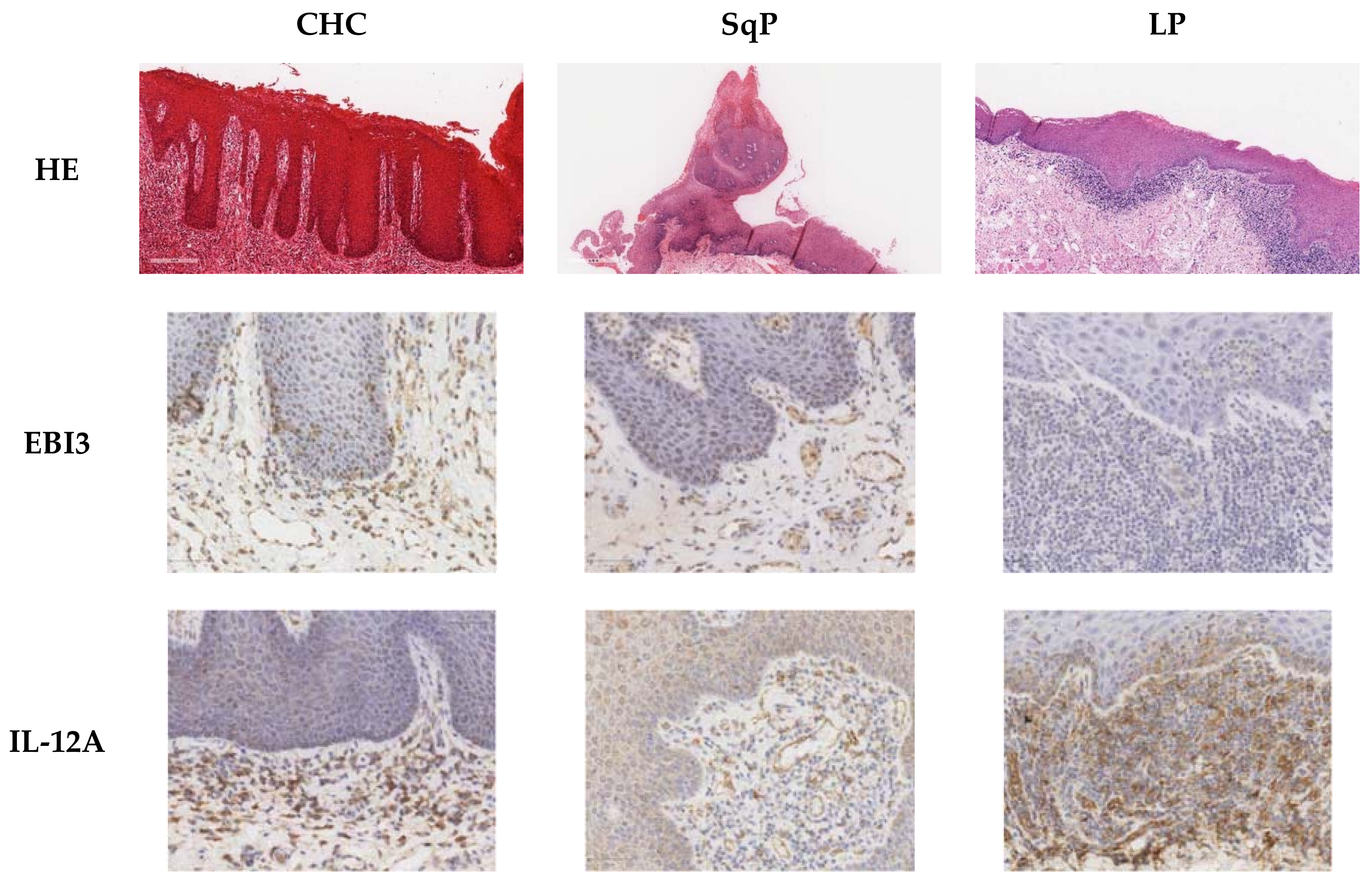
3.1. Detection of IL-17A, EBI,3 and IL-12A Cytokine-Producing Cells, and Foxp3+ Cells in the Corium and Epithelial Layer of the Oral Mucosa in CHC, SqP, and LP
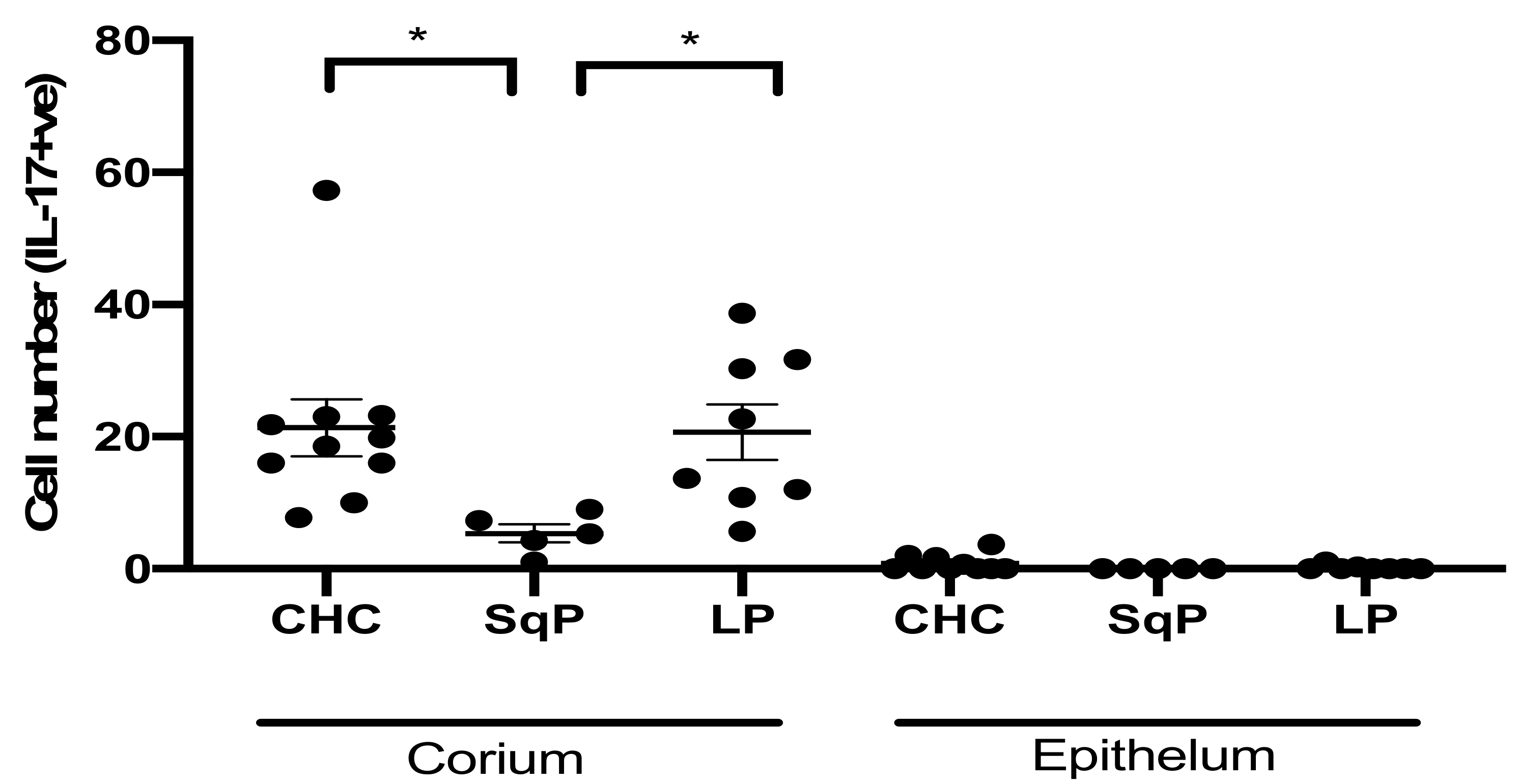
3.2. Comparison of IL-17A-Producing Cell Numbers in CHC, LP, and SqP
3.3. Comparison of Foxp3+ Cell Numbers in CHC, LP, and SqP
3.4. Comparison of EBI3- and IL-12A-Producing Cell Numbers in CHC, LP, and SqP
3.5. Correlation between EBI3- and IL-17A-Producing Cell Numbers in Tissue Sections
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dongari-Bagtzoglou, A.; Dwivedi, P.; Ioannidou, E.; Shaqman, M.; Hull, D.; Burleson, J. Oral Candida infection and colonization in solid organ transplant recipients. Oral Microbiol. Immunol. 2009, 24, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Merelli, M.; Righi, E.; Diaz-Martin, A.; Rosello, E.M.; Luzzati, R.; Parra, A.; Trecarichi, E.M.; Sanguinetti, M.; Posteraro, B.; et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J. Clin. Microbiol. 2013, 51, 4167–4172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, M.A.O.; Williams, D.W. Diagnosis and management of oral candidosis. Br. Dent. J. 2017, 223, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Banoczy, J.; Gintner, Z.; Dombi, C. Tobacco use and oral leukoplakia. J. Dent. Educ. 2001, 65, 322–327. [Google Scholar] [CrossRef]
- Dilhari, A.; Weerasekera, M.M.; Siriwardhana, A.; Maheshika, O.; Gunasekara, C.; Karunathilaka, S.; Nagahawatte, A.; Fernando, N. Candida infection in oral leukoplakia: An unperceived public health problem. Acta Odontol. Scand. 2016, 74, 565–569. [Google Scholar] [CrossRef]
- Williams, D.W.; Potts, A.J.; Wilson, M.J.; Matthews, J.B.; Lewis, M.A. Characterisation of the inflammatory cell infiltrate in chronic hyperplastic candidosis of the oral mucosa. J. Oral Pathol. Med. 1997, 26, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mohd Bakri, M.; Mohd Hussaini, H.; Rachel Holmes, A.; David Cannon, R.; Mary Rich, A. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J. Oral Microbiol. 2010, 2. [Google Scholar] [CrossRef]
- Sankari, S.L.; Gayathri, K.; Balachander, N.; Malathi, L. Candida in potentially malignant oral disorders. J. Pharm. Bioallied Sci. 2015, 7, S162–S164. [Google Scholar] [CrossRef]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Zhu, H.W.; McMillan, A.S.; McGrath, C.; Li, L.S.; Samaranayake, L.P. Oral carriage of yeasts and coliforms in stroke sufferers: A prospective longitudinal study. Oral Dis. 2008, 14, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Lamey, P.J.; Lewis, M.A.; MacDonald, D.G. Treatment of candidal leukoplakia with fluconazole. Br. Dent. J. 1989, 166, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Peterson, A.C.; Brane, L.; Huppler, A.R.; Hernandez-Santos, N.; Whibley, N.; Garg, A.V.; Simpson-Abelson, M.R.; Gibson, G.A.; Mamo, A.J.; et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J. Exp. Med. 2014, 211, 2075–2084. [Google Scholar] [CrossRef]
- Kashem, S.W.; Riedl, M.S.; Yao, C.; Honda, C.N.; Vulchanova, L.; Kaplan, D.H. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 2015, 43, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Trautwein-Weidner, K.; Gladiator, A.; Nur, S.; Diethelm, P.; LeibundGut-Landmann, S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015, 8, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Shears, R.K.; Sharma, R.; Krishna, M.; Webb, C.; Ali, R.; Wei, X.; Kadioglu, A.; Zhang, Q. IL-35 is critical in suppressing superantigenic Staphylococcus aureus-driven inflammatory Th17 responses in human nasopharynx-associated lymphoid tissue. Mucosal Immunol. 2020, 13, 460–470. [Google Scholar] [CrossRef]
- Williams, A.; Williams, D.; Rogers, H.; Wei, X.; Lewis, M.; Wozniak, S.; Farnell, D.; Jones, A. Immunohistochemical Expression Patterns of Inflammatory Cells Involved in Chronic Hyperplastic Candidosis. Pathogens 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.F.; Hong, Y.X.; Feng, G.J.; Zhang, G.F.; Rogers, H.; Lewis, M.A.; Williams, D.W.; Xia, Z.F.; Song, B.; Wei, X.Q. Lipopolysaccharide-induced M2 to M1 macrophage transformation for IL-12p70 production is blocked by Candida albicans mediated up-regulation of EBI3 expression. PLoS ONE 2013, 8, e63967. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.Q.; Rogers, H.; Lewis, M.A.; Williams, D.W. The role of the IL-12 cytokine family in directing T-cell responses in oral candidosis. Clin. Dev. Immunol. 2011, 2011, 697340. [Google Scholar] [CrossRef] [Green Version]
- Salkowska, A.; Karas, K.; Karwaciak, I.; Walczak-Drzewiecka, A.; Krawczyk, M.; Sobalska-Kwapis, M.; Dastych, J.; Ratajewski, M. Identification of Novel Molecular Markers of Human Th17 Cells. Cells 2020, 9, 1611. [Google Scholar] [CrossRef]
- Zhang, S. The role of transforming growth factor beta in T helper 17 differentiation. Immunology 2018, 155, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Casanova, J.L.; Puel, A. Mucocutaneous IL-17 immunity in mice and humans: Host defense vs. excessive inflammation. Mucosal Immunol. 2018, 11, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.H.; Richardson, J.P.; Zhou, C.; Coleman, B.M.; Moyes, D.L.; Ho, J.; Huppler, A.R.; Ramani, K.; McGeachy, M.J.; Mufazalov, I.A.; et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| IL-17A in Lamina Propria of CHC | EBI3 | Foxp3 |
| ρ | 0.55 | 0.08 |
| p = | 0.13 | 0.85 |
| n | 9 | 9 |
| IL-17A in Lamina Propria of Lichen Planus | EBI3 | Foxp3 |
| ρ | 0.116 | 0.524 |
| p = | 0.827 | 0.183 |
| n | 6 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.; Rogers, H.; Williams, D.; Wei, X.-Q.; Farnell, D.; Wozniak, S.; Jones, A. Higher Number of EBI3 Cells in Mucosal Chronic Hyperplastic Candidiasis May Serve to Regulate IL-17-Producing Cells. J. Fungi 2021, 7, 533. https://doi.org/10.3390/jof7070533
Williams A, Rogers H, Williams D, Wei X-Q, Farnell D, Wozniak S, Jones A. Higher Number of EBI3 Cells in Mucosal Chronic Hyperplastic Candidiasis May Serve to Regulate IL-17-Producing Cells. Journal of Fungi. 2021; 7(7):533. https://doi.org/10.3390/jof7070533
Chicago/Turabian StyleWilliams, Ailish, Helen Rogers, David Williams, Xiao-Qing Wei, Damian Farnell, Sue Wozniak, and Adam Jones. 2021. "Higher Number of EBI3 Cells in Mucosal Chronic Hyperplastic Candidiasis May Serve to Regulate IL-17-Producing Cells" Journal of Fungi 7, no. 7: 533. https://doi.org/10.3390/jof7070533
APA StyleWilliams, A., Rogers, H., Williams, D., Wei, X.-Q., Farnell, D., Wozniak, S., & Jones, A. (2021). Higher Number of EBI3 Cells in Mucosal Chronic Hyperplastic Candidiasis May Serve to Regulate IL-17-Producing Cells. Journal of Fungi, 7(7), 533. https://doi.org/10.3390/jof7070533






