Making Traditional Japanese Distilled Liquor, Shochu and Awamori, and the Contribution of White and Black Koji Fungi
Abstract
1. Introduction
2. Making Method of Single Distilled Shochu
3. Koji Fungi Used for Shochu and Awamori
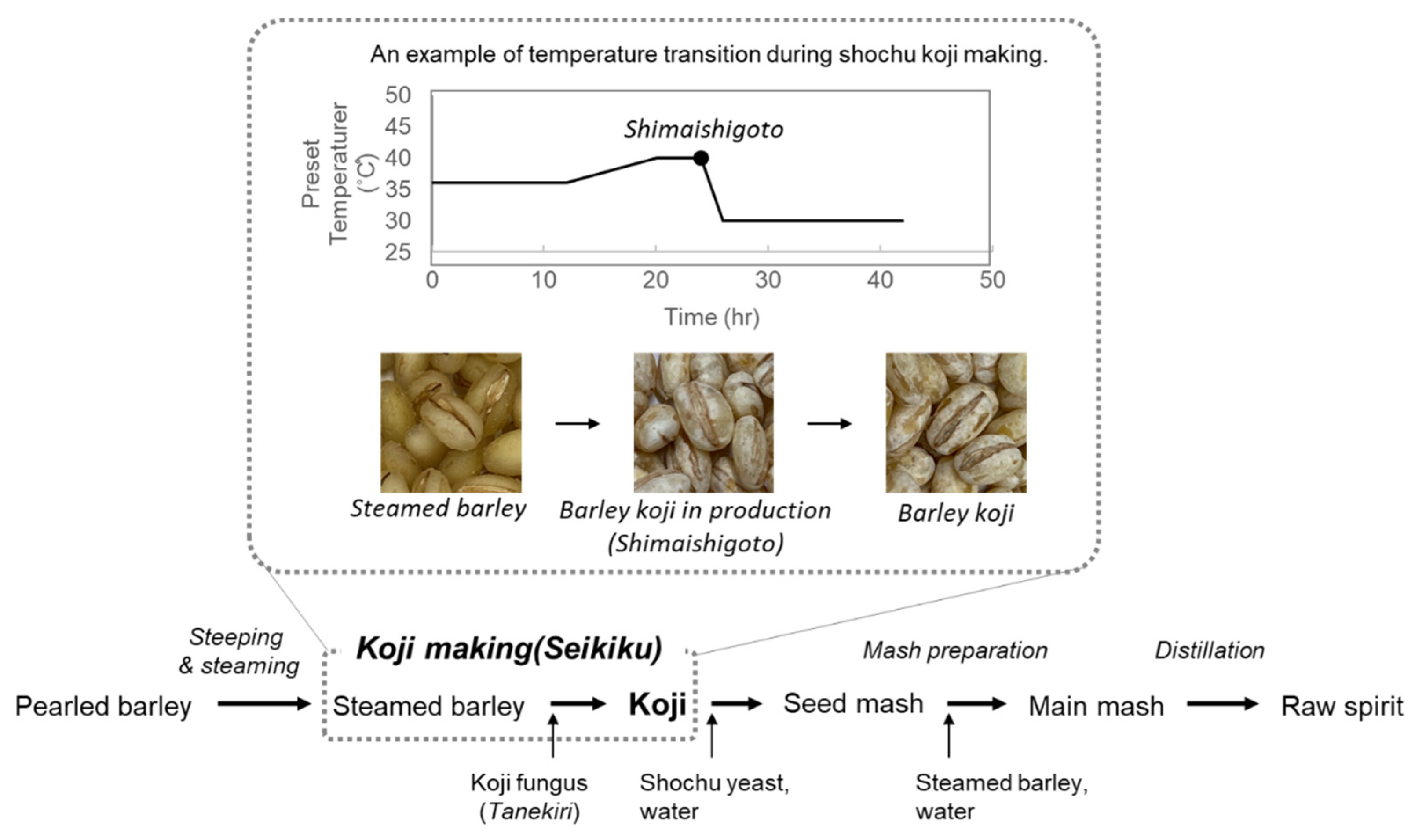
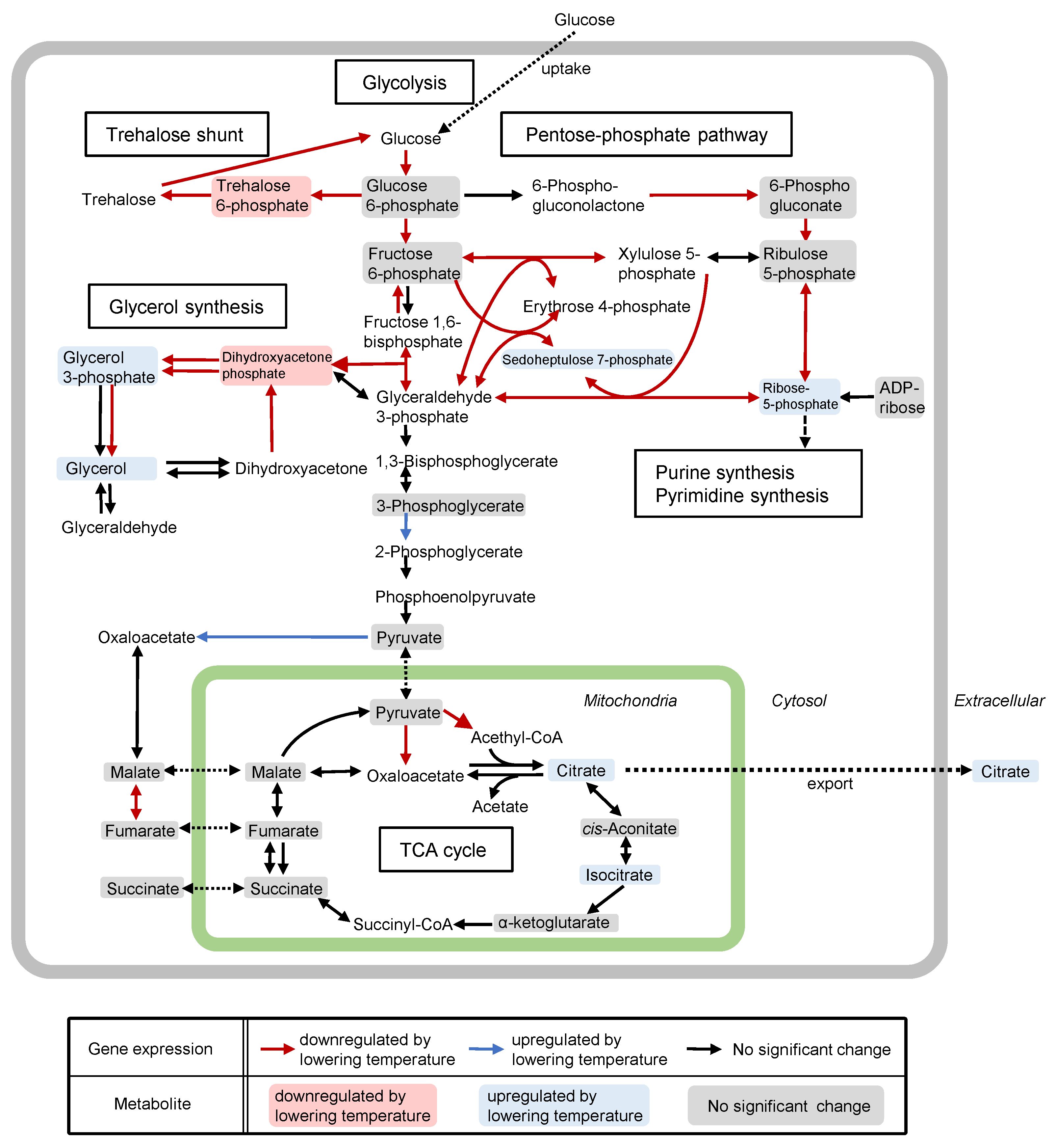
4. Temperature Control for Shochu Koji Making
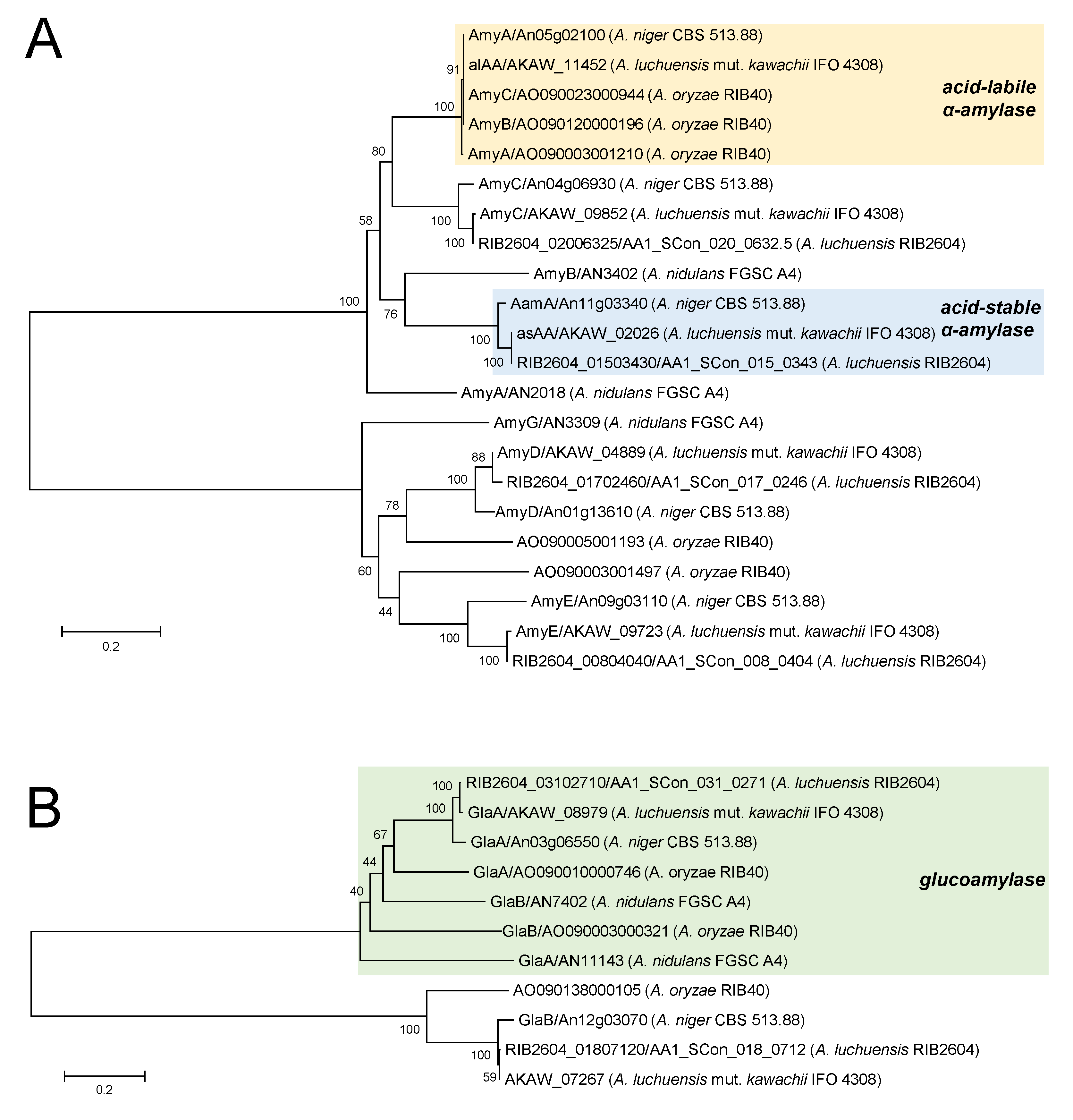
5. Amylolytic Enzyme System in Koji
6. Citric Acid Production in Koji Fungi
7. Genome Analysis and Editing of Koji Fungi
8. Diversity of Shochu Flavor
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitamura, Y.; Kusumoto, K.; Oguma, T.; Nagai, T.; Furukawa, S.; Suzuki, C.; Satomi, M.; Magariyama, Y.; Takamine, K.; Tamaki, H. Ethnic Fermented Foods and Alcoholic Beverages of Japan. Ethn. Fermented Foods Alcohol. Beverages Asia 2016, 193–236. [Google Scholar] [CrossRef]
- Iwano, K.; Mikami, S.; Fukuda, K.; Shiinoki, S.; Shimada, T. Correlation among the various enzyme activities of shochu koji and dissolution, saccharification and fermentation. J. Brew. Soc. Jpn. 1986, 81, 554–557. [Google Scholar] [CrossRef]
- Yamaoka, H. Brewing of awamori. J. Brew. Soc. Jpn. 2001, 96, 736–742. [Google Scholar] [CrossRef][Green Version]
- Nunokawa, Y. Seishukoji, Koji-gaku; Murakami, H., Ed.; Brewing Society of Japan: Tokyo, Japan, 1986; pp. 217–219. [Google Scholar]
- Nishiya, N. Shochukoji, Koji-gaku; Murakami, H., Ed.; Brewing Society of Japan: Tokyo, Japan, 1986; pp. 296–300. [Google Scholar]
- Shirakami, H. Moromi, Manufacturing Technology of Honkaku Shochu; Brewing Society of Japan: Tokyo, Japan, 1991; pp. 114–120. [Google Scholar]
- Nagatani, M. Joryu, Manufacturing Technology of Honkaku Shochu; Brewing Society of Japan: Tokyo, Japan, 1991; pp. 165–173. [Google Scholar]
- Kawachi, G. Kurokoji; Kagoshimakenshuzokumiairengokai: Kagoshima, Japan, 1919; pp. 1–2. [Google Scholar] [CrossRef]
- Kitahara, K.; Kurushima, M. Is Aspergillus kawachii really a mutant of black Aspergillus? J. Ferment. Technol. 1949, 27, 182–183. [Google Scholar]
- Futagami, T.; Tamaki, H.; Goto, M.; Takamine, K. Shochugaku-eno Izanai. Seibutsu-Kogaku Kaishi 2019, 97, 82–86. (In Japanese) [Google Scholar]
- Yamada, O.; Takara, R.; Hamada, R.; Hayashi, R.; Tsukahara, M.; Mikami, S. Molecular biological researches of Kuro-Koji molds, their classification and safety. J. Biosci. Bioeng. 2011, 112, 233–237. [Google Scholar] [CrossRef]
- Hong, S.B.; Lee, M.; Kim, D.H.; Varga, J.; Frisvad, J.C.; Perrone, G.; Gomi, K.; Yamada, O.; Machida, M.; Houbraken, J.; et al. Aspergillus luchuensis, an industrially important black Aspergillus in East Asia. PLoS ONE 2013, 8, e63769. [Google Scholar] [CrossRef]
- Hong, S.B.; Yamada, O.; Samson, R.A. Taxonomic re-evaluation of black koji molds. Appl. Microbiol. Biotechnol. 2014, 98, 555–561. [Google Scholar] [CrossRef]
- Iwano, K.; Mikami, S.; Fukuda, K.; Nose, A.; Shiinoki, S. Influence of cultural conditions on various enzyme activities of shochu koji. J. Brew. Soc. Jpn. 1987, 82, 200–204. [Google Scholar] [CrossRef]
- Omori, T.; Takeshima, N.; Shimoda, M. Formation of acid-labile α-amylase during barley-koji production. J. Ferment. Bioeng. 1994, 78, 27–30. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Takeshima, N.; Ohba, H.; Omori, T.; Shimoda, M.; Wada, H. Production of acid-stable α-amylase by Aspergillus kawachii during barley Shochu-Koji production. J. Ferment. Bioeng. 1997, 84, 224–227. [Google Scholar] [CrossRef]
- Futagami, T.; Mori, K.; Wada, S.; Ida, H.; Kajiwara, Y.; Takashita, H.; Tashiro, K.; Yamada, O.; Omori, T.; Kuhara, S.; et al. Transcriptomic analysis of temperature responses of Aspergillus kawachii during barley koji production. Appl. Environ. Microbiol. 2015, 81, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, C.; Izumitsu, K.; Onoue, M.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. Mitochondrial citrate transporters CtpA and YhmA are required for extracellular citric acid accumulation and contribute to cytosolic acetyl coenzyme A generation in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2019, 85, e03136-18. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, C.; Nakamura, E.; Mori, K.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. LaeA controls citric acid production through regulation of the citrate exporter-encoding cexA gene in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2020, 86, e01950-19. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K. Regulatory mechanisms for amylolytic gene expression in the koji mold Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2019, 83, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Hata, Y.; Ichikawa, E.; Kawato, A.; Suginami, K.; Imayasu, S. Regulation of the glucoamylase-encoding gene (glaB), expressed in solid-state culture (koji) of Aspergillus oryzae. J. Ferment. Bioeng. 1998, 86, 301–307. [Google Scholar] [CrossRef]
- Kaneko, A.; Sudo, S.; Takayasu-Sakamoto, Y.; Tamura, G.; Ishikawa, T.; Oba, T. Molecular cloning and determination of the nucleotide sequence of a gene encoding an acid-stable α-amylase from Aspergillus kawachii. J. Ferment. Bioeng. 1996, 81, 292–298. [Google Scholar] [CrossRef]
- Suganuma, T.; Fujita, K.; Kitahara, K. Some distinguishable properties between acid-stable and neutral types of alpha-amylases from acid-producing koji. J. Biosci. Bioeng. 2007, 104, 353–362. [Google Scholar] [CrossRef]
- Hayashida, S.; Nakahara, K.; Kuroda, K.; Miyata, T.; Iwanaga, S. Structure of the raw-starch-affinity site on the Aspergillus awamori var. kawachi glucoamylase I molecule. Agric. Biol. Chem. 1989, 53, 135–141. [Google Scholar] [CrossRef]
- Goto, M.; Semimaru, T.; Furukawa, K.; Hayashida, S. Analysis of the raw starch-binding domain by mutation of a glucoamylase from Aspergillus awamori var. kawnchi expressed in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1994, 60, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Tagawa, Y.; Yoshizaki, Y.; Wang, T.; Yamaguchi, M.; Kadooka, C.; Okutsu, K.; Futagami, T.; Tamaki, H.; Takamine, K. The expression profiles of acid-stable α-amylase and acid-labile α-amylase of Aspergillus luchuensis mut. kawachii effect on the microstructure of koji and alcohol fermentation. LWT 2021, 139, 110580. [Google Scholar] [CrossRef]
- de Groot, P.W.; Brandt, B.W.; Horiuchi, H.; Ram, A.F.; de Koster, C.G.; Klis, F.M. Comprehensive genomic analysis of cell wall genes in Aspergillus nidulans. Fungal. Genet. Biol. 2009, 46 (Suppl. 1), S72–S81. [Google Scholar] [CrossRef]
- Karaffa, L.; Kubicek, C.P. Aspergillus niger citric acid accumulation: Do we understand this well working black box? Appl. Microbiol. Biotechnol. 2003, 61, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Torres, N. Modelling approach to control of carbohydrate metabolism during citric acid accumulation by Aspergillus niger. I. Model definition and stability of the steady state. Biotechnol. Bioeng. 1994, 44, 104–111. [Google Scholar] [CrossRef]
- Torres, N. Modelling approach to control of carbohydrate metabolism during citric acid accumulation by Aspergillus niger. II. Sensitivity analysis. Biotechnol. Bioeng. 1994, 44, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Vasquez, F.; González-Alcón, C.; Torres, N.V. Metabolism of citric acid production by Aspergillus niger: Model definition, steady-state analysis and constrained optimization of citric acid production rate. Biotechnol. Bioeng. 2000, 70, 82–108. [Google Scholar] [CrossRef]
- Odoni, D.I.; Vazquez-Vilar, M.; van Gaal, M.P.; Schonewille, T.; Martins Dos Santos, V.A.P.; Tamayo-Ramos, J.A.; Suarez-Diez, M.; Schaap, P.J. Aspergillus niger citrate exporter revealed by comparison of two alternative citrate producing conditions. FEMS Microbiol. Lett. 2019, 366, fnz071. [Google Scholar] [CrossRef]
- Steiger, M.G.; Rassinger, A.; Mattanovich, D.; Sauer, M. Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger. Metab. Eng. 2019, 52, 224–231. [Google Scholar] [CrossRef]
- Nakamura, E.; Kadooka, C.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. Citrate exporter enhances both extracellular and intracellular citric acid accumulation in the koji fungi Aspergillus luchuensis mut. kawachii and Aspergillus oryzae. J. Biosci. Bioeng. 2021, 131, 68–76. [Google Scholar] [CrossRef]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef]
- Futagami, T.; Mori, K.; Yamashita, A.; Wada, S.; Kajiwara, Y.; Takashita, H.; Omori, T.; Takegawa, K.; Tashiro, K.; Kuhara, S.; et al. Genome sequence of the white koji mold Aspergillus kawachii IFO 4308, used for brewing the Japanese distilled spirit shochu. Eukaryot. Cell. 2011, 10, 1586–1587. [Google Scholar] [CrossRef] [PubMed]
- Yamada, O.; Machida, M.; Hosoyama, A.; Goto, M.; Takahashi, T.; Futagami, T.; Yamagata, Y.; Takeuchi, M.; Kobayashi, T.; Koike, H.; et al. Genome sequence of Aspergillus luchuensis NBRC 4314. DNA Res. 2016, 23, 507–515. [Google Scholar] [CrossRef][Green Version]
- Takahashi, T.; Mizutani, O.; Shiraishi, Y.; Yamada, O. Development of an efficient gene-targeting system in Aspergillus luchuensis by deletion of the non-homologous end joining system. J. Biosci. Bioeng. 2011, 112, 529–534. [Google Scholar] [CrossRef]
- Tashiro, S.; Futagami, T.; Wada, S.; Kajiwara, Y.; Takashita, H.; Omori, T.; Takahashi, T.; Yamada, O.; Takegawa, K.; Goto, M. Construction of a ligD disruptant for efficient gene targeting in white koji mold, Aspergillus kawachii. J. Gen. Appl. Microbiol. 2013, 59, 257–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadooka, C.; Yamaguchi, M.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Katayama, T.; Maruyama, J.; Tamaki, H.; Futagami, T. A CRISPR/Cas9-mediated gene knockout system in Aspergillus luchuensis mut. kawachii. Biosci. Biotechnol. Biochem. 2020, 84, 2179–2183. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Kida, M.; Maki, N.; Isogai, A.; Iwata, H.; Nishiya, T. Analysis of Shochu and White Spirits and Classification by Their Compounds. J. Brew. Soc. Jpn. 2006, 101, 446–457. [Google Scholar] [CrossRef][Green Version]
- Takashita, H. Study on Flavor-Taste of Barley-Shochu. J. Brew. Soc. Jpn. 2012, 107, 381–388. [Google Scholar] [CrossRef]
- Takamine, K.; Sameshima, Y. The Contribution Factor for Flavor of Sweetpotato Shochu. J. Brew. Soc. Jpn. 2008, 103, 601–606. [Google Scholar] [CrossRef][Green Version]
- Ando, Y. Search for Characteristic Flavor and Taste of Shochu. J. Brew. Soc. Jpn. 2012, 107, 300–305. [Google Scholar] [CrossRef][Green Version]
- Sakaida, H.; Nakahara, N.; Watashi, N.; Kai, T.; Nakashima, Y.; Sakakibara, Y.; Nishiyama, K.; Fukuda, N.; Suiko, M. Characteristic Flavor of Buckwheat Shochu and Comparison of Volatile Compounds from Variety Cereal Shochu. Nippon Shokuhin Kogaku Kaishi 2003, 50, 555–562. [Google Scholar] [CrossRef]
- Kataoka, R.; Watanabe, T.; Hayashi, R.; Isogai, A.; Yamada, O.; Ogihara, J. Awamori fermentation test and 1-octen-3-ol productivity analysis using fatty acid oxygenase disruptants of Aspergillus luchuensis. J. Biosci. Bioeng. 2020, 130, 489–495. [Google Scholar] [CrossRef]
- Kataoka, R.; Watanabe, T.; Yano, S.; Mizutani, O.; Yamada, O.; Kasumi, T.; Ogihara, J. Aspergillus luchuensis fatty acid oxygenase ppoC is necessary for 1-octen-3-ol biosynthesis in rice koji. J. Biosci. Bioeng. 2020, 129, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Koseki, T.; Furuse, S.; Iwano, K.; Matsuzawa, H. Purification and characterization of a feruloylesterase from Aspergillus awamori. Biosci. Biotechnol. Biochem. 1998, 62, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Tokashiki, M.; Tokashiki, M.; Uechi, K.; Ito, S.; Taira, T. Characterization and induction of phenolic acid decarboxylase from Aspergillus luchuensis. J. Biosci. Bioeng. 2018, 126, 162–168. [Google Scholar] [CrossRef]
- Maeda, M.; Motosoko, M.; Tokashiki, T.; Tokashiki, J.; Mizutani, O.; Uechi, K.; Goto, M.; Taira, T. Phenolic acid decarboxylase of Aspergillus luchuensis plays a crucial role in 4-vinylguaiacol production during awamori brewing. J. Biosci. Bioeng. 2020, 130, 352–359. [Google Scholar] [CrossRef]
- Futagami, T.; Kadooka, C.; Ando, Y.; Okutsu, K.; Yoshizaki, Y.; Setoguchi, S.; Takamine, K.; Kawai, M.; Tamaki, H. Multi-gene phylogenetic analysis reveals that shochu-fermenting Saccharomyces cerevisiae strains form a distinct sub-clade of the Japanese sake cluster. Yeast 2017, 34, 407–415. [Google Scholar] [CrossRef]
- Mori, K.; Kadooka, C.; Masuda, C.; Muto, A.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Futagami, T.; Tamaki, H. Genome sequence of Saccharomyces cerevisiae strain Kagoshima No. 2, used for brewing the Japanese distilled spirit shōchū. Genome Announc. 2017, 5, e01126-17. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Mori, K.; Tashiro, K.; Higuchi, Y.; Takegawa, K.; Takashita, H. Genomic sequence of Saccharomyces cerevisiae BAW-6, a yeast strain optimal for brewing barley shochu. Genome Announc. 2018, 6, e00228-18. [Google Scholar] [CrossRef]
- Oishi, M.; Tanoue, Y.; Kajiwara, Y.; Takashita, H.; Okazaki, N. Sensory attributs of furfural formed in barley-shochu making and its formation factors. J. Brew. Soc. Jpn. 2008, 103, 730–734. [Google Scholar] [CrossRef][Green Version]
- Oishi, M.; Nekogaki, K.; Kajiwara, Y.; Takashita, H.; Shimoda, M.; Okazaki, N. Sensory attributes and classification of odor compounds in barley-shochu. J. Brew. Soc. Jpn. 2013, 108, 113–121. [Google Scholar] [CrossRef]
- Miyamoto, M. The Awamori Flavor Wheel. J. Brew. Soc. Jpn. 2018, 113, 536–543. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Kajiwara, Y.; Futagami, T.; Goto, M.; Takashita, H. Making Traditional Japanese Distilled Liquor, Shochu and Awamori, and the Contribution of White and Black Koji Fungi. J. Fungi 2021, 7, 517. https://doi.org/10.3390/jof7070517
Hayashi K, Kajiwara Y, Futagami T, Goto M, Takashita H. Making Traditional Japanese Distilled Liquor, Shochu and Awamori, and the Contribution of White and Black Koji Fungi. Journal of Fungi. 2021; 7(7):517. https://doi.org/10.3390/jof7070517
Chicago/Turabian StyleHayashi, Kei, Yasuhiro Kajiwara, Taiki Futagami, Masatoshi Goto, and Hideharu Takashita. 2021. "Making Traditional Japanese Distilled Liquor, Shochu and Awamori, and the Contribution of White and Black Koji Fungi" Journal of Fungi 7, no. 7: 517. https://doi.org/10.3390/jof7070517
APA StyleHayashi, K., Kajiwara, Y., Futagami, T., Goto, M., & Takashita, H. (2021). Making Traditional Japanese Distilled Liquor, Shochu and Awamori, and the Contribution of White and Black Koji Fungi. Journal of Fungi, 7(7), 517. https://doi.org/10.3390/jof7070517





