Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections
Abstract
1. Introduction
2. Literature Review
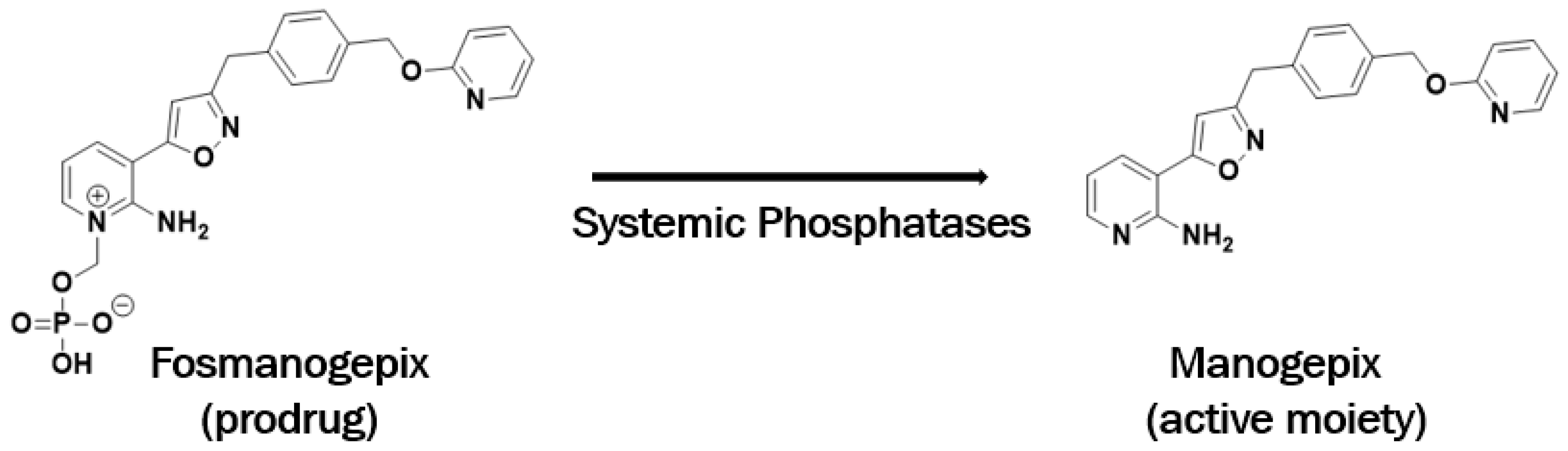
3. Discovery of Manogepix
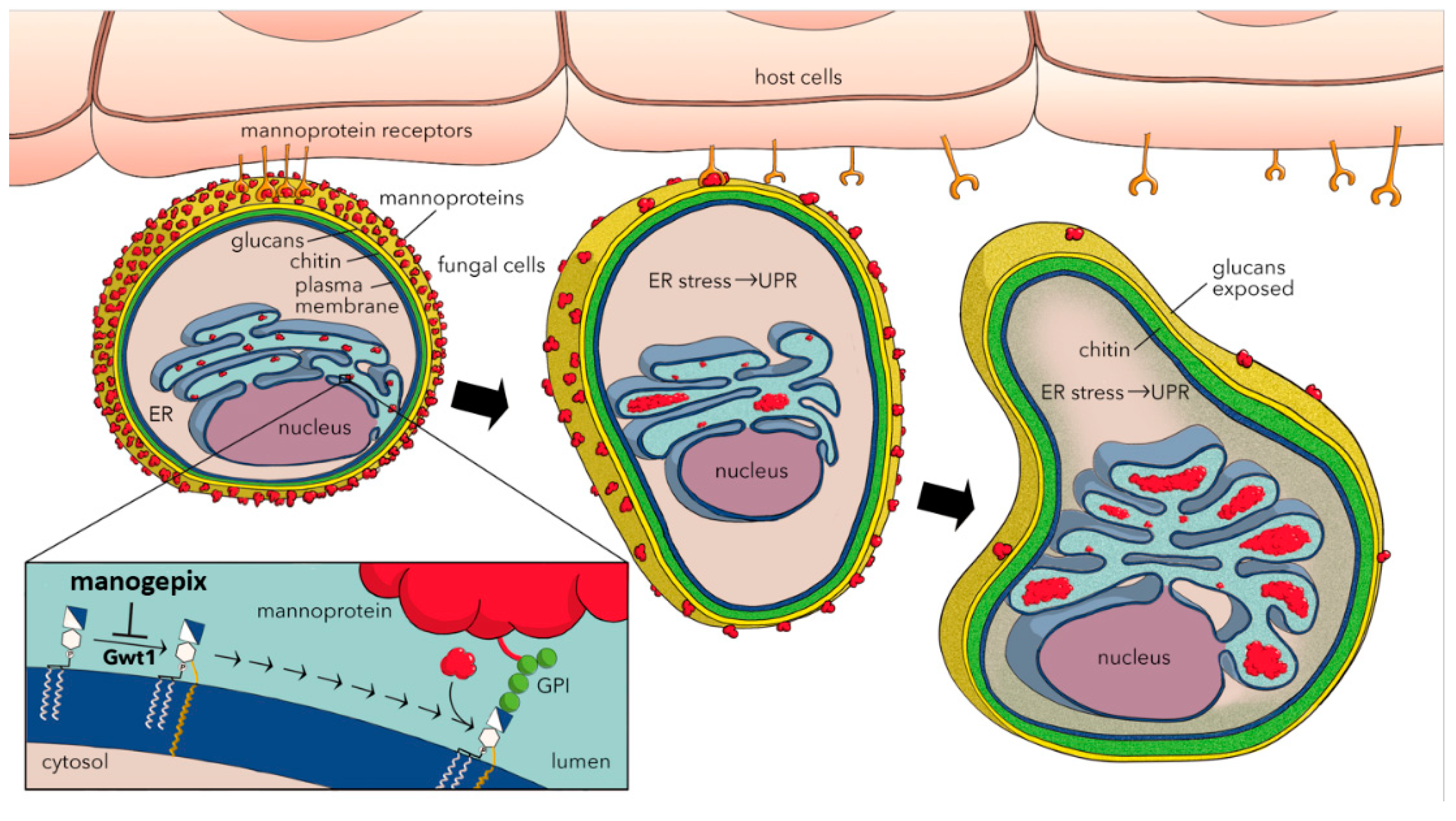
4. Mechanism of Action
5. Effects on Virulence Factors and Biofilms
6. Time Kill and Post Antifungal Effects
7. MGX In Vitro Activity against Yeasts
8. MGX In Vitro Activity against Molds
9. Resistance
10. Activity against Echinocandin-, Azole- and AMB-Resistant Candida spp.
11. Activity against Azole- and AMB-Resistant Aspergillus spp.
12. Pharmacokinetics/Pharmacodynamics
13. In Vivo Efficacy
14. Tissue Distribution
15. Phase 1 Clinical Trials
16. Phase 2 Clinical Trials
17. Regulatory Submissions
18. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Laniado-Laborin, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Revista Iberoamericana de Micología 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Chiller, T. The Rise in Antifungal Resistance. 2016. Available online: https://www.medscape.com/viewarticle/861041#vp_1 (accessed on 4 April 2016).
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida auris Isolates Resistant to Three Classes of Antifungal Medications—New York, 2019. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory Analysis of an Outbreak of Candida auris in New York from 2016 to 2018: Impact and Lessons Learned. J. Clin. Microbiol. 2019, 58, 58. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Patterson, T.F. Emergence of Azole Resistance in Aspergillus. Semin. Respir. Crit. Care Med. 2015, 36, 673–680. [Google Scholar] [CrossRef]
- Liu, Y.; Mittal, R.; Solis, N.V.; Prasadarao, N.V.; Filler, S.G. Mechanisms of Candida albicans Trafficking to the Brain. PLOS Pathog. 2011, 7, e1002305. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance. Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar] [CrossRef]
- Osherov, N.; Kontoyiannis, D.P. The anti-Aspergillus drug pipeline: Is the glass half full or empty? Med. Mycol. 2017, 55, 118–124. [Google Scholar] [CrossRef][Green Version]
- Cortez, K.J.; Roilides, E.; Quiroz-Telles, F.; Meletiadis, J.; Antachopoulos, C.; Knudsen, T.; Buchanan, W.; Milanovich, J.; Sutton, D.A.; Fothergill, A.; et al. Infections Caused by Scedosporium spp. Clin. Microbiol. Rev. 2008, 21, 157–197. [Google Scholar] [CrossRef]
- Bhatt, V.R.; Viola, G.M.; Ferrajoli, A. Invasive fungal infections in acute leukemia. Ther. Adv. Hematol. 2011, 2, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, B.; Schilling, A.; Anagnostopolous, I.; Siehl, I.; Thiel, E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma. 2004, 45, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A. Zygomycosis: Reemergence of an old pathogen. Clin. Infect. Dis. 2004, 39, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Edwards, J.; Ibrahim, A. Novel Perspectives on Mucormycosis: Pathophysiology, Presentation, and Management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Hori, T.; Miyazaki, M.; Watanabe, N. In vitro and in vivo antifungal activities of E1211, a water-soluble prodrug of E1210, F1–1377. In Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 17–20 September 2011; ASM: Chicago, IL, USA, 2011. [Google Scholar]
- Tsukahara, K.; Hata, K.; Nakamoto, K.; Sagane, K.; Watanabe, N.-A.; Kuromitsu, J.; Kai, J.; Tsuchiya, M.; Ohba, F.; Jigami, Y.; et al. Medicinal genetics approach towards identifying the molecular target of a novel inhibitor of fungal cell wall assembly. Mol. Microbiol. 2003, 48, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Mann, P.A.; McLellan, C.A.; Koseoglu, S.; Si, Q.; Kuzmin, E.; Flattery, A.; Harris, G.; Sher, X.; Murgolo, N.; Wang, H.; et al. Chemical Genomics-Based Antifungal Drug Discovery: Targeting Glycosylphosphatidylinositol (GPI) Precursor Biosynthesis. ACS Infect. Dis. 2014, 1, 59–72. [Google Scholar] [CrossRef]
- Nakamoto, K.; Tsukada, I.; Tanaka, K.; Matsukura, M.; Haneda, T.; Inoue, S.; Murai, N.; Abe, S.; Ueda, N.; Miyazaki, M.; et al. Synthesis and evaluation of novel antifungal agents-quinoline and pyridine amide derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 4624–4626. [Google Scholar] [CrossRef]
- Miyazaki, M.; Horii, T.; Hata, K.; Watanabe, N.-A.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; Inoue, S.; Matsukura, M.; et al. In VitroActivity of E1210, a Novel Antifungal, against Clinically Important Yeasts and Molds. Antimicrob. Agents Chemother. 2011, 55, 4652–4658. [Google Scholar] [CrossRef]
- Watanabe, N.-A.; Miyazaki, M.; Horii, T.; Sagane, K.; Tsukahara, K.; Hata, K. E1210, a New Broad-Spectrum Antifungal, Suppresses Candida albicans Hyphal Growth through Inhibition of Glycosylphosphatidylinositol Biosynthesis. Antimicrob. Agents Chemother. 2011, 56, 960–971. [Google Scholar] [CrossRef]
- Orlean, P.; Menon, A.K. Thematic review series: Lipid Posttranslational Modifications.GPI anchoring of protein in yeast and mammalian cells, or: How we learned to stop worrying and love glycophospholipids. J. Lipid Res. 2007, 48, 993–1011. [Google Scholar] [CrossRef]
- Umemura, M.; Okamoto, M.; Nakayama, K.-I.; Sagane, K.; Tsukahara, K.; Hata, K.; Jigami, Y. GWT1 Gene Is Required for Inositol Acylation of Glycosylphosphatidylinositol Anchors in Yeast. J. Biol. Chem. 2003, 278, 23639–23647. [Google Scholar] [CrossRef]
- Sharp, M.; Soltow, Q.; Shaw, K.J.; Pogliano, J. Fungal Cytological Profiling of Candida albicans Exposed to Diverse Antifungal Agents Including the Novel Gwt1 inhibitor APX001A. Open Forum Infect. Dis. 2017, 4, S475–S476. [Google Scholar] [CrossRef][Green Version]
- McLellan, C.A.; Whitesell, L.; King, O.D.; Lancaster, A.K.; Mazitschek, R.; Lindquist, S. Inhibiting GPI Anchor Biosynthesis in Fungi Stresses the Endoplasmic Reticulum and Enhances Immunogenicity. ACS Chem. Biol. 2012, 7, 1520–1528. [Google Scholar] [CrossRef]
- Covel, J.A.; Soltow, Q.A.; Kapoor, M.; Moloney, M.K.; Webb, P.J.; Trzoss, M.; Sharp, M.; Shaw, K.J. The Discovery of Manogepix/Fosmanogepix and Other Gwt1 Inhibitors for the Treatment of Invasive Fungal Infections. Med. Chem. Rev. 2019, 221–237. [Google Scholar] [CrossRef]
- Horii, T.; Okubo, M.; Miyazaki, M.; Hata, K.; Watanabe, N.-A. In vivo pharmacodynamic correlates of success for E1210 treatment of disseminated candidiasis. F1–843. In Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, USA, 12–15 September 2010; ASM: Boston, MA, USA, 2010. [Google Scholar]
- Huband, M.D.; Pfaller, M.A.; Flamm, R.K.; Messer, S.A.; Schaefer, B.; Bien, P.A.; Castaneira, M. Activity of manogepix (APX001A) against 2669 fungal isolates from the SENTRY surveillance program (2018–2019) stratified by infection type. In Proceedings of the IDWeek, Virtual Conference, 21–22 October 2020. Online. [Google Scholar]
- Jørgensen, K.M.; Astvad, K.M.T.; Arendrup, M. In vitro Activity of Manogepix (APX001A) and Comparators against Contemporary Molds: MEC Comparison and Preliminary Experience with Colorimetric MIC Determination. Antimicrob. Agents Chemother. 2020, 64, 64. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Huband, M.D.; Flamm, R.K.; Bien, P.A.; Castanheira, M. In vitro Activity of APX001A (Manogepix) and Comparator Agents against 1706 Fungal Isolates Collected during an International Surveillance Program in 2017. Antimicrob. Agents Chemother. 2019, 63, 63. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In vitro activity of APX001A against rare moulds using EUCAST and CLSI methodologies. J. Antimicrob. Chemother. 2019, 74, 1295–1299. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Hata, K.; Jones, R.N.; Messer, S.A.; Moet, G.J.; Castanheira, M. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Candida spp. as determined by CLSI broth microdilution method. Diagn. Microbiol. Infect. Dis. 2011, 71, 167–170. [Google Scholar] [CrossRef]
- Hager, C.L.; Larkin, E.L.; Long, L.; Abidi, F.Z.; Shaw, K.J.; Ghannoum, M.A. In vitro and In Vivo Evaluation of the Antifungal Activity of APX001A/APX001 against Candida auris. Antimicrob. Agents Chemother. 2018, 62, e02319-17. [Google Scholar] [CrossRef]
- Arendrup, M.; Jørgensen, K.M. Manogepix (APX001A) Displays Potent In vitro Activity against Human Pathogenic Yeast, but with an Unexpected Correlation to Fluconazole MICs. Antimicrob. Agents Chemother. 2020, 64, 64. [Google Scholar] [CrossRef]
- Zhu, Y.; Kilburn, S.; Kapoor, M.; Chaturvedi, S.; Shaw, K.J.; Chaturvedi, V. Evaluation of in vitro activity of manogepix against multidrug-resistant and pan-resistant Candida auris from the New York Outbreak. Antimicrob. Agents Chemother. 2020. [Google Scholar] [CrossRef]
- Castanheira, M.; Duncanson, F.P.; Diekema, D.J.; Guarro, J.; Jones, R.N.; Pfaller, M.A. Activities of E1210 and Comparator Agents Tested by CLSI and EUCAST Broth Microdilution Methods against Fusarium and Scedosporium Species Identified Using Molecular Methods. Antimicrob. Agents Chemother. 2011, 56, 352–357. [Google Scholar] [CrossRef]
- Arendrup, M.; Chowdhary, A.; Jørgensen, K.M.; Meletiadis, J. Manogepix (APX001A) in vitro activity against Candida auris. A head to head comparison of EUCAST and CLSI MICs. Antimicrob. Agents Chemother. 2020. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI standard M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI standard M38; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Wiederhold, N.P.; Najvar, L.K.; Fothergill, A.W.; McCarthy, D.I.; Bocanegra, R.; Olivo, M.; Kirkpatrick, W.R.; Everson, M.P.; Duncanson, F.P.; Patterson, T.F. The Investigational Agent E1210 Is Effective in Treatment of Experimental Invasive Candidiasis Caused by Resistant Candida albicans. Antimicrob. Agents Chemother. 2014, 59, 690–692. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J. Antimicrob. Chemother. 2018, 73, 3060–3062. [Google Scholar] [CrossRef]
- Kapoor, M.; Moloney, M.; Soltow, Q.A.; Pillar, C.M.; Shaw, K.J. Evaluation of Resistance Development to the Gwt1 Inhibitor Manogepix (APX001A) in Candida Species. Antimicrob. Agents Chemother. 2019, 64, 64. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Shaw, K.J.; Jaramillo, R.; Patterson, H.; Olivo, M.; Catano, G.; Patterson, T.F. Efficacy of Delayed Therapy with Fosmanogepix (APX001) in a Murine Model of Candida auris Invasive Candidiasis. Antimicrob. Agents Chemother. 2019, 63, 63. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Duncanson, F.; Messer, S.A.; Moet, G.J.; Jones, R.N.; Castanheira, M. In vitro Activity of a Novel Broad-Spectrum Antifungal, E1210, Tested against Aspergillus spp. Determined by CLSI and EUCAST Broth Microdilution Methods. Antimicrob. Agents Chemother. 2011, 55, 5155–5158. [Google Scholar] [CrossRef]
- Badali, H.; Patterson, H.; Sanders, C.; Mermella, B.; Gibas, C.; Mele, J.; Fan, H.; Ibrahim, A.S.; Shaw, K.J.; Wiederhold, N.P. Manogepix, the active moiety of the investigational agent fosmanogepix, demonstrates in vitro activity against members of the Fusarium oxysporum and Fusarium solani species complexes. In Proceedings of the IDweek 2020, Philadelphia, PA, USA, 21–25 October 2020. Poster 1282, IDSA: Online. [Google Scholar]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Wiederhold, N.P.; Shaw, K.J.; Patterson, T.F.; Filler, S.; Ibrahim, A. APX001A Protects Immunosuppressed Mice from Rhizopus delemar Infection. Open Forum Infect. Dis. 2017, 4, S475. [Google Scholar] [CrossRef]
- Viriyakosol, S.; Kapoor, M.; Okamoto, S.; Covel, J.; Soltow, Q.A.; Trzoss, M.; Shaw, K.J.; Fierer, J. APX001 and Other Gwt1 Inhibitor Prodrugs Are Effective in Experimental Coccidioides immitis Pneumonia. Antimicrob. Agents Chemother. 2018, 63, e01715-18. [Google Scholar] [CrossRef]
- Locke, J.B.; Almaguer, A.L.; Zuill, D.E.; Bartizal, K. Characterization of In vitro Resistance Development to the Novel Echinocandin CD101 in Candida Species. Antimicrob. Agents Chemother. 2016, 60, 6100–6107. [Google Scholar] [CrossRef] [PubMed]
- Liston, S.D.; Whitesell, L.; Kapoor, M.; Shaw, K.J.; Cowen, L.E. Enhanced Efflux Pump Expression in Candida Mutants Results in Decreased Manogepix Susceptibility. Antimicrob. Agents Chemother. 2020, 64, 64. [Google Scholar] [CrossRef]
- Arendrup, M.; Chowdhary, A.; Astvad, K.M.T.; Jørgensen, K.M. APX001A In vitro Activity against Contemporary Blood Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob. Agents Chemother. 2018, 62, AAC.01225-18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lee, M.H.; Paderu, P.; Lee, A.; Jimenez-Ortigosa, C.; Park, S.; Mansbach, R.S.; Shaw, K.J.; Perlin, D.S. Significantly Improved Pharmacokinetics Enhances In Vivo Efficacy of APX001 against Echinocandin- and Multidrug-Resistant Candida Isolates in a Mouse Model of Invasive Candidiasis. Antimicrob. Agents Chemother. 2018, 62, AAC.00425-18. [Google Scholar] [CrossRef]
- Ferrari, S.; Sanguinetti, M.; Torelli, R.; Posteraro, B.; Sanglard, D. Contribution of CgPDR1-Regulated Genes in Enhanced Virulence of Azole-Resistant Candida glabrata. PLoS ONE 2011, 6, e17589. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Horii, T.; Miyazaki, M.; Watanabe, N.-A.; Okubo, M.; Sonoda, J.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; et al. Efficacy of Oral E1210, a New Broad-Spectrum Antifungal with a Novel Mechanism of Action, in Murine Models of Candidiasis, Aspergillosis, and Fusariosis. Antimicrob. Agents Chemother. 2011, 55, 4543–4551. [Google Scholar] [CrossRef]
- Zhao, M.; Lepak, A.J.; Vanscoy, B.; Bader, J.C.; Marchillo, K.; VanHecker, J.; Ambrose, P.G.; Andes, D.R. In Vivo Pharmacokinetics and Pharmacodynamics of APX001 against Candida spp. in a Neutropenic Disseminated Candidiasis Mouse Model. Antimicrob. Agents Chemother. 2018, 62, e02542-17. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Maung, B.B.W.; Mansbach, R.; Shaw, K.J.; Walsh, T.J. Efficacy of APX001 in treatment of Candida endophthalmitis and haematagenous meningoencephalitis in experimental non-neutropenic rabbit model. In Proceedings of the 28th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID 2018), Madrid, Spain, 21–24 April 2018. [Google Scholar]
- Shaw, K.J.; Schell, W.A.; Covel, J.; Duboc, G.; Giamberardino, C.; Kapoor, M.; Moloney, M.; Soltow, Q.A.; Tenor, J.L.; Toffaletti, D.L.; et al. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against Cryptococcus. Antimicrob. Agents Chemother. 2018, 62, e00523-18. [Google Scholar] [CrossRef]
- Watanabe, N.-A.; Horii, T.; Miyazaki, M.; Hata, K. In vitro activity of E1210 and in vivo activity of E1211, a water-soluble prodrug of E1210, in combination with other antifungals. In Proceedings of the F1–1378, in Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 17 September 2011; ASM: Chicago, IL, USA, 2011. [Google Scholar]
- Zhao, M.; Lepak, A.J.; Marchillo, K.; VanHecker, J.; Sanchez, H.; Ambrose, P.G.; Andes, D. APX001 Pharmacokinetic/Pharmacodynamic Target Determination against Aspergillus fumigatus in an In Vivo Model of Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2019, 63, 63. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Jeon, H.H.; Gu, Y.; Kapoor, M.; Shaw, K.J.; Ibrahim, A.S. APX001 Is Effective in the Treatment of Murine Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2018, 63, e01713-18. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Gu, Y.; Singh, S.; Alqarihi, A.; Shaw, K.J.; Ibrahim, A.S. Galactomannan Is a Biomarker of Fosmanogepix (APX001) Efficacy in Treating Experimental Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2019, 64, 64. [Google Scholar] [CrossRef] [PubMed]
- Alkhazraji, S.; Gebremariam, T.; Alqarihi, A.; Gu, Y.; Mamouei, Z.; Singh, S.; Wiederhold, N.P.; Shaw, K.J.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Immunocompromised Mice Infected with Invasive Pulmonary Scedosporiosis or Disseminated Fusariosis. Antimicrob. Agents Chemother. 2019, 64, 64. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Wiederhold, N.P.; Shaw, K.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Pulmonary Murine Mucormycosis Due to Rhizopus arrhizus. Antimicrob. Agents Chemother. 2020, 64, 64. [Google Scholar] [CrossRef]
- Hodges, M.R.; Ople, E.; Shaw, K.J.; Mansbach, R.; Van Marle, S.P.; Van Hoogdalem, E.-J.; Kramer, W.; Wedel, P. Phase 1 Study to Assess Safety, Tolerability and Pharmacokinetics of Single and Multiple Oral Doses of APX001 and to Investigate the Effect of Food on APX001 Bioavailability. Open Forum Infect. Dis. 2017, 4. [Google Scholar] [CrossRef]
- Hodges, M.R.; Ople, E.; Shaw, K.J.; Mansbach, R.; Van Marle, S.J.; Van Hoogdalem, E.-J.; Wedel, P.; Kramer, W. First-in-Human Study to Assess Safety, Tolerability and Pharmacokinetics of APX001 Administered by Intravenous Infusion to Healthy Subjects. Open Forum Infect. Dis. 2017, 4. [Google Scholar] [CrossRef]
- Hope, W.; McEntee, L.; Livermore, J.; Whalley, S.; Johnson, A.; Farrington, N.; Kolamunnage-Dona, R.; Schwartz, J.; Kennedy, A.; Law, D.; et al. Pharmacodynamics of the Orotomides against Aspergillus fumigatus: New Opportunities for Treatment of Multidrug-Resistant Fungal Disease. mBio 2017, 8, e01157-17. [Google Scholar] [CrossRef] [PubMed]
- Mercier, T.; Guldentops, E.; Lagrou, K.; Maertens, J. Galactomannan, a Surrogate Marker for Outcome in Invasive Aspergillosis: Finally Coming of Age. Front. Microbiol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Mansbach, R.; Shaw, K.J.; Hodges, M.R.; Coleman, S.; E Fitzsimmons, M. Absorption, Distribution, and Excretion of 14C-APX001 after Single-Dose Administration to Rats and Monkeys. Open Forum Infect. Dis. 2017, 4, S472. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Viscoli, C.; Pappas, P.G.; Vazquez, J.; Ostrosky-Zeichner, L.; Rotstein, C.; Sobel, J.D.; Herbrecht, R.; Rahav, G.; Jaruratanasirikul, S.; et al. Isavuconazole Versus Caspofungin in the Treatment of Candidemia and Other Invasive Candida Infections: The ACTIVE Trial. Clin. Infect. Dis. 2018, 68, 1981–1989. [Google Scholar] [CrossRef]
- Amplyx website. Amplyx Announces Positive Top-Line Data in Phase 2 Clinical Trial of Novel Antifungal Fosmanogepix. 2020. Available online: https://amplyx.com/amplyx-announces-positive-top-line-data-in-phase-2-clinical-trial-of-novel-antifungal-fosmanogepix/ (accessed on 20 July 2020).
- Amplyx website. FDA Grants Fast Track Status to Amplyx Pharmaceuticals for IV and Oral Formulation of Fosmanogepix (APX001) for Seven Different Indications. 2019. Available online: https://amplyx.com/fda-grants-fast-track-status-to-amplyx-pharmaceuticals-for-iv-and-oral-formulations-of-fosmanogepix-apx001-for-seven-different-indications/ (accessed on 9 September 2019).
| Organism (of Strains) | Method | MIC90 | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MGX | FLC | VRC | POS | ITC | CAS | AFG | AMB | |||
| C. albicans (21) | CLSI | 0.06 | 64 | 0.5 | 0.5 | nt | 4 | nt | nt | [32] |
| C. albicans (402) | EUCAST | 0.016 | 0.25 | 0.008 | nt | nt | nt | 0.008 | 0.25 | [34] |
| C. albicans (414) | CLSI | 0.008 | 0.25 | 0.015 | 0.06 | nt | nt | 0.03 | 1 | [30] |
| C. albicans (29) | CLSI | ≤0.03 | >64 | nt | nt | nt | 2 | nt | nt | [40] |
| C. auris (16) | CLSI | 0.03 | >64 | 1 | 1 | 1 | 1 | 0.25 | 4 | [33] |
| C. auris (122) | CLSI | 0.03 | 512 | 4 | 0.12 | 0.25 | nt | 0.5 | 1 | [37] |
| C. auris (200) | CLSI | 0.03 | 256 | 2 | 0.5 | 1 | 0.25 | 1 | 2 | [35] |
| C. auris (100) | CLSI | 0.008 | nt | nt | nt | nt | nt | nt | nt | [41] |
| C. dubliniensis (48) | EUCAST | 0.008 | 0.5 | 0.016 | nt | nt | nt | 0.016 | 0.06 | [34] |
| C. dubliniensis (49) | CLSI | 0.008 | 0.25 | 0.015 | 0.06 | nt | nt | 0.12 | 0.06 | [30] |
| C. glabrata (321) | CLSI | 0.12 | 32 | 1 | 1 | nt | nt | 0.12 | 1 | [30] |
| C. glabrata (20) | CLSI | 0.06 | 32 | 1 | 1 | nt | 2 | nt | nt | [32] |
| C. glabrata (188) | EUCAST | 0.125 | 32 | 0.5 | nt | nt | nt | 0.03 | 0.5 | [34] |
| C. kefyr (12) | EUCAST | 0.5 | 0.5 | 0.016 | nt | nt | nt | 0.03 | 0.5 | [34] |
| C. kefyr (13) | CLSI | 0.5 | 0.25 | ≤0.008 | 0.12 | nt | nt | 0.12 | 1 | [30] |
| C. krusei (43) | CLSI | >2 | 32 | 0.5 | 0.5 | nt | nt | 0.12 | 1 | [30] |
| C. krusei (54) | EUCAST | >0.5 | 64 | 0.5 | nt | nt | nt | 0.06 | 1 | [34] |
| C. lusitaniae (39) | CLSI | 0.12 | 2 | 0.015 | 0.12 | nt | nt | 0.5 | 1 | [30] |
| C. lusitaniae (12) | EUCAST | 0.016 | 0.5 | 0.016 | nt | nt | nt | 0.06 | 0.25 | [34] |
| C. parapsilosis (270) | CLSI | 0.015 | 2 | 0.06 | 0.12 | nt | nt | 1 | 1 | [30] |
| C. parapsilosis (25) | CLSI | 0.06 | 16 | 0.25 | 0.12 | nt | 4 | nt | nt | [32] |
| C. parapsilosis (39) | EUCAST | 0.03 | 2 | 0.03 | nt | nt | nt | 2 | 0.5 | [34] |
| C. tropicalis (24) | CLSI | 0.06 | 64 | 2 | 0.5 | nt | 4 | nt | nt | [32] |
| C. tropicalis (151) | CLSI | 0.03 | 0.5 | 0.06 | 0.5 | nt | nt | 0.06 | 1 | [30] |
| C. tropicalis (41) | EUCAST | 0.016 | 1 | 0.03 | nt | nt | nt | 0.03 | 0.5 | [34] |
| C. neoformans (30) | CLSI | 0.5 | 4 | 0.06 | 0.25 | nt | nt | >4 | 1 | [30] |
| Strain Source | Strains | MIC50 (µg/mL) | MIC90 (µg/mL) | MIC Range (µg/mL) | Reference |
|---|---|---|---|---|---|
| Worldwide | 100 | 0.004 | 0.008 | <0.0005–0.015 | [41] |
| India, Japan, Korea, Germany | 16 | 0.004 | 0.03 | 0.002–0.015 | [33] |
| Worldwide | 13 | 0.03 | 0.03 | ≤0.002–0.03 | [43] |
| USA | 1 | -- | -- | 0.06 | [30] |
| USA | 5 | 0.03 | -- | 0.03–0.06 | [28] |
| Panama | 6 | 0.004 | -- | ≤0.002–0.015 | [28] |
| India | 122 | 0.008 | 0.03 | 0.001–0.25 | [37] |
| NY/NJ, USA | 200 | 0.016 | 0.03 | 0.008 -0.03 | [35] |
| Organism (of Strains) | Method | MIC90/MEC90 | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MGX | VRC | POS | ITC | CAS | AFG | AMB | |||
| Alternaria alternata (10) | CLSI | 4 | nt | 16 | nt | nt | nt | 4 | [31] |
| A. alliaceus (10) | CLSI | 0.03 | nt | 0.12 | nt | nt | nt | 32 | [31] |
| A. calidoustus (10) | CLSI | 0.03 | nt | 16 | nt | nt | nt | 1 | [31] |
| A. flavus (20) | CLSI | 0.03 | 1 | 1 | 1 | 0.12 | ≤0.008 | nt | [44] |
| A. flavus (18) | CLSI | 0.015 | 1 | 0.5 | 1 | nt | 0.03 | 2 | [30] |
| A. fumigatiaffinis (10) | CLSI | 0.03 | nt | 0.5 | nt | nt | nt | 4 | [31] |
| A. fumigatus (182) | CLSI | 0.03 | 0.5 | 0.5 | 1 | nt | 0.03 | 2 | [30] |
| A. fumigatus (22) | CLSI | 0.06 | 2 | 1 | >8 | 0.12 | 0.015 | nt | [44] |
| A. fumigatus (121) | EUCAST | 0.06 | 0.5 | 0.12 | 0.25 | nt | nt | 0.5 | [29] |
| A. lentulus (10) | CLSI | 0.03 | nt | 1 | nt | nt | nt | 1 | [31] |
| A. niger (18) | EUCAST | 0.03 | 2 | 0.25 | 1 | nt | nt | 0.25 | [29] |
| A. niger (13) | CLSI | 0.015 | 2 | 1 | 4 | 0.12 | ≤0.008 | nt | [44] |
| A. niger (23) | CLSI | 0.015 | 2 | 1 | 4 | nt | 0.015 | 1 | [30] |
| A. terreus (23) | CLSI | 0.06 | 1 | 0.5 | 1 | 0.12 | 0.015 | nt | [44] |
| A. terreus (10) | CLSI | 0.03 | 0.5 | 0.25 | 0.5 | nt | 0.015 | 2 | [30] |
| A. thermomutatus (10) | CLSI | 0.25 | nt | 1 | nt | nt | nt | 2 | [31] |
| A. udagawae (10) | CLSI | 0.06 | nt | 0.5 | nt | nt | nt | 1 | [31] |
| Cunninghamella bertholletiae (10) | CLSI | 16 | nt | 2 | nt | nt | nt | 4 | [31] |
| F. solani (15) | CLSI | 0.06 | >8 | >8 | >8 | >8 | >8 | 2 | [36] |
| F. oxysporum (15) | CLSI | 0.25 | 4 | 2 | >8 | >8 | >8 | 4 | [36] |
| F. oxysporum (10) | CLSI | 16 | nt | 16 | nt | nt | nt | 1 | [31] |
| F. verticilloides (10) | CLSI | 16 | nt | 16 | nt | nt | nt | 32 | [31] |
| Gibberella fujikuroi (30) | CLSI | 0.12 | 8 | >8 | >8 | >8 | >8 | 4 | [36] |
| Lichtheimia corymbifera (10) | CLSI | 16 | nt | 2 | nt | nt | nt | 0.12 | [31] |
| Lichtheimia ramosa (10) | CLSI | 16 | nt | 0.5 | nt | nt | nt | 0.06 | [31] |
| Lomentospora prolificans (10) | CLSI | 0.06 | nt | 16 | nt | nt | nt | 8 | [31] |
| M. circinelloides (10) | CLSI | 8 | nt | 1 | nt | nt | nt | 0.03 | [31] |
| Rhizomucor pusillus (10) | CLSI | 16 | nt | 2 | nt | nt | nt | 0.06 | [31] |
| R. arrhizus (10) | CLSI | 16 | nt | 0.5 | nt | nt | nt | 0.12 | [31] |
| R. microsporus (10) | CLSI | 16 | nt | 2 | nt | nt | nt | 0.12 | [31] |
| S. apiospermum (28) | CLSI | 0.12 | 1 | 2 | 4 | >8 | 4 | >4 | [36] |
| S. apiospermum (10) | CLSI | 16 | nt | 8 | nt | nt | nt | 32 | [31] |
| S. aurantiacum (10) | CLSI | 0.03 | nt | 16 | nt | nt | nt | 16 | [31] |
| S. boydii (10) | CLSI | 0.12 | nt | 2 | nt | nt | nt | 2 | [31] |
| S. prolificans (28) | CLSI | 0.12 | >8 | >8 | >8 | >8 | 4 | >4 | [36] |
| Pathogen | Infection Type | Efficacy Endpoint (Special Study) | Reference |
|---|---|---|---|
| Manogepix | |||
| C. albicans | oropharyngeal | Oral CFU | [53] |
| C. albicans | disseminated | Survival | [53] |
| C. albicans | disseminated | Survival, kidney CFU | [40] |
| C. tropicalis | disseminated | Survival | [53] |
| A. fumigatus | pulmonary | Survival | [53] |
| A. flavus | pulmonary | Survival | [53] |
| F. solani | disseminated | Survival | [53] |
| Fosmanogepix | |||
| C. albicans | oropharyngeal | Oral CFU | [16] |
| C. albicans | disseminated | Survival | [16] |
| C. albicans | disseminated | Kidney CFU | [51] |
| C. albicans | disseminated | Kidney CFU (PK/PD) | [54] |
| C. albicans1 | disseminated | Brain, eye CFU | [55] |
| C. auris | disseminated | Survival; kidney, lung, brain CFU | [33] |
| C. auris | disseminated | Survival; kidney, lung, brain CFU | [43] |
| C. auris | disseminated | Kidney CFU (PK/PD) | [54] |
| C. glabrata | disseminated | Kidney CFU (PK/PD) | [54] |
| C. glabrata | disseminated | Kidney CFU | [51] |
| C. immitis | pulmonary | Survival; lung, spleen CFU | [47] |
| C. neoformans | disseminated | Brain, lung CFU | [56] |
| A. flavus | pulmonary | Survival | [16] |
| A. flavus | pulmonary | Survival (combination MFG, AFG) | [57] |
| A. fumigatus | pulmonary | Survival | [16] |
| A. fumigatus | pulmonary | Lung CFU (PK/PD) | [58] |
| A. fumigatus | pulmonary | Lung CFU, survival, histology | [59] |
| A. fumigatus | pulmonary | Lung CFU; serum, BAL GM (GM biomarker) | [60] |
| F. solani | disseminated | Survival | [16] |
| F. solani | disseminated | Survival; kidney, brain CFU; histology | [61] |
| R. arrhizus var. arrhizus | pulmonary | Survival; lung, brain CFU | [62] |
| R. arrhizus var. delemar | pulmonary | Survival; lung, brain CFU | [62] |
| S. apiospermum | pulmonary | Survival; kidney, lung, brain CFU; histology | [61] |
| S. prolificans | pulmonary | Survival | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, K.J.; Ibrahim, A.S. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. J. Fungi 2020, 6, 239. https://doi.org/10.3390/jof6040239
Shaw KJ, Ibrahim AS. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. Journal of Fungi. 2020; 6(4):239. https://doi.org/10.3390/jof6040239
Chicago/Turabian StyleShaw, Karen Joy, and Ashraf S. Ibrahim. 2020. "Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections" Journal of Fungi 6, no. 4: 239. https://doi.org/10.3390/jof6040239
APA StyleShaw, K. J., & Ibrahim, A. S. (2020). Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. Journal of Fungi, 6(4), 239. https://doi.org/10.3390/jof6040239






