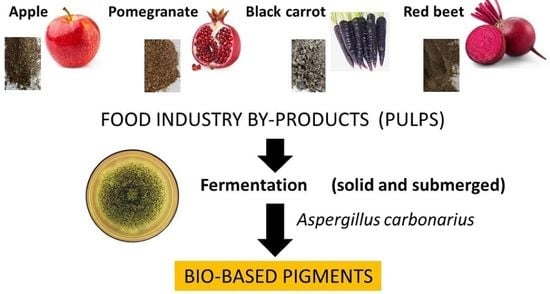
Production of Bio-Based Pigments from Food Processing Industry By-Products (Apple, Pomegranate, Black Carrot, Red Beet Pulps) Using Aspergillus carbonarius
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Species and Inoculum
2.2. Substrate
2.3. Selection of Pulp Type
2.4. Effect of Fermentation Strategy and Incubation Period
2.5. Effect of Substrate Particle Size
2.6. Effect of Initial Substrate pH
2.7. Instrumental Analysis
2.8. Analytical Techniques
2.9. Dyeing Tests
3. Results and Discussion
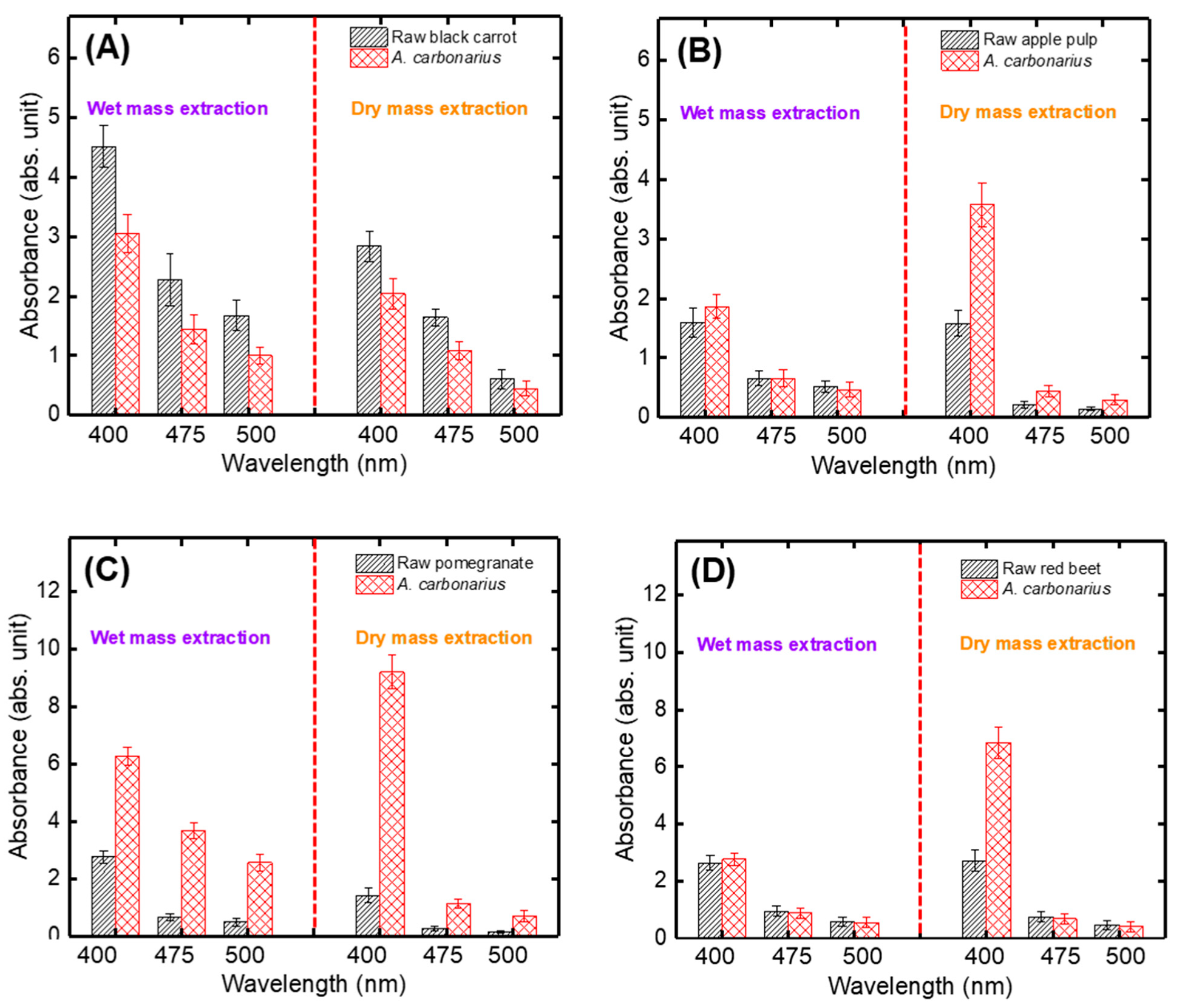
3.1. Optimal Fungal Species and Pulp Type
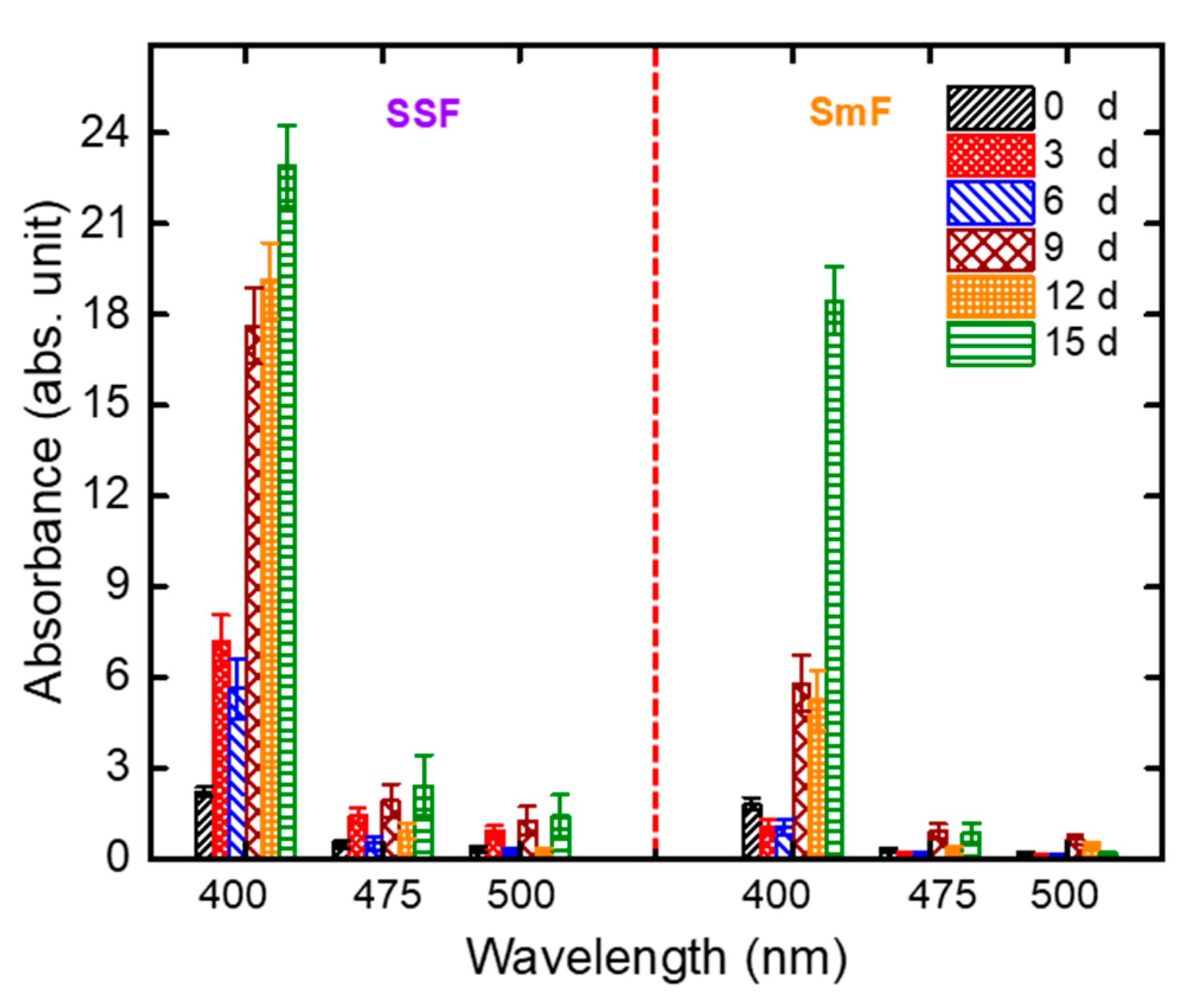
3.2. Optimal Fermentation Strategy and Incubation Period
3.3. Optimal Substrate Particle Size
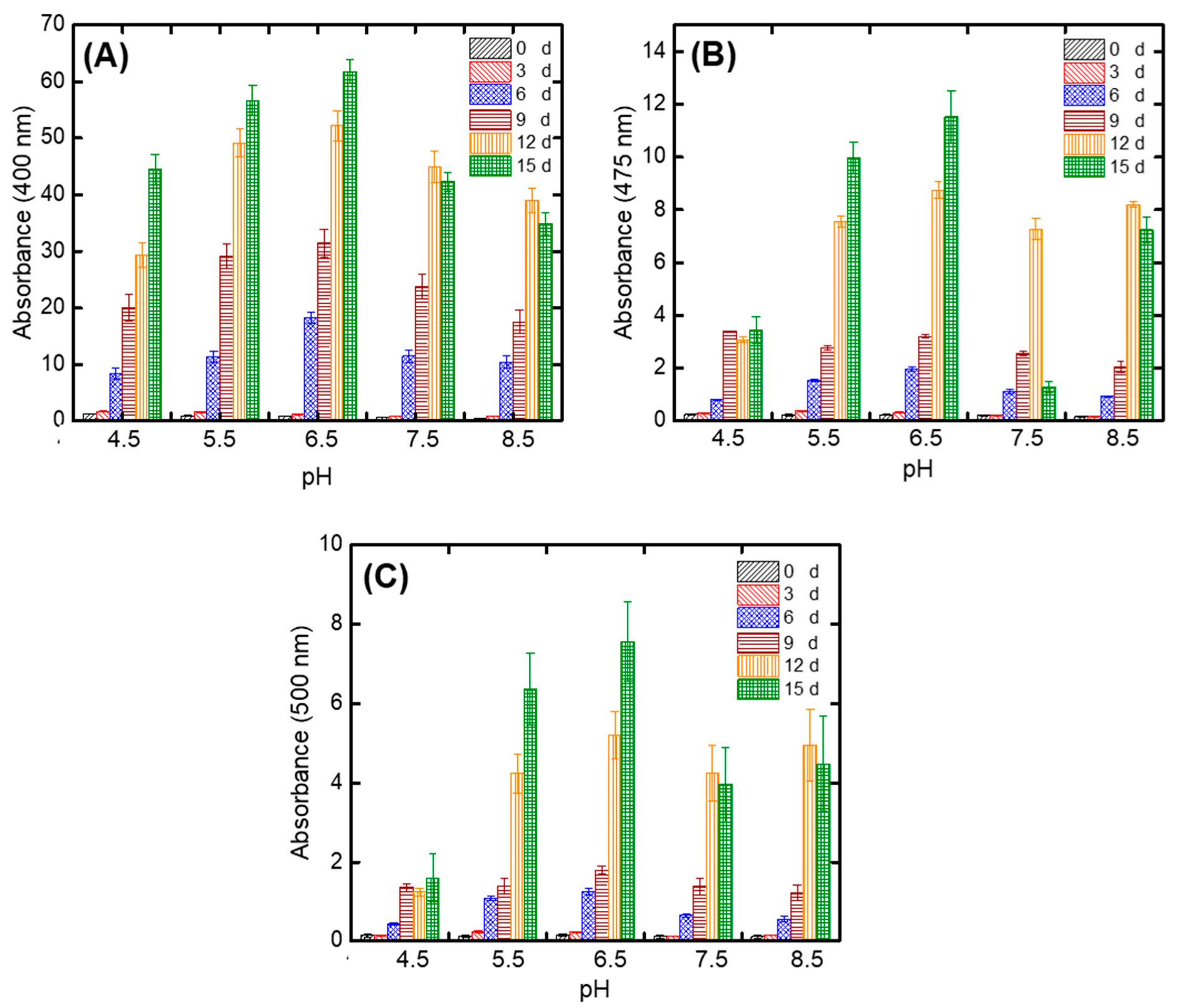
3.4. Optimal Initial Substrate pH
3.5. C:N Ratio Utilization
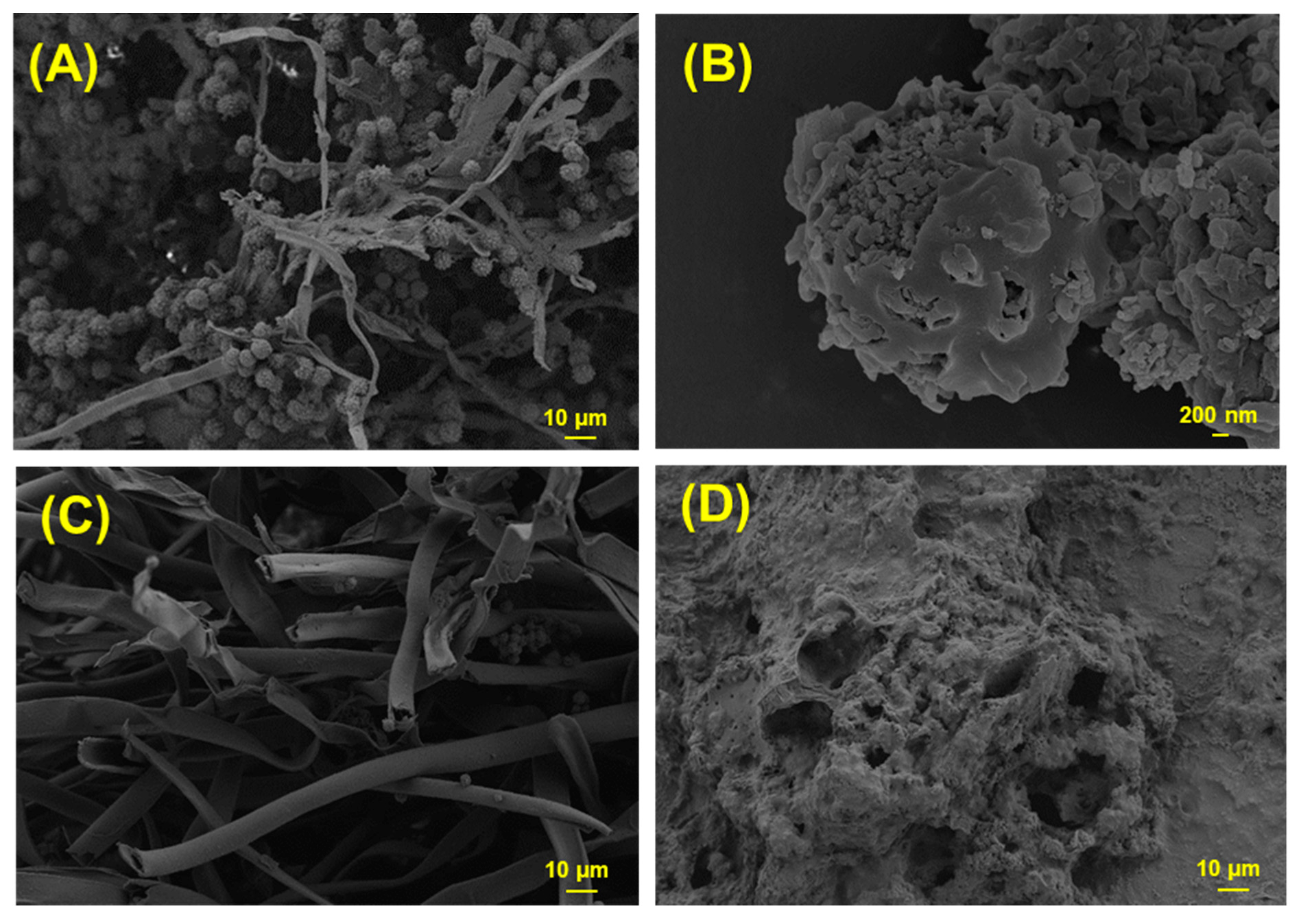
3.6. Characterization of Fungal Biomass
3.7. Potential Usage of Fungal Pigment in Textile Industry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vendruscolo, F.; Luise Müller, B.; Esteves Moritz, D.; de Oliveira, D.; Schmidell, W.; Luiz Ninow, J. Thermal stability of natural pigments produced by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2013, 2, 278–284. [Google Scholar] [CrossRef]
- Shahid, M.; ul-Islam, S.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Karger-Kocsis, J. Paints, coatings and solvents. Compos. Sci. Technol. 1994, 51, 613–614. [Google Scholar] [CrossRef]
- Barnett, J.R.; Miller, S.; Pearce, E. Colour and art: A brief history of pigments. Opt. Laser Technol. 2006, 38, 445–453. [Google Scholar] [CrossRef]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- US FDA. Laws & Regulations-FDA Authority over Cosmetics: How Cosmetics Are not FDA-Approved, but Are FDA-Regulated. 2016. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated (accessed on 14 October 2020).
- FAO. WHO. Safety Evaluation of Certain Food Additives. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/258934/9789241660730-eng.pdf;jsessionid=CEC17CE6D1D291C848E8289CFCD2A713?sequence=1 (accessed on 14 October 2020).
- Embaby, A.M.; Hussein, M.N.; Hussein, A. Monascus orange and red pigments production by Monascus purpureus ATCC16436 through co-solid state fermentation of corn cob and glycerol: An eco-friendly environmental low cost approach. PLoS ONE 2018, 13, e0207755. [Google Scholar] [CrossRef]
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Guo, T.; Tang, C.; Chai, X.; Zhao, W.; Bai, J.; Lin, Q. Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef]
- Thejus, P.K.; Krishnapriya, K.V.; Nishanth, K.G. A cost-effective intense blue colour inorganic pigment for multifunctional cool roof and anticorrosive coatings. Sol. Energy Mater. Sol. Cells 2021, 219, 110778. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current perspective of yellowish-orange pigments from microorganisms—A review. J. Clean. Prod. 2018, 180, 168–182. [Google Scholar] [CrossRef]
- Tirumale, S.; Wani, N.A. Biopigments: Fungal Pigments. In Fungi and Their Role in Sustainable Development: Current Perspectives, 1st ed.; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; Chapter 23; pp. 413–426. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.-J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry-Challenges and the way forward. Front. Nutr. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Nirlane da Costa Souza, P.; Luiza Bim Grigoletto, T.; Alberto Beraldo de Moraes, L.; Abreu, L.M.; Henrique Souza Guimarães, L.; Santos, C.; Ribeiro Galvão, L.; Gomes Cardoso, P. Production and chemical characterization of pigments in filamentous fungi. Microbiology 2016, 162, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Tichota, D.M.; Pereira, J.Q.; Segalin, J.; de Oliveira Rios, A.; Brandelli, A. Pigment Production by Filamentous Fungi on Agro-Industrial Byproducts: An Eco-Friendly Alternative. Appl. Biochem. Biotechnol. 2013, 171, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Mahboobi Soofiani, N.; Mahboubi, A.; Taherzadeh, M. Use of Organic Wastes and Industrial By-Products to Produce Filamentous Fungi with Potential as Aqua-Feed Ingredients. Sustainability 2018, 10, 3296. [Google Scholar] [CrossRef]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and Colorants from Filamentous Fungi. In Fungal Metabolites, 1st ed.; Merillon, J.M., Ramawat, K., Eds.; Springer International Publishing: Chambridge, UK, 2017; pp. 499–568. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Carvalho, S.A. Nutritional potential of biomass and metabolites from filamentous fungi. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2010; Volume 2, pp. 1126–1135. [Google Scholar]
- Teixeira, M.F.S.; Martins, M.S.; da Silva, J.C.; Kirsch, L.S.; Fernandes, O.C.C.; Carneiro, A.L.B.; de Conti, R.; Durán, N. Amazonian biodiversity: Pigments from Aspergillus and Penicillium-characterizations, antibacterial activities and their toxicities. Curr. Trends Biotechnol. Pharm. 2012, 6, 2230–7303. [Google Scholar]
- Mostafa, M.E.; Saad Abbady, M. Secondary Metabolites and Bioactivity of the Monascus Pigments Review Article. Glob. J. Biotechnol. Biochem. 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Heo, Y.M.; Kim, K.; Kwon, S.L.; Na, J.; Lee, H.; Jang, S.; Kim, C.H.; Jung, J.; Kim, J.-J. Investigation of Filamentous Fungi Producing Safe, Functional Water-Soluble Pigments. Mycobiology 2018, 46, 269–277. [Google Scholar] [CrossRef]
- Saravanan, A.; Jayasree, R.; Senthil Kumar, P.; Varjani, S.; Hemavathy, R.V.; Jeevanantham, S.; Yaashikaa, P.R. Production of pigment using Aspergillus tamarii: New potentials for synthesizing natural metabolites. Environ. Technol. Innov. 2020, 19, 100967. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Pal, A.K.; Gajjar, D.U.; Vasavada, A.R. DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med. Mycol. 2014, 52, 10–18. [Google Scholar] [CrossRef]
- Geib, E.; Gressler, M.; Viediernikova, I.; Hillmann, F.; Jacobsen, I.D.; Nietzsche, S.; Hertweck, C.; Brock, M. A non-canonical melanin biosynthesis pathway protects Aspergillus terreus conidia from environmental stress. Cell Chem. Biol. 2016, 23, 587–597. [Google Scholar] [CrossRef]
- Narendrababu, B.N.; Shishupala, S. Spectrophotometric detection of Pigments from Aspergillus and Penicillium isolates. J. Appl. Biol. Biotechnol. 2017, 5, 53–58. [Google Scholar] [CrossRef]
- Bouras, H.D.; Yeddou, A.R.; Bouras, N.; Hellel, D.; Holtz, M.D.; Sabaou, N.; Chergui, A.; Nadjemi, B. Biosorption of Congo red dye by Aspergillus carbonarius M333 and Penicillium glabrum Pg1: Kinetics, equilibrium and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2017, 80, 915–923. [Google Scholar] [CrossRef]
- Kantifedaki, A.; Kachrimanidou, V.; Mallouchos, A.; Papanikolaou, S.; Koutinas, A.A. Orange processing waste valorisation for the production of bio-based pigments using the fungal speciess Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 2018, 185, 882–890. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.-B.; Chen, D.; Zhang, A.-M.; Niu, S.-Q. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Babitha, S.; Soccol, C.R.; Pandey, A. Solid-state fermentation for the production of Monascus pigments from jackfruit seed. Bioresour. Technol. 2007, 98, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.R.; Stuart, D.M. Production of pigments by Monascus purpureus in solid culture. J. Ind. Microbiol. 1991, 8, 23–28. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Cardoso, L.C.; Ghiggi, V.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P.; Soccol, C.R. Microbial Pigments. In Biotransformation of Waste Biomass into High Value Biochemicals, 1st ed.; Brar, S.K., Dhillon, G.S., Soccol, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 73–97. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, A.; Khan, S.A.; Yusuf, M.; Shahid, M.; Manzoor, N.; Mohammad, F. Assessment of antimicrobial activity of Catechu and its dyed substrate. J. Clean. Prod. 2011, 19, 1385–1394. [Google Scholar] [CrossRef]
- Shibila, S.D.; Nanthini, A.U.R. Extraction and characterization of red pigment from Talaromyces australis and its application in dyeing cotton yarn. Int. Arch. App. Sci. Technol. 2019, 10, 81–91. [Google Scholar] [CrossRef]
- Sankaran, S.; Khanal, S.K.; Jasti, N.; Jin, B.; Pometto, A.L.; Van Leeuwen, J.H. Use of Filamentous Fungi for Wastewater Treatment and Production of High Value Fungal Byproducts: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 400–449. [Google Scholar] [CrossRef]
- Akilandeswari, P.; Pradeep, B.V. Aspergillus terreus Kmbf1501 A Potential Pigment Producer Under Submerged Fermentation. Int. J. Pharm. Pharm. Sci. 2017, 9, 38–43. [Google Scholar] [CrossRef]
- Raman, N.M.; Shah, P.H.; Mohan, M.; Ramasamy, S. Improved production of melanin from Aspergillus fumigatus AFGRD105 by optimization of media factors. AMB Express 2015, 5, 72. [Google Scholar] [CrossRef]
- Palacio-Barrera, A.M.; Areiza, D.; Zapata, P.; Atehortúa, L.; Correa, C.; Peñuela-Vásquez, M. Induction of pigment production through media composition, abiotic and biotic factors in two filamentous fungi. Biotechnol. Rep. 2019, 21, e00308. [Google Scholar] [CrossRef]
- Said, F.M.; Brooks, J.; Chisti, Y. Optimal C:N ratio for the production of red pigments by Monascus ruber. World J. Microbiol. Biotechnol. 2014, 30, 2471–2479. [Google Scholar] [CrossRef]
- Dufossé, L. Pigments, Microbial. Reference Module in Life Sciences. 2016. Available online: https://hal.archives-ouvertes.fr/hal-01734750/document (accessed on 14 October 2020).
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Gmoser, R.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Post-treatment of Fungal Biomass to Enhance Pigment Production. Appl. Biochem. Biotechnol. 2019, 189, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, T.; Prabhudessai, T. A Solid Liquid State Culture Method to Stimulate Monascus Pigments by Intervention of Different Substrates. Int. Res. J. Biol. Sci. 2013, 2, 22–29. [Google Scholar]
- Satyanarayana, T.; Deshmukh, S.K.; Johri, B.N. Developments in Fungal Biology and Applied Mycology; Springer: Singapore, 2017; pp. 525–541. [Google Scholar]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between Secondary Metabolism and Fungal Development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef]
- Baker, S.E.; Bennett, J. An Overview of the Genus Aspergillus. In The Aspergilli: Genomics, Medical Aspects, Biotechnology, and Research Methods; Machida, M., Gomi, K., Eds.; Caister Academic Press: Poole, UK, 2007; Volume 26, pp. 3–13. [Google Scholar]
- Teertstra, W.R.; Tegelaar, M.; Dijksterhuis, J.; Golovina, E.A.; Ohm, R.A.; Wösten, H.A.B. Maturation of conidia on conidiophores of Aspergillus niger. Fungal Genet. Biol. 2017, 98, 61–70. [Google Scholar] [CrossRef]
- Zalokar, M. Studies on biosynthesis of carotenoids in Neurospora crassa. Arch. Biochem. Biophys. 1954, 50, 71–80. [Google Scholar] [CrossRef]
- Babitskaya, V.G.; Shcherba, V.V.; Filimonova, T.V.; Grigorchyuk, E.A. Melanin pigments from the fungi Paecilomyces variotii and Aspergillus carbonarius. Appl. Biochem. Microbiol. 2000, 36, 128. [Google Scholar] [CrossRef]
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.R.; Lisboa, H.F.; Gonçalves, R.C.R. Production of Melanin Pigment by Fungi and Its Biotechnological Applications. In Melanin; Blumenber, M., Ed.; IntechOpen: London, UK, 2017; p. 3760. Available online: https://www.intechopen.com/books/melanin/production-of-melanin-pigment-by-fungi-and-its-biotechnological-applications (accessed on 14 October 2020).
- Afshari, M.; Shahidi, F.; Mortazavi, S.A.; Tabatabai, F.; Es’haghi, Z. Investigating the influence of pH, temperature and agitation speed on yellow pigment production by Penicillium aculeatum ATCC 10409. Nat. Prod. Res. 2015, 29, 1300–1306. [Google Scholar] [CrossRef]
- Arora, D.S.; Chandra, P. Antioxidant Activity of Aspergillus fumigatus. ISRN Pharmacol. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, J.P.; Hwang, H.J.; Kim, S.W.; Choi, J.W.; Yun, J.W. Production of red pigment by submerged culture of Paecilomyces sinclairii. Lett. Appl. Microbiol. 2002, 35, 195–202. [Google Scholar] [CrossRef]
- Pihet, M.; Vandeputte, P.; Tronchin, G.; Renier, G.; Saulnier, P.; Georgeault, S.; Mallet, R.; Chabasse, D.; Symoens, F.; Bouchara, J.-P. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 2009, 9, 177. [Google Scholar] [CrossRef]
- Hernández, F.; Ibáñez, M.; Portolés, T.; Cervera, M.I.; Sancho, J.V.; López, F.J. Advancing towards universal screening for organic pollutants in waters. J. Hazard. Mater. 2015, 282, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Schiweck, H.; Bär, A.; Vogel, R.; Schwarz, E.; Kunz, M.; Dusautois, C.; Clement, A.; Lefranc, C.; Lüssem, B.; Moser, M.; et al. Sugar Alcohols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Sinha, M.; Weyda, I.; Sørensen, A.; Bruno, K.S.; Ahring, B.K. Alkane biosynthesis by Aspergillus carbonarius ITEM 5010 through heterologous expression of Synechococcus elongatus acyl-ACP/CoA reductase and aldehyde deformylating oxygenase genes. AMB Express 2017, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Mellado, E.; Garcia-Effron, G.; Lopez, J.F.; Grimalt, J.O.; Cuenca-Estrella, J.M.; Rodriguez-Tudela, J.L. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 2008, 73, 339–347. [Google Scholar] [CrossRef]
- Vadlapudi, V.; Borah, N.; Yellusani, K.R.; Gade, S.; Reddy, P.; Rajamanikyam, M.; Vempati, L.N.S.; Gubbala, S.P.; Chopra, P.; Upadhyayula, S.M.; et al. Aspergillus Secondary Metabolite Database, a resource to understand the Secondary metabolome of Aspergillus genus. Sci. Rep. 2017, 7, 7325. [Google Scholar] [CrossRef]
- Gealt, M.A. Isolation of p-Amyrin from the Fungus Aspergillus nidulans. J. Gen. Microbiol. 1983, 129, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.M. Flavoring and medicinal values of the yellow pigment produced by Monascus ruber 4066 species cultivated on static malt agar medium. Int. Res. J. Biochem. Biotechnol. 2016, 3, 37–43. [Google Scholar]
- Caligiani, A.; Bonzanini, F.; Palla, G.; Cirlini, M.; Bruni, R. Characterization of a Potential Nutraceutical Ingredient: Pomegranate (Punica granatum L.) Seed Oil Unsaponifiable Fraction. Plant Foods Hum. Nutr. 2010, 65, 277–283. [Google Scholar] [CrossRef]
- Yin, S.J.; Si, Y.X.; Qian, G.Y. Inhibitory Effect of Phthalic Acid on Tyrosinase: The Mixed-Type Inhibition and Docking Simulations. Enzym. Res. 2011, 294724, 7. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Topical treatment of melasma. Indian J. Dermatol. 2009, 54, 303–309. [Google Scholar] [CrossRef]
- Lu, Y.H.; Tao, L.; Wang, Z.T.; Wei, D.Z.; Xiang, H.B. Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J. Enzym. Inhib. Med. Chem. 2007, 22, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Shcherba, V.V.; Babitskaya, V.G.; Kurchenko, V.P.; Ikonnikova, N.V.; Kukulyanskaya, T.A. Antioxidant Properties of Fungal Melanin Pigments. Appl. Biochem. Microbiol. 2000, 36, 491–495. [Google Scholar] [CrossRef]
- Zeidan, R.; Ul-Hassan, Z.; Al-Thani, R.; Migheli, Q.; Jaoua, S. In-Vitro Application of a Qatari Burkholderia cepacia species (QBC03) in the Biocontrol of Mycotoxigenic Fungi and in the Reduction of Ochratoxin A biosynthesis by Aspergillus carbonarius. Toxins 2019, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Bruno, K.S.; Solfrizzo, M.; Perrone, G.; Mulè, G.; Visconti, A.; Baker, S.E. New Insight into the Ochratoxin A Biosynthetic Pathway through Deletion of a Nonribosomal Peptide Synthetase Gene in Aspergillus carbonarius. Appl. Environ. Microbiol. 2012, 78, 8208–8218. [Google Scholar] [CrossRef]
- Castellá, G.; Bragulat, M.R.; Puig, L.; Sanseverino, W.; Cabañes, F.J. Genomic diversity in ochratoxigenic and non ochratoxigenic strains of Aspergillus carbonarius. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Moghaddam, M.K.; Adivi, M.G.; Dehkord, M.T. Effect of Acids and Different Mordanting Procedures on Color Characteristics of Dyed Wool Fibers Using Eggplant Peel (Solanum melongena L). Prog. Color Colorants Coat. 2019, 12, 219–230. [Google Scholar]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From Extraction to Advanced Analytical Methods: The Challenges of Melanin Analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef]

| Type of Pulp | C (%) | H (%) | N (%) | S (%) | C:N |
|---|---|---|---|---|---|
| Black carrot | 29.79 | 4.27 | 1.32 | 0.00 | 22.57/1 |
| Red beet | 37.54 | 5.43 | 1.82 | 0.00 | 20.63/1 |
| Pomegranate | 50.94 | 6.08 | 3.02 | 0.02 | 16.87/1 |
| Apple | 52.12 | 7.64 | 4.52 | 0.11 | 11.53/1 |
| Sample | C (%) | H (%) | N (%) | S (%) | C:N |
|---|---|---|---|---|---|
| Unfermented PP | 50.94 | 6.08 | 3.02 | 0.02 | 16.87/1 |
| Fermented PP | 42.46 | 5.09 | 1.60 | 0.00 | 26.54/1 |
| Name of Compounds | Chemical Structure | Formula | Classification | S |
|---|---|---|---|---|
| Xylitol | 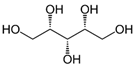 | C5H12O5 | Alcohol | 951 |
| L-Arabinitol | 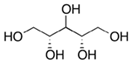 | C5H12O5 | Alcohol | 946 |
| Sorbitol | 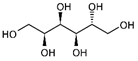 | C6H14O6 | Alcohol | 796 |
| Ethanol | 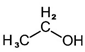 | C2H5OH | Alcohol | 949 |
| Linoleic acid | 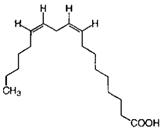 | C18H32O2 | Fatty acid | 851 |
| Oleic acid | 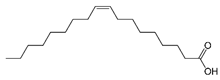 | C18H34O2 | Fatty acid | 829 |
| Erucic acid | 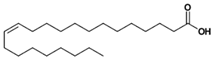 | C22H42O2 | Fatty acid | 753 |
| Ergosterol | 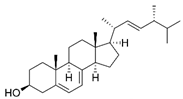 | C28H44O | Sterol | 794 |
| Squalene | 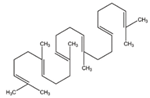 | C30H50 | Triterpene | 778 |
| β-Amyrin | 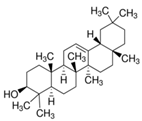 | C30H50O | Triterpene | 887 |
| 2,6-diisopropylnaphthalene | 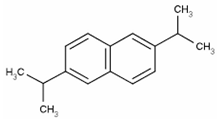 | C16H26 | Naphthalene | 796 |
| γ-Tocopherol | 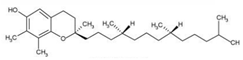 | C28H48O2 | Vitamin E | 899 |
| Galangin | 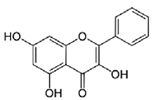 | C15H10O5 | Flavonoid | 754 |
| Phthalic acid | 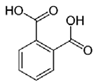 | C6H4(CO2) | Dicarboxylic acid | 884 |
| Mequinol (4-methoxyphenol) | 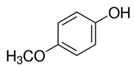 | C7H8O2 | Methoxyphenol | 723 |
| 1,8-dihydroxynaphthalene | 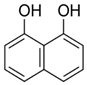 | C10H8O2 | Naphthalene | 941 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezirhan Arikan, E.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of Bio-Based Pigments from Food Processing Industry By-Products (Apple, Pomegranate, Black Carrot, Red Beet Pulps) Using Aspergillus carbonarius. J. Fungi 2020, 6, 240. https://doi.org/10.3390/jof6040240
Bezirhan Arikan E, Canli O, Caro Y, Dufossé L, Dizge N. Production of Bio-Based Pigments from Food Processing Industry By-Products (Apple, Pomegranate, Black Carrot, Red Beet Pulps) Using Aspergillus carbonarius. Journal of Fungi. 2020; 6(4):240. https://doi.org/10.3390/jof6040240
Chicago/Turabian StyleBezirhan Arikan, Ezgi, Oltan Canli, Yanis Caro, Laurent Dufossé, and Nadir Dizge. 2020. "Production of Bio-Based Pigments from Food Processing Industry By-Products (Apple, Pomegranate, Black Carrot, Red Beet Pulps) Using Aspergillus carbonarius" Journal of Fungi 6, no. 4: 240. https://doi.org/10.3390/jof6040240
APA StyleBezirhan Arikan, E., Canli, O., Caro, Y., Dufossé, L., & Dizge, N. (2020). Production of Bio-Based Pigments from Food Processing Industry By-Products (Apple, Pomegranate, Black Carrot, Red Beet Pulps) Using Aspergillus carbonarius. Journal of Fungi, 6(4), 240. https://doi.org/10.3390/jof6040240