Functional Defect of Neutrophils Causing Dermatophytosis: Case Report
Abstract
1. Introduction
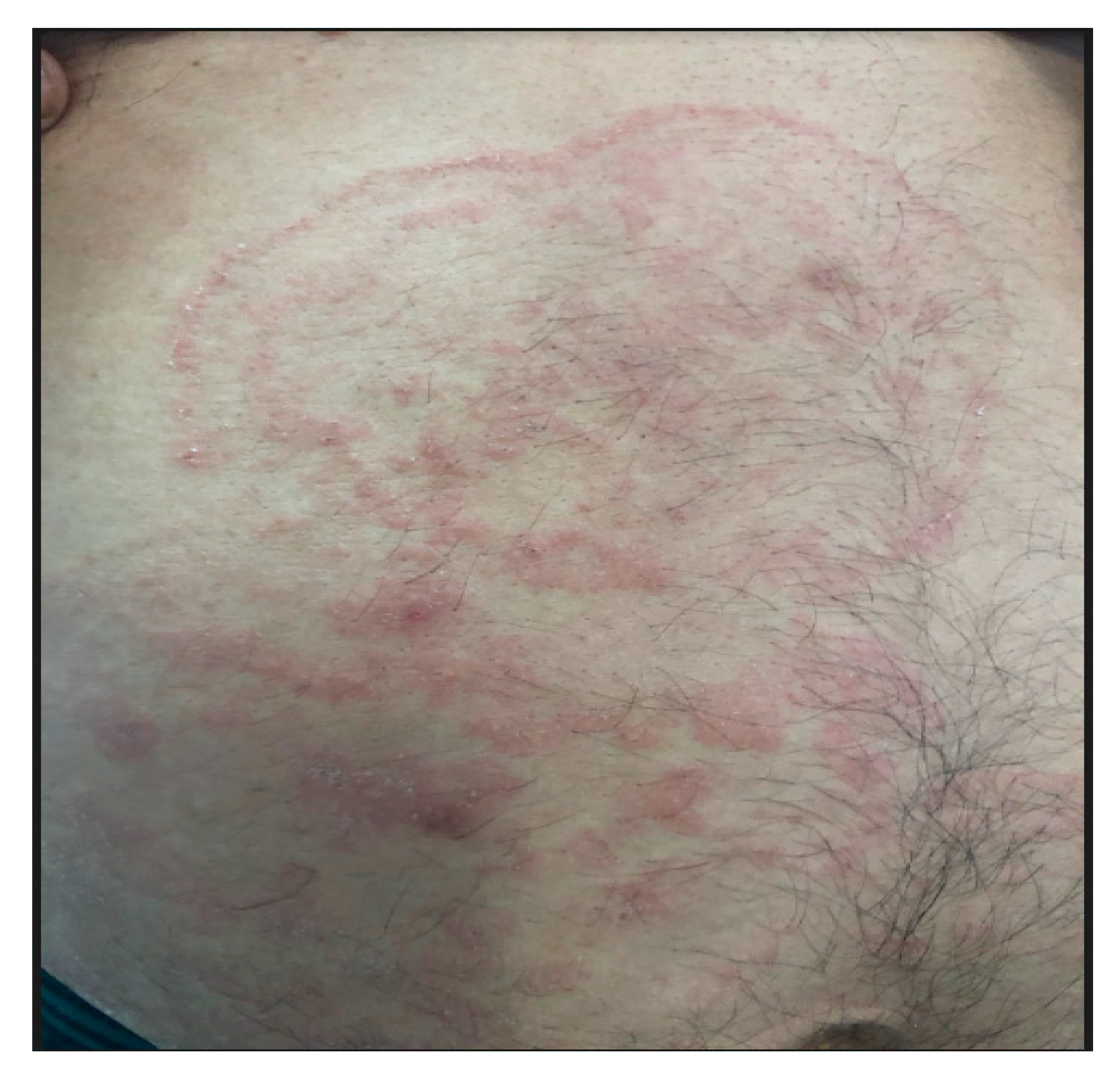
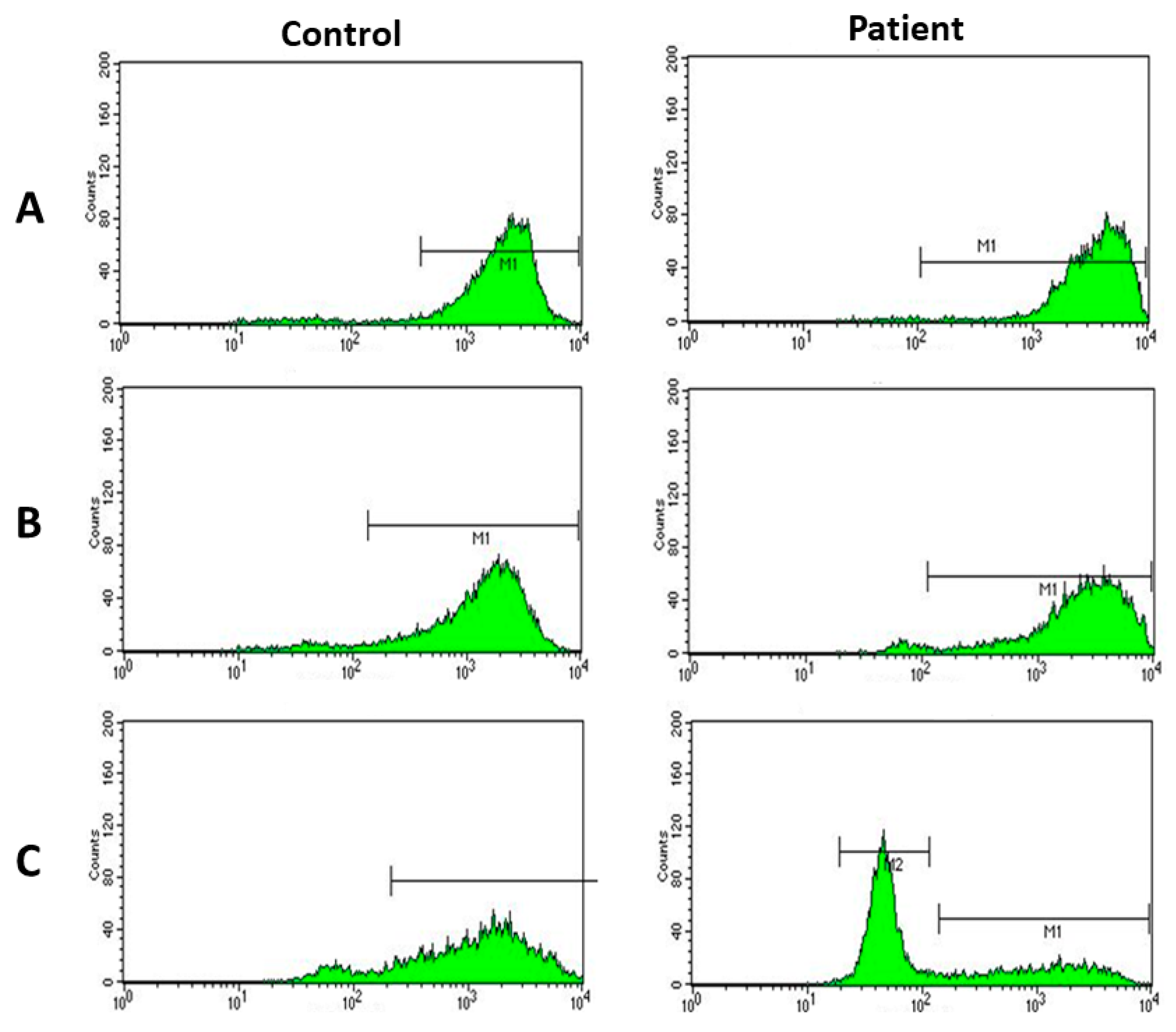
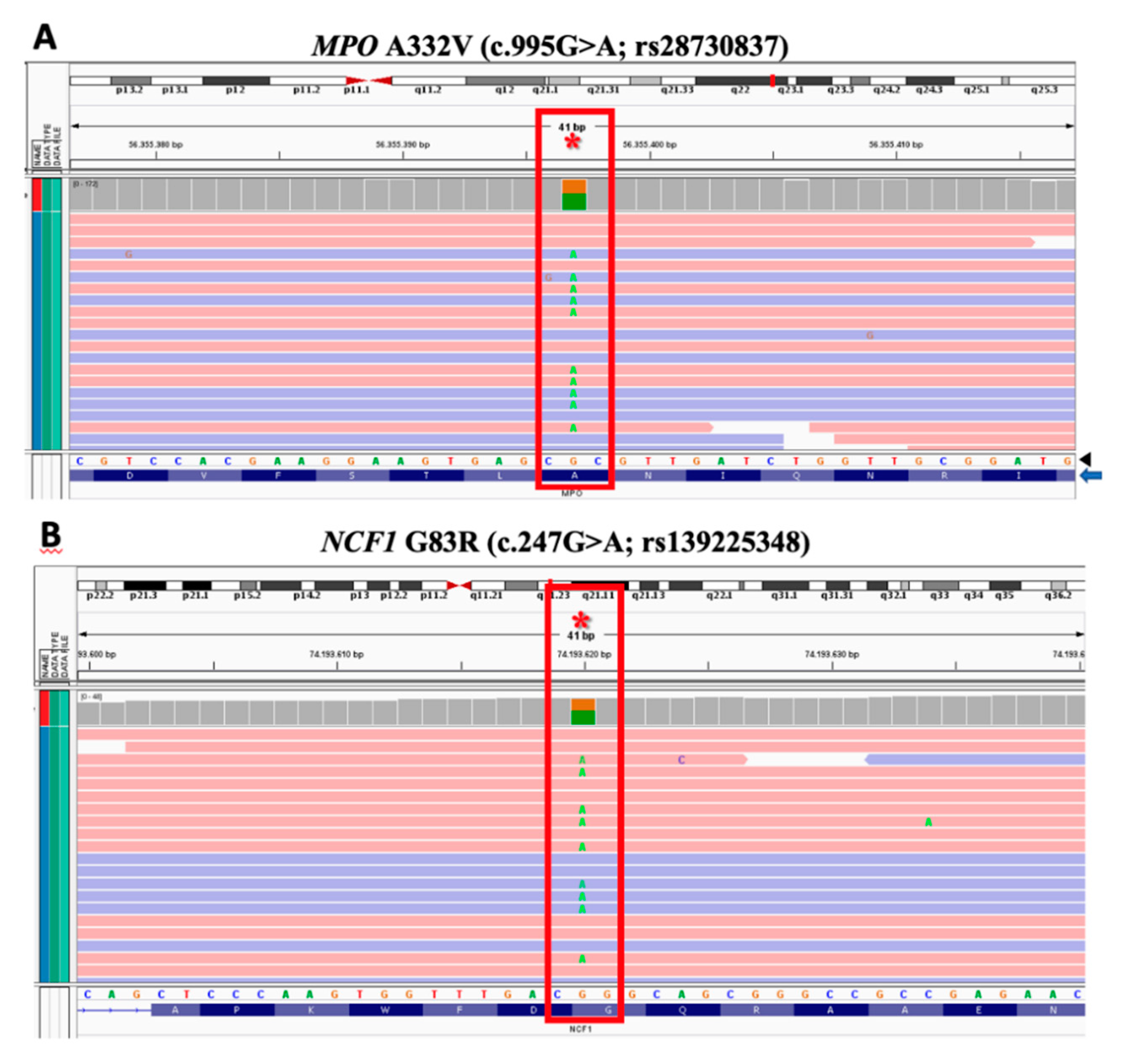
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roos, D. Chronic Granulomatous Disease. Methods Mol. Biol. 2019, 1982, 531–542. [Google Scholar] [PubMed]
- Abid, M.R.; Spokes, K.C.; Shih, S.C.; Aird, W.C. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J. Biol. Chem. 2007, 282, 35373–35385. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D.; Kawahara, T.; Diebold, B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007, 43, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Curnutte, J.T. Chronic granulomatous disease: The solving of a clinical riddle at the molecular level. Clin. Immunol. Immunopathol. 1993, 67, S2–S15. [Google Scholar] [CrossRef]
- Winkelstein, J.A.; Marino, M.C.; Johnston, R.B., Jr.; Boyle, J.; Curnutte, J.; Gallin, J.I.; Malech, H.L.; Holland, S.M.; Ochs, H.; Quie, P.; et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Med. Baltim. 2000, 79, 155–169. [Google Scholar] [CrossRef]
- Segal, B.H.; Leto, T.L.; Gallin, J.I.; Malech, H.L.; Holland, S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Med. Baltim. 2000, 79, 170–200. [Google Scholar] [CrossRef]
- Hansson, M.; Olsson, I.; Nauseef, W.M. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch. Biochem. Biophys. 2006, 445, 214–224. [Google Scholar] [CrossRef]
- Lau, D.; Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 2006, 111, 16–26. [Google Scholar] [CrossRef]
- Nauseef, W.M. Myeloperoxidase biosynthesis by a human promyelocytic leukemia cell line: Insight into myeloperoxidase deficiency. Blood 1986, 67, 865–872. [Google Scholar] [CrossRef]
- Cech, P.; Papathanassiou, A.; Boreux, G.; Roth, P.; Miescher, P.A. Hereditary myeloperoxidase deficiency. Blood 1979, 53, 403–411. [Google Scholar] [CrossRef]
- Edwards, S.W.; Hart, C.A.; Davies, J.M.; Pattison, J.; Hughes, V.; Sills, J.A. Impaired neutrophil killing in a patient with defective degranulation of myeloperoxidase. J. Clin. Lab. Immunol. 1988, 25, 201–206. [Google Scholar]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun. 1996, 64, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.M.; van Koppen, E.; Ahlin, A.; Belohradsky, B.H.; Bernatowska, E.; Corbeel, L.; Espanol, T.; Fischer, A.; Kurenko-Deptuch, M.; Mouy, R.; et al. Chronic granulomatous disease: The European experience. PLoS ONE 2009, 4, e5234. [Google Scholar] [CrossRef]
- De Souza Ferreira, C.; Araújo, T.H.; Ângelo, M.L.; Pennacchi, P.C.; Okada, S.S.; de Araujo Paula, F.B.; Migliorini, S.; Rodrigues, M.R. Neutrophil dysfunction induced by hyperglycemia: Modulation of myeloperoxidase activity. Cell. Biochem. Funct. 2012, 30, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Brinkmann Frye, E.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997, 324, 565–570. [Google Scholar] [CrossRef]
- Marchetti, C.; Patriarca, P.; Pietro Solero, G.; Baralle, F.E.; Romano, M. Genetic Characterization of Myeloperoxidase Deficiency in Italy. Hum. Mut. 2004, 23, 496–505. [Google Scholar] [CrossRef] [PubMed]
- MacCarthy, K.G.; Dahl, M.V. Inhibition of growth of Trichophyto rubrum by the myeloperoxidase-hydrogen peroxide-chloride system. J. Investig. Dermatol. 1989, 92, 639–641. [Google Scholar] [CrossRef]
- Nguyen, C.; Katner, H.P. Myeloperoxidase deficiency manifesting as pustular candida dermatitis. Clin. Infect. Dis. 1997, 24, 258–260. [Google Scholar] [CrossRef]
- Parry, M.F.; Root, R.K.; Metcalf, J.A.; Delaney, K.K.; Kaplow, L.S.; Richar, W.J. Myeloperoxidase deficiency: Prevalence and clinical significance. Ann. Intern. Med. 1981, 95, 293–301. [Google Scholar] [CrossRef]
- Weber, M.L.; Abela, A.; de Repentigny, L.; Garel, L.; Lapointe, N. Myeloperoxidase deficiency with extensive candidal osteomyelitis of the base of the skull. Pediatrics 1987, 80, 876–879. [Google Scholar]
- El-Benna, J.; Dang, P.M.C.; Gougerot-Pocidalo, M.A.; Marie, J.C.; Braut-Boucher, F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: Structure, phosphorylation and implication in diseases. Exp. Mol. Med. 2009, 41, 217–225. [Google Scholar] [CrossRef] [PubMed]
- De Leo, F.R.; Ulman, K.V.; Davis, A.R.; Jutila, K.L.; Quinn, M.T. Assembly of the human neutrophil NADPH oxidase involves binding of p67phox and flavocytochrome b to a common functional domain in p47phox. J. Biol. Chem. 1996, 271, 17013–17020. [Google Scholar] [CrossRef]
- Dang, P.M.; Cross, A.R.; Babior, B.M. Assembly of the neutrophil respiratory burst oxidase: A direct interaction between p67PHOX and cytochrome b558. Proc. Natl. Acad. Sci. USA 2001, 98, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Motalebi, S.; Han, C.H.; Lambeth, J.D. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b (558). J. Biol. Chem. 1999, 274, 22999–23005. [Google Scholar] [CrossRef]
- Jackson, S.H.; Gallin, J.I.; Holland, S.M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 1995, 182, 751–758. [Google Scholar] [CrossRef]
- Denson, L.A.; Jurickova, I.; Karns, R.; Shaw, K.A.; Cutler, D.J.; Okou, D.T.; Dodd, A.; Quinn, K.; Mondal, K.; Aronow, B.J.; et al. Clinical and Genomic Correlates of Neutrophil Reactive Oxygen Species Production in Pediatric Patients With Crohn’s Disease. Gastroenterol 2018, 154, 2097–2110. [Google Scholar] [CrossRef]
- Bielinski, S.J.; Hall, J.L.; Pankow, J.S.; Boerwinkle, E.; Matijevic-Aleksic, N.; He, M.; Chambless, L.; Folsom, A.R. Genetic variants in TLR2 and TLR4 are associated with markers of monocyte activation: The Atherosclerosis Risk in Communities MRI Study. Hum. Genet. 2011, 129, 655–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lek, L.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- D’Ostiani, C.F.; Del Sero, G.; Bacci, A.; Montagnoli, C.; Spreca, A.; Mencacci, A.; Ricciardi-Castagnolic, P.; Romania, L. Dendritic cells discriminate between yeast and hyphae of the fungus Candida albicans: Implications for initiation of T Helper immunity in vitro and in vivo. J. Exp. Med. N. Y. 2000, 191, 1661–1674. [Google Scholar] [CrossRef]
- Blake, J.S.; Dahl, M.V.; Herron, M.J.; Nelson, R.D. An immunoinhibitory cell wall glycoprotein (mannan) from Trichophyton rubrum. J. Investig. Dermatol. 1991, 96, 657–661. [Google Scholar] [CrossRef]
| Parameters | Patient | Reference Range |
|---|---|---|
| IgG (mg/dL) | 1070 | 384–1200 |
| IgM (mg/dL) | 85 | 40–230 |
| IgA (mg/dL) | 339 | 22–176 |
| IgE (UI/mL) | 3829 | <100 |
| Leukocytes (cells/mm3) | 12,960 | 6000–11,000 |
| Isoagglutinin | Anti-B: 1/32 | >1/4 |
| Serology for rubella | IgG + IgM- | Positive IgG |
| Serology for CMV | IgG + IgM- | Positive IgG |
| Antipneumococcal antibodies | <0.5 for 7 serotypes | Positive response |
| B cells cells/mm3 (%) | 117 (8.04) | 161–979 (12.8–38.4) |
| T CD3+ cells/mm3 (%) | 1187 (81.24) | 605–2460 (60–87) |
| T CD4+ cells/mm3 (%) | 787 (53.85) | 493–1666 (32–61) |
| T CD8+ cells/mm3 (%) | 400 (27.4) | 224–1112 (34–43) |
| Proliferative response to PHA (s.i.) * | 24.1 | Control = 128.9 |
| Proliferative response to CMV (s.i.) * | 5.7 | Control = 99.8 |
| Proliferative response to tetanus toxoid (s.i.) * | 0.5 | Control = 50.1 |
| Proliferative response to candidin (s.i.) * | 4.6 | Control = 80.5 |
| NK cells cells/mm3 (%) | 134 | 73–654 (4–28) |
| C3 level (mg/dL) | 105 | 88–165 |
| C4 level (mg/dL) | 27 | 14–44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantino-Silva, R.N.; Perazzio, S.F.; Weidebach, N.d.A.; Grumach, A.S. Functional Defect of Neutrophils Causing Dermatophytosis: Case Report. J. Fungi 2020, 6, 238. https://doi.org/10.3390/jof6040238
Constantino-Silva RN, Perazzio SF, Weidebach NdA, Grumach AS. Functional Defect of Neutrophils Causing Dermatophytosis: Case Report. Journal of Fungi. 2020; 6(4):238. https://doi.org/10.3390/jof6040238
Chicago/Turabian StyleConstantino-Silva, Rosemeire N., Sandro F. Perazzio, Nicolas de Albuquerque Weidebach, and Anete S. Grumach. 2020. "Functional Defect of Neutrophils Causing Dermatophytosis: Case Report" Journal of Fungi 6, no. 4: 238. https://doi.org/10.3390/jof6040238
APA StyleConstantino-Silva, R. N., Perazzio, S. F., Weidebach, N. d. A., & Grumach, A. S. (2020). Functional Defect of Neutrophils Causing Dermatophytosis: Case Report. Journal of Fungi, 6(4), 238. https://doi.org/10.3390/jof6040238





