Rhizoferrin Glycosylation in Rhizopus microsporus
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Cultivation, Fungal Extraction, and Metabolite Analysis
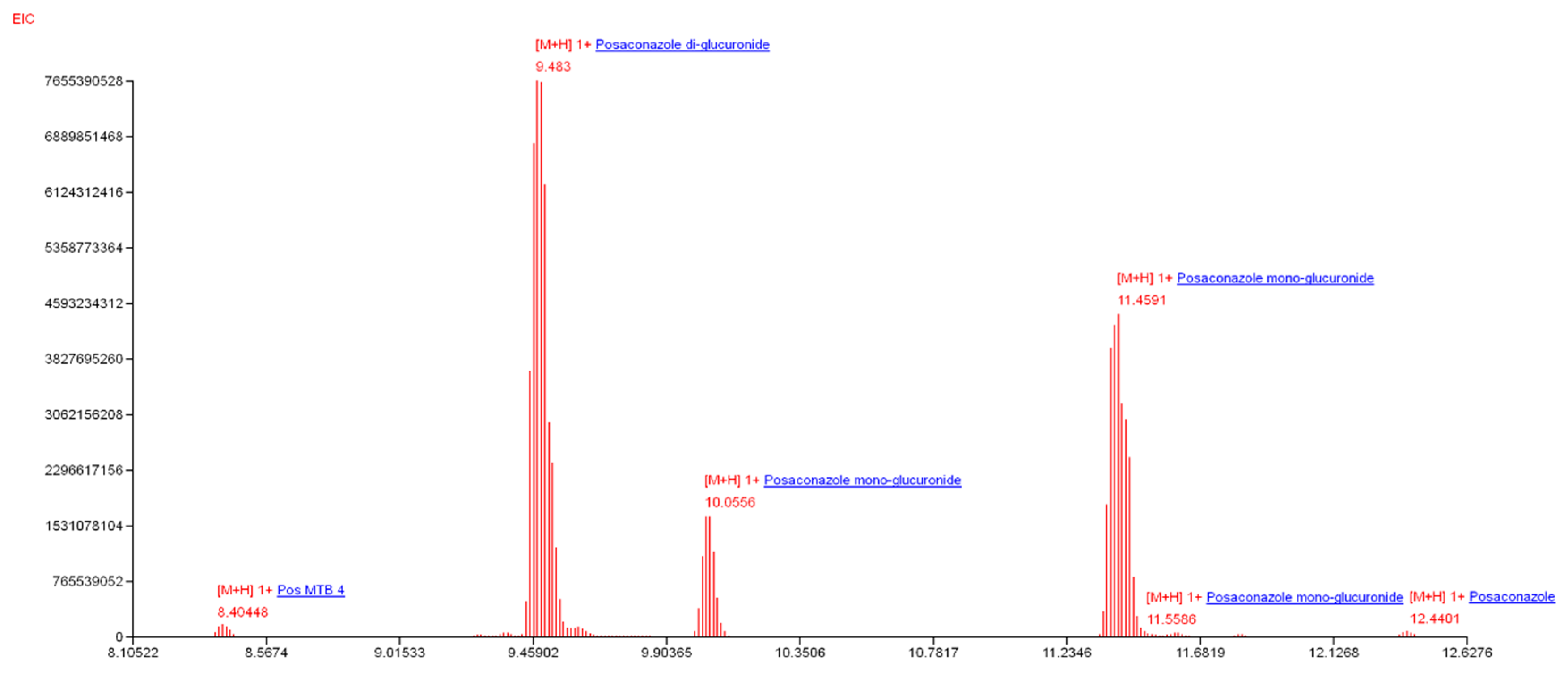
2.2. Molecular Identification and Human Sample Collection
3. Results
3.1. HPLC Separation and Annotation of Rhizoferrin Analogs
3.2. Quantitation of Rhizoferrins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morin-Sardin, S.; Nodet, P.; Coton, E.; Jany, J.-L. Mucor: A Janus-faced fungal genus with human health impact and industrial applications. Fungal Biol. Rev. 2017, 31, 12–32. [Google Scholar] [CrossRef]
- Voigt, K.; Wolf, T.; Ochsenreiter, K.; Nagy, G.; Kaerger, K.; Shelest, E.; Papp, T. Genetic and metabolic aspects of primary and secondary metabolism of the zygomycetes. In Biochemistry and Molecular Biology; Hoffmeister, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 361–385. [Google Scholar]
- Müller, C.; Neugebauer, T.; Zill, P.; Lass-Flörl, C.; Bracher, F.; Binder, U. Sterol composition of clinically relevant mucorales and changes resulting from posaconazole treatment. Molecules 2018, 23, 1218. [Google Scholar] [CrossRef] [PubMed]
- Gooday, G.W.; Carlile, M.J. The discovery of fungal sex hormones: III. Trisporic acid and its precursors. Mycologist 1997, 11, 126–130. [Google Scholar] [CrossRef]
- Hollmann, M.; Razzazi-Fazeli, E.; Grajewski, J.; Twaruzek, M.; Sulyok, M.; Böhm, J. Detection of 3-nitropropionic acid and cytotoxicity in Mucor circinelloides. Mycotoxin Res. 2008, 24, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Jennessen, J.; Nielsen, K.F.; Houbraken, J.; Lyhne, E.K.; Schnürer, J.; Frisvad, J.C.; Samson, R.A. Secondary metabolite and mycotoxin production by the Rhizopus microsporus group. J. Agric. Food Chem. 2005, 53, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Partida-Martinez, L.P.; Flores de Looß, C.; Ishida, K.; Ishida, M.; Roth, M.; Buder, K.; Hertweck, C. Rhizonin, the first mycotoxin isolated from the zygomycota, is not a fungal metabolite but is produced by bacterial endosymbionts. Appl. Environ. Microbiol. 2007, 73, 793–797. [Google Scholar] [CrossRef]
- Winkelmann, G. A search for glomuferrin: A potential siderophore of arbuscular mycorrhizal fungi of the genus Glomus. Biometals 2017, 30, 559–564. [Google Scholar] [CrossRef]
- Carroll, C.S.; Grieve, C.L.; Murugathasan, I.; Bennet, A.J.; Czekster, C.M.; Lui, H.; Naismith, J.; Moore, M.M. The rhizoferrin biosynthetic gene in the fungal pathogen Rhizopus delemar is a novel member of the NIS gene family. Int. J. Biochem. Cell Biol. 2017, 89, 136–146. [Google Scholar] [CrossRef]
- Polaino, S.; Gonzalez-Delgado, J.A.; Arteaga, P.; Herrador, M.M.; Barrero, A.F.; Cerdá-Olmedo, E. Apocarotenoids in the sexual interaction of Phycomyces blakesleeanus. Org. Biomol. Chem. 2012, 10, 3002–3009. [Google Scholar] [CrossRef]
- Lee, S.C.; Heitman, J. Sex in the Mucoralean fungi. Mycoses 2014, 57 (Suppl. 3), 18–24. [Google Scholar] [CrossRef]
- Sutter, R.P.; Capage, D.A.; Harrison, T.L.; Keen, W.A. Trisporic acid biosynthesis in separate plus and minus cultures of Blakeslea trispora: Identification by Mucor assay of two mating-type-specific components. J. Bacteriol. 1973, 114, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Shoolery, J.N.; Schwyn, B.; Holden, I.; Neilands, J.B. Rhizobactin, a structurally novel siderophore from Rhizobium meliloti. J. Am. Chem. Soc. 1985, 107, 1739–1743. [Google Scholar] [CrossRef]
- Drechsel, H.; Tschierske, M.; Thieken, A.; Jung, G.; Zähner, H.; Winkelmann, G. The carboxylate type siderophore rhizoferrin and its analogs produced by directed fermentation. J. Ind. Microbiol. 1995, 14, 105–112. [Google Scholar] [CrossRef]
- Haselwandter, K.; Haas, H.; Häninger, G.; Winkelmann, G. Siderophores in plant root tissue: Tagetes patula nana colonized by the arbuscular mycorrhizal fungus Gigaspora margarita. Biometals 2020. [Google Scholar] [CrossRef]
- Gao, S.-S.; Li, X.-M.; Williams, K.; Proksch, P.; Ji, N.-Y.; Wang, B.-G. Rhizovarins A–F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189. J. Nat. Prod. 2016, 79, 2066–2074. [Google Scholar] [CrossRef]
- Schwartze, V.U.; Winter, S.; Shelest, E.; Marcet-Houben, M.; Horn, F.; Wehner, S.; Linde, J.; Valiante, V.; Sammeth, M.; Riege, K.; et al. Gene expansion shapes genome architecture in the human pathogen Lichtheimia corymbifera: An evolutionary genomics analysis in the ancient terrestrial Mucorales (Mucoromycotina). PLoS Genet. 2014, 10, e1004496. [Google Scholar] [CrossRef]
- Drechsel, H.; Metzger, J.; Freund, S.; Jung, G.; Boelaert, J.R.; Winkelmann, G. Rhizoferrin—A novel siderophore from the fungus Rhizopus microsporus var. rhizopodiformis. Biol. Met. 1991, 4, 238–243. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Jeffery, E.F.; Shannon, J.D.; Ramakrishnan, G. Characterization of the siderophore of Francisella tularensis and role of fslA in siderophore production. J. Bacteriol. 2006, 188, 3785–3795. [Google Scholar] [CrossRef]
- Carroll, C.S.; Moore, M.M. Ironing out siderophore biosynthesis: A review of non-ribosomal peptide synthetase (NRPS)-independent siderophore synthetases. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 356–381. [Google Scholar] [CrossRef]
- Pluhacek, T.; Lemr, K.; Ghosh, D.; Milde, D.; Novak, J.; Havlicek, V. Characterization of microbial siderophores by mass spectrometry. Mass Spectrom. Rev. 2016, 35, 35–47. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Felsenfeld, A.J.; Rodriguez, M.; Coleman, M.; Ross, D.; Llach, F. Desferrioxamine therapy in hemodialysis patients with aluminum-associated bone disease. Kidney Int. 1989, 35, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.A. Cefiderocol: A novel siderophore cephalosporin defeating carbapenem-resistant pathogens. Clin. Infect. Dis. 2019, 69, S519–S520. [Google Scholar] [CrossRef] [PubMed]
- Dobias, R.; Havlicek, V. Microbial siderophores: Markers of infectious diseases. In Microbial and Natural Macromolecules: Synthesis and Applications; Das, S., Das, H.R., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2020. [Google Scholar]
- Hoenigl, M.; Orasch, T.; Faserl, K.; Prattes, J.; Loeffler, J.; Springer, J.; Gsaller, F.; Reischies, F.; Duettmann, W.; Raggam, R.B.; et al. Triacetylfusarinine C: A urine biomarker for diagnosis of invasive aspergillosis. J. Infect. 2019, 78, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pluhacek, T.; Skriba, A.; Novak, J.; Luptakova, D.; Havlicek, V. Analysis of microbial siderophores by mass spectrometry. In Methods Molecular Biology; Bhattacharya, S.K., Ed.; Springer Nature: New York, NY, USA, 2019. [Google Scholar]
- Devireddy, L.R.; Hart, D.O.; Goetz, D.H.; Green, M.R. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell 2010, 141, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Clifton, M.; Hoette, T.M.; Mori, K.; Deng, S.-X.; Qiu, A.; Viltard, M.; Williams, D.; Paragas, N.; Leete, T.; et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol. 2010, 6, 602–609. [Google Scholar] [CrossRef]
- Münzinger, M.; Taraz, K.; Budzikiewicz, H.; Drechsel, H.; Heymann, P.; Winkelmann, G.; Meyer, J.-M. S, S-rhizoferrin (enantio-rhizoferrin)–a siderophore of Ralstonia (Pseudomonas) pickettii DSM 6297–the optical antipode of R, R-rhizoferrin isolated from fungi. Biometals 1999, 12, 189–193. [Google Scholar] [CrossRef]
- Burnside, D.M.; Wu, Y.; Shafaie, S.; Cianciotto, N.P. The Legionella pneumophila siderophore legiobactin is a polycarboxylate that is identical in structure to rhizoferrin. Infect. Immun. 2015, 83, 3937–3945. [Google Scholar] [CrossRef]
- Kühn, S.; Braun, V.; Köster, W. Ferric rhizoferrin uptake into Morganella morganii: Characterization of genes involved in the uptake of a polyhydroxycarboxylate siderophore. J. Bacteriol. 1996, 178, 496–504. [Google Scholar] [CrossRef]
- Matzanke, B.F.; Böhnke, R.; Möllmann, U.; Schünemann, V.; Schumann, G.; Trautwein, A.X.; Winkelmann, G. Transport and utilization of rhizoferrin bound iron in Mycobacterium smegmatis. Biometals 1999, 12, 315–321. [Google Scholar] [CrossRef]
- Dadwal, S.S.; Kontoyiannis, D.P. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev. Mol. Diagn. 2018, 18, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Lynch, J.P.; Fishbein, M.C.; Clark, N.M. Mucormycosis. Semin Respir. Crit. Care Med. 2020, 41, 99–114. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Larcher, G.; Dias, M.; Razafimandimby, B.; Bomal, D.; Bouchara, J.-P. Siderophore production by pathogenic mucorales and uptake of deferoxamine B. Mycopathologia 2013, 176, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kousser, C.; Clark, C.; Sherrington, S.; Voelz, K.; Hall, R.A. Pseudomonas aeruginosa inhibits Rhizopus microsporus germination through sequestration of free environmental iron. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Boukhalfa, H.; Reilly, S.D.; Michalczyk, R.; Iyer, S.; Neu, M.P. Iron(III) coordination properties of a pyoverdin siderophore produced by Pseudomonas putida ATCC 33015. Inorg. Chem. 2006, 45, 5607–5616. [Google Scholar] [CrossRef] [PubMed]
- Novák, J.; Škríba, A.; Havlíček, V. CycloBranch 2: Molecular formula annotations applied to imzML data sets in bimodal fusion and LC-MS data files. Anal. Chem. 2020, 92, 6844–6849. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Kamimura, H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl. Environ. Microbiol. 1986, 52, 515–519. [Google Scholar] [CrossRef]
- Li, P.; Sun, C.; Wang, Y.; Wang, S.; Yan, C.; Deng, S.; Huo, X.; Feng, L.; Wang, C.; Tian, Y.; et al. Efficiently regioselective glucosylation of estrogen analogues mediated by fungus Rhizopus oryzae AS 3.2380. Catal. Commun. 2017, 97, 106–110. [Google Scholar] [CrossRef]
- Li, Y.; Theuretzbacher, U.; Clancy, C.J.; Nguyen, M.H.; Derendorf, H. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin. Pharmacokinet. 2010, 49, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, G.; Samonis, G.; Kontoyiannis, D.P. Pulmonary mucormycosis. Semin Respir. Crit. Care Med. 2011, 32, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, G. Iron and virulence in Francisella tularensis. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]

| M | Metabolite | Formula | R. microsporus | M. irregularis | R. delemar | L. corymbifera | Rhizobium meliloti | C. echinulata | C. elegans | Rhizomucor pusillus | M. circinelloides | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 119.0219 | 3-Nitropropionic acid | C3H5NO4 | [5] | |||||||||
| 262.1165 | RHF citryl intermediate | C10H18N2O6 | [9] | |||||||||
| 289.1804 | Trisporic acid E1 | C18H25O3 | [10] | |||||||||
| 290.1882 | Trisporic acid A | C18H26O3 | [11] | |||||||||
| 304.1675 | Trisporic acid B | C18H24O4 | [11] | |||||||||
| 306.1831 | Trisporic acid C | C18H26O4 | [11] | |||||||||
| 320.1624 | Trisporic acid D2 | C18H24O5 | [12] | |||||||||
| 376.1720 | Rhizobactin | C15H26N3O8 | [13] | |||||||||
| 384.1169 | Desoxy-bis-imido-RHF | C16H20N2O9 | [14] | |||||||||
| 400.1118 | Bis-imido-RHF 3 | C16H20N2O10 | [14,15] | |||||||||
| 404.1431 | Di-desoxy-RHF | C16H24N2O10 | [14] | |||||||||
| 418.1224 | Imido-RHF | C16H22N2O11 | [14,15] | |||||||||
| 420.1380 | Mono-desoxy-RHF | C16H24N2O11 | [14] | |||||||||
| 422.1173 | Nor-RHF 4 | C15H22N2O12 | [14] | |||||||||
| 432.1744 | Di-methyl-di-desoxy-RHF | C18H28N2O10 | [14] | |||||||||
| 434.1537 | Methyl-desoxy-RHF | C17H26N2O11 | [14] | |||||||||
| 435.2404 | Rhizovarin F | C27H33NO4 | [16] | |||||||||
| 436.1329 | Rhizoferrin (RHF) | C16H24N2O12 | [9,14] | |||||||||
| 450.1122 | 2-Oxo-RHF | C16H22N2O13 | [14] | |||||||||
| 450.1486 | Homo-RHF | C17H26N2O12 | [14] | |||||||||
| 452.1278 | 2-Oxa-homo-RHF | C16H24N2O13 | [14] | |||||||||
| 464.1642 | 2-Methyl-homo-RHF | C18H28N2O12 | [14] | |||||||||
| 603.3560 | Rhizovarin D | C37H49NO6 | [16] | |||||||||
| 615.3560 | Rhizovarin E | C38H49NO6 | [16] | |||||||||
| 645.3302 | Rhizovarin C | C38H47NO8 | [16] | |||||||||
| 769.2111 | Rhizovarin A | C37H44ClNO8 | [16] | |||||||||
| 783.2268 | Rhizovarin B | C38H46ClNO8 | [16] | |||||||||
| 742.2280 | Di-Hex-imido-RHF | C28H42N2O21 | * | |||||||||
| 760.2386 | Di-Hex-RHF | C28H44N2O22 | * | |||||||||
| 1084.3442 | Tetra-Hex-RHF | C40H64N2O32 | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škríba, A.; Patil, R.H.; Hubáček, P.; Dobiáš, R.; Palyzová, A.; Marešová, H.; Pluháček, T.; Havlíček, V. Rhizoferrin Glycosylation in Rhizopus microsporus. J. Fungi 2020, 6, 89. https://doi.org/10.3390/jof6020089
Škríba A, Patil RH, Hubáček P, Dobiáš R, Palyzová A, Marešová H, Pluháček T, Havlíček V. Rhizoferrin Glycosylation in Rhizopus microsporus. Journal of Fungi. 2020; 6(2):89. https://doi.org/10.3390/jof6020089
Chicago/Turabian StyleŠkríba, Anton, Rutuja Hiraji Patil, Petr Hubáček, Radim Dobiáš, Andrea Palyzová, Helena Marešová, Tomáš Pluháček, and Vladimír Havlíček. 2020. "Rhizoferrin Glycosylation in Rhizopus microsporus" Journal of Fungi 6, no. 2: 89. https://doi.org/10.3390/jof6020089
APA StyleŠkríba, A., Patil, R. H., Hubáček, P., Dobiáš, R., Palyzová, A., Marešová, H., Pluháček, T., & Havlíček, V. (2020). Rhizoferrin Glycosylation in Rhizopus microsporus. Journal of Fungi, 6(2), 89. https://doi.org/10.3390/jof6020089





