Abstract
Mucormycosis is an emerging fungal infection caused by Mucorales with an unacceptable high mortality rate. Mucorales is a complex fungal group, including eleven different genera that can infect humans. This heterogeneity is associated with species-specific invasion pathways and responses to the host defense mechanisms. The host innate immune system plays a major role in preventing Mucorales growth and host invasion. In this system, macrophages are the main immune effector cells in controlling these fungi by rapid and efficient phagocytosis of the spores. However, Mucorales have evolved mechanisms to block phagosomal maturation and species-specific mechanisms to either survive as dormant spores inside the macrophage, as Rhizopus species, or geminate and escape, as Mucor species. Classical fungal models of mucormycosis, mostly Rhizopus, have made important contributions to elucidate key aspects of the interaction between Mucorales and macrophages, but they lack robust tools for genetic manipulation. The recent introduction of the genetically tractable Mucor circinelloides as a model of mucormycosis offers the possibility to analyze gene function. This has allowed the identification of regulatory pathways that control the fungal response to phagocytosis, including a non-canonical RNAi pathway (NCRIP) that regulates the expression of most genes regulated by phagocytosis.
1. Introduction.
Fungal spores are ubiquitous threats to human health that cause diverse pathologies ranging from mild and treatable allergic diseases to lethal infections. Fungi colonize both indoor and outdoor environments, and their spores pullulate at levels 1000-fold higher than other common airborne allergens [1], making the interaction between fungal pathogens and human hosts virtually inescapable. In this scenario, evolution has forced the development of molecular mechanisms to both attack fungal pathogens and defend the barrier surfaces in the hosts [2]. In these barrier surfaces (skin, pulmonary epithelium, and mucous membranes), the recruitment of the innate immune cells is highly effective to protect humans from thousands of fungal species.
The regular functioning of the innate immune system is sufficiently efficient to inactivate most of the fungal threats that are continuously encountering the barrier surfaces of the host. However, fungal infections represent a serious concern for immunocompromised individuals who must entirely rely on antifungal treatments. In this sense, this review focuses on the fungal order Mucorales, an ancient group of fungi that comply with both an opportunistic preference for immunocompromised hosts [3] and a natural antifungal resistant to most of the compounds regularly used for other fungal groups [4]. Once Mucorales overcome the innate immune system, they establish an infection known as mucormycosis, a highly lethal disease that presents an overall mortality rate of around 50% [5]. Mucorales present specific features such as coenocytic hyphae and a cell wall containing polysaccharide chitosan (an N-deacetylated version of chitin), whereas other distant groups of fungi like Ascomycetes and Basidiomycetes have septate hyphae with chitin as the main polysaccharide [6]. Nonetheless, there are no studies directly relating these specific features of Mucorales to their intrinsic antifungal resistance and high mortality rates. More recent studies have found new molecular particularities of Mucorales that could partially explain the antifungal resistance observed in these fungi [7]. This is the case of the ergosterol synthesis system, a frequent target for many of the current antifungal compounds. In this system, the lanosterol 14α-demethylase CYP51 F5 presents two amino acid substitutions conserved only in Mucorales, which could explain their intrinsic antifungal resistance to short-tailed azole compounds [7]. Another specific feature of Mucorales related to their antifungal resistance is a unique mechanism for the generation of epimutants [8,9]. These epimutants are strains temporarily adapted to the presence of different antifungal compounds, showing an acquired resistance that is lost once the compound disappears. The mechanism for this acquired resistance is based on the RNA interference (RNAi) mechanism of these fungi, which specifically destroys the mRNA of the gene that encodes the corresponding protein targeted by the antifungal compound [8,10,11].
The resistant of Mucorales to antifungals comes along with a reduced knowledge on the interaction of the host phagocytic cells with the spore and its implications in the pathophysiology of mucormycosis. This review will summarize current information related to macrophage–Mucorales interactions, describing and discussing new advances and future perspectives.
2. Mucoralean Species Involved in Mucormycosis
The order Mucorales is a group of ancient fungi classified in the subphylum Mucoromycotina. They are commonly known as “early-diverging fungi” because they are in a basal position with respect to Basidiomycota and Ascomycota in the fungal tree of life [12]. Last reports delimitate the order Mucorales to 55 genera and 260 species [13], including 25 species from 11 genera that cause infections in humans [14]. The most prevalent genus causing mucormycosis is Rhizopus, followed by the genera Mucor, Lichtheimia, and Rhizomucor [5,15,16]. Other less frequent pathogenic species can be found in the genera Syncephalastrum, Cunninghamella, Apophysomyces, and Saksenaea [17]. Traditionally, mucormycosis is defined generally as a fungal infection caused by any species of the order Mucorales, characterized by accelerated tissue invasion and necrosis followed by angioinvasion and dissemination. However, recent studies are unveiling particularities of this disease, depending on the fungal species that cause the infection. For instance, mucormycosis can manifest as a cutaneous, rinocerebral, or pulmonary infection, which recently has been related to the infecting species that is involved and the route of infection [15]. In this sense, Mucorales can be classified attending to the primary route of infection: either airborne or by contact. Rhizopus and Rhizomucor are primarily airborne, whereas Mucor and Lichtheimia infect the hosts mostly by contact [15]. This preference for the route of infection can be explained by the differences found in the sporangia of these genera [15]. Thus, Rhizopus and Rhizomucor present dry sporangia over long and thin sporangiophores fully adapted to release the spores in the air. On the contrary, Mucor and Lichtheimia generate wet sporangia that release the spores in small droplets when several sporangia stick together. Nonetheless, dried mass of spores can also be dispersed by air using the airborne route as a secondary way of infection [15]. These considerations might explain the prevalence of Mucor and Lichtheimia species in infected burn wounds and trauma injuries, usually caused by contact with soil or contaminated surfaces [15]. Another important feature of Mucorales directly related to their ability to produce mucormycosis is their differential thermotolerance. Rhizopus and Lichtheimia are thermotolerant and can grow at 37 °C [18,19], Rhizomucor species are thermophilic and can grow up to 55 °C [20], and Mucor species are usually mesophilic [21]. All the mucoralean strains causing mucormycosis in humans must be adapted to grow at 37 °C, which means that species isolated from patients that do not belong to the genera Rhizopus and Lichtheimia must present additional molecular adaptations to allow germination inside the phagocytic cells of the host. These molecular adaptations and the mechanisms to overcome the fungicidal attack of macrophages are widely uncharacterized in Mucorales, and their future unveiling will uncover the pathways that different mucoralean species chose to produce mucormycosis.
3. Host Defense against Mucorales
Mucormycosis is initiated by asexual resting spores that get in contact with skin or mucosa. Tissue invasion is largely prevented by physical barriers of the innate immune system, but a defective function of the immune cell effectors or damage of the skin or mucosa may result in mucormycosis. Lungs deserve special attention because they are the main entrance of the spores in the body and are equipped with specialized macrophages, alveolar macrophages, which are in the airway lumen in close contact with the respiratory epithelium [22]. These specialized macrophages and other macrophages play a pivotal role, with other innate immune cells having a minor role. Here, we outline the most recent signs of progress in the function of the different cells involved in the defense against Mucorales. The detailed function of these cells can be found in recent extensive reviews [23,24,25].
3.1. Epithelial Cells
Epithelial cells make up an external barrier of defense against any type of infection, including mucormycosis. Indeed, lesions in their integrity are considered as one of the risk factors of Mucorales infection [26]. Studies performed in Rhizopus oryzae revealed that the spore adhesion to the basement membrane proteins exposed after epithelial damage is the initial step to active spore germination and host infection [27]. Interaction between Mucorales spores and epithelial cells has been studied frequently in vitro using the alveolar epithelial cell line A-549 [28,29] and also in vivo [29]. Analyses of the host transcription response to Mucorales revealed the activation of several signaling pathways well known as part of the host response to fungal pathogens, including tumor necrosis factor, interleukin-1 (IL-1) alpha and beta, nuclear factor kappa B, mitogen-activated protein kinase, IL-22, and IL-17A [28,29]. In addition, an initial analysis suggested that the platelet-derived growth factor receptor B (PDGFRB) signaling is used by Mucorales as a mechanism to damage barrier host cells because its inhibition reduces epithelial damage [28]. Interestingly, a second transcriptomic analysis unveiled the activation of epidermal growth factor receptor (EGFR) signaling in alveolar epithelial cells, which is phosphorylated upon in vitro infection of these cells with Rhizopus delemar spores. It is postulated that EGFR signaling promotes the disease, as specific inhibition of this pathway with cetuximab or gefitinib reduces invasion and damage in the airway epithelial cells. Moreover, in vivo experiments showed that gefitinib treatment increases the survival of infected mice [29].
3.2. Phagocytes
Neutrophils and macrophages are essential components of an early immune response in fungal killing [30] promptly recruited in the point of infection in Mucorales infection [31,32]. Macrophages play a pivotal role in the defense against these fungi because their specific depletion in larval zebrafish and mouse models results in a higher susceptibility to infection [32,33]. Analysis of the kinetics of recruitment and degree of association with immune cells in immunocompetent mice infected intratracheally with R. oryzae revealed that, although a significant influx of neutrophils in the lungs, most of the spores were associated with alveolar and interstitial macrophages and dendritic cells. Confocal images in sorted cells showed that the spores were predominantly phagocytosed by alveolar macrophages followed by interstitial macrophages, highlighting the importance of alveolar macrophages in lung infection [32]. This study and others have shown that healthy alveolar macrophages fail to kill Rhizopus resting spores, although they can produce spore clearance of swollen and active spores [34,35]. Despite the inability to kill resting Rhizopus spores, alveolar macrophages from healthy mouse and rats block germination [36,37]. In rats, the inhibition of Rhizopus spore germination by alveolar macrophages is mediated by the generation of nitric oxide from oxidation of L-arginine, but the mechanism remains poorly understood [37]. In addition, phagocytosis in macrophage activates an iron restriction response as a part of the nutritional immunity [33]. Other Mucorales, exampled by Mucor species, probably also confront nutritional stress in the phagosome (see below), but they are able to overcome this defense mechanism and germinate inside the macrophage in vitro [38,39,40].
Neutrophils are important in host defense against Mucorales, considering that neutropenia is a risk factor for mucormycosis [41,42]. These cells may cause both spore and hyphae damage by releasing reactive oxygen metabolites, cationic peptides, and perforin. Neutrophil-spore interaction leads to the expression of toll-like receptors and the production of necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-1b that facilitate the activation of other immune cells [43]. Besides the spore developmental stage or the hots immunocompetence, phagocytosis by neutrophils can be altered by other factors such as hyperglycemia, acidosis, and high iron levels that may be contributed to Mucorales infection [44,45].
In addition to the mechanisms described above, phagocytes can restrict the spores to the point of the infection by forming tight cell clusters around spores resembling early granulomas [33,46]. Spores are contained in these granulomas, where there is a lack of reactive oxygen burst, and failure to kill fungal spores as mucormycosis was reactivated by dexamethasone-induced immunosuppression [46].
3.3. Natural Killer Cells
Natural killer (NK) cells play a role in the immunosuppression of Mucorales infection by hyphae damage. The interaction between hyphae and NK cells induces their activation and perforin release [47]. This response is generalized among Mucorales species but only for the fungal hyphae and does not affect spores [48]. Nevertheless, studies in R. oryzae showed that hyphae have an immunosuppressive effect because hyphae can inhibit the NK cell secretion of immunomodulatory chemokines IFN-γ and RANTES (Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted) protein [47,49].
3.4. Platelets
Recent studies reported that, beyond the homeostasis function, platelets have antimicrobial and antifungal properties. Platelets inhibit spore germination and hyphal grow in Rhizopus, Rhizomucor, Mucor, and Absidia species via granular secretion of proinflammatory and anti-inflammatory cytokines and chemokines. Besides, platelets bind to other cells to produce phagocytosis and dendritic cells and B and T lymphocyte activation [48,50,51].
3.5. Dendritic and Lymphocyte T Cells
The adaptative immune response during Mucorales infection has been poorly studied. Nevertheless, recent reports highlight a higher role of this specific immune system. Among components of the adaptative immune system, T cells have an essential role against mucormycosis. The presence of Mucorales hyphae induces CD4+ and CD8+ T cell production that release cytokines such as IL-4, IFN-γ, IL-10, and IL-17, and mediate hyphal damage [52]. Consequently, lymphopenia has been associated with increase mortality in pulmonary mucormycosis and hematological malignancies [53,54]. Moreover, dendritic cells can trigger T cell response playing as intermediate cells between the innate and adaptative responses [55]. The presence of R. oryzae hyphae actives IL-23 production by dendritic cells, inducing T helper 17 cells (Th-17). Th-17 cells secreted IL-17 promoting neutrophils response [56].
4. The Responses of Mucorales Spores to Phagocytosis
Host innate immune cells display many available arms to block the fungal invasion, but fungi have evolved strategies to counteract this attack, which is also true for Mucorales. Despite that macrophages and neutrophils are rapidly recruited to the infection point in mammals and zebrafish infection models [30,31,33], Mucorales spores are phagocytized predominantly by macrophages [32]. Therefore, numerous works have analyzed the responses of the engulfed spores to the phagocytosis, where different types of macrophages have been analyzed (Table 1), including alveolar macrophages [32], bone marrow-derived macrophages (BMDMs) [32], murine alveolar cell lines [34], and murine macrophage cell lines [57,58]. Many fungal pathogens, including Mucorales, have evolved mechanisms to avoid or survive the macrophage confrontation following different strategies (Table 1) [30]. A critical point in fungal invasion is the germination to produce hyphae that cannot be engulfed by the macrophages because of their size [30]. Therefore, macrophages rapidly engulf fungal spores once they get in contact with the host, but they are unable to kill Mucorales spores, resulting in two main species-specific outcomes. In some species, represented by R. oryzae and R. delemar, phagocytosis blocks germination of resting spores remaining viable inside alveolar macrophages in the lungs of immunocompetent mice at least for 10 days post-infection [32]. Mucorales spores inside macrophages have been observed also in human histopathology samples of surgical specimens from a patient with disseminated mucormycosis [32]. Persistence inside the macrophage may facilitate Mucorales spore dissemination, as has been suggested for Cryptococcus neoformans [59], and this could explain how spores of a L. corymbifera virulent strain are more readily phagocytosed than those of attenuated strains [34].

Table 1.
Results of the interactions between macrophages and mucoralean species.
Other Mucorales, typified by Mucor species, are also resistant to phagocytosis, but in this case, they can germinate inside the phagosome and kill macrophages in vitro and in vivo [31,38,39]. Infection of adult zebrafish with M. circinelloides spores induced cell death mediated by apoptosis in macrophages but not neutrophils [31]. Conversely, R. oryzae does not induce either apoptosis or necrosis of host cell during interaction in vitro with murine BMDMs [32]. However, it induces phagosome maturation arrest via targeting LC3-associated phagocytosis (LAP), which is a major antifungal pathway regulating early events in the biogenesis of Aspergillus fumigatus phagosome [61,62]. Phagosome maturation arrest is mediated by cell wall melanin [32]. Similarly, Mucor induces blockage of phagosome maturation in a murine macrophage cell line and BMDMs [39], but in this case, it depends on the calcineurin signal pathway (Figure 1) [63].
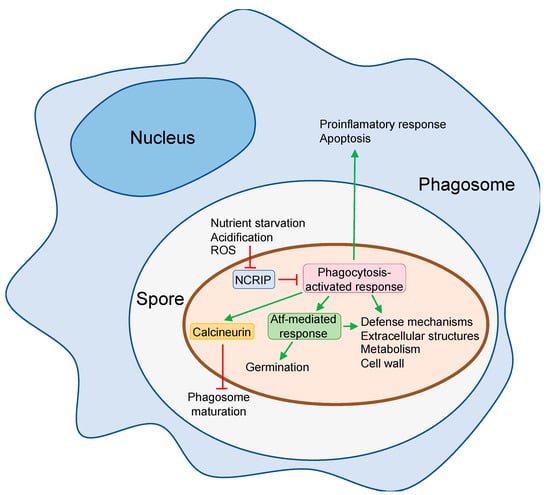
Figure 1.
Mechanisms of Mucor circinelloides to escape from macrophage fungistatic activity. When M. circinelloides spores are phagocytized, a complex gene response is activated to survive macrophages attack that encompasses the differential expression of genes involved in defense mechanisms, extracellular structures, metabolism, and cell wall [57]. This response also up-regulates transcription factors atf1 and atf2 that regulate germination at low pH and other processes involved in the response to phagocytosis [57]. Calcineurin pathway is also activated and promotes phagosome maturation arrest [63]. The spore response to phagocytosis also comprises the activation of macrophage genes involved in apoptosis and proinflammatory response [57]. The NCRIP represses most of the genes of the phagocytosis-activated response in the absence of macrophages [58], suggesting that some of the signals present in the phagosome repress this RNAi-related pathway to allow the activation of the spore response to phagocytosis. Green arrow, activation; red T, repression.
Despite the evident proof that Mucorales can escape from the macrophages attack, their mechanisms are poorly characterized. The study of the host-pathogen interaction at a transcriptomic level may elucidate the regulatory pathways involved during phagocytosis. A transcriptomic analysis of the interaction between R. delemar (strain 99-880) and BMDMs revealed that the majority of genes previously implicated in the iron acquisition were up-regulated during infection, including fet3 and ftr1 [32]. These genes code for a multicopper ferroxidase and a high-affinity iron permease and comprise the major iron assimilation pathway of Rhizopus and M. circinelloides, which has a pivotal pathogenetic role during in vivo infection [60,64]. Reduction of ftr1 expression in R. delemar and deletion of fet3 in M. circinelloides compromised their ability to acquire iron and reduced their virulence in diabetic ketoacidotic (DKA) and immunocompromised mice, respectively [60,64]. Importantly, immunization with anti-Ftr1 immune sera protected DKA mice from infection with R. delemar [60].
Other transcriptome studies have focused on the characterization of mechanisms used by Mucorales species that are able to germinate and grow inside the macrophages. These species should be able to mount an active defense to grow in a harsh and poor environment as the phagosome. The transcriptomic response to phagocytosis has been studied using virulent and attenuated strains of M. circinelloides, followed by the study of gene function by the generation of knockout mutants [57]. This analysis identified a large number of genes (>9% of total genes) differentially expressed in the two M. circinelloides pathotypes after 5 h of interaction with a murine macrophage cell line, revealing a general response showed by both strains and a virulence-specific response. The general response comprises an enrichment in genes involved in nutrient assimilation and metabolism, suggesting that M. circinelloides can shift toward alternative nutrient sources to allow germination inside the phagosome. The virulence-specific response highlighted an increase in biological processes involved in regulating cAMP-dependent intracellular signal transduction pathways and the response to oxidative stress that could explain the M. circinelloides ability to survive macrophage attack [57]. Functional characterization of M. circinelloides response to macrophages by deleting genes encoding transcription factors (atf1, atf2, and gcn4), extracellular proteins (chi1 and pps1), and an aquaporin (aqp1) revealed that defects in most of the genes were associated with impaired germination inside the phagosome, which resulted in a decreased virulence in mice [57]. The two atf1 and atf2 genes code for two basic leucine zipper transcription factors similar to Schizosaccharomyces pombe Atf1, which is involved in oxidative stress [65]. The further transcriptomic analysis of the mutants in atf1 and atf2 suggested the existence of an Atf-mediated pathway that is involved not only in the response to oxidative stress but also in the macronutrient metabolism (Figure 1). In addition, this Atf-mediated pathway regulates germination at low pH because mutants in atf1, atf2, and in genes controlled by the pathway showed germination defects at acidic pH, suggesting that the pathway is activated by the low pH in the phagosome [57].
During mice infection, M. circinelloides undergoes a novel RNAi epimutation phenomenon that silences target genes, developing a transient an adaptive advantage to a hostile environment [66]. This novel epimutational mechanism of drug resistance interacts with a non-canonical RNAi pathway (NCRIP) [9]. The NCRIP regulates the mRNA levels of specific genes post-transcriptionally by degradation using a mechanism that includes RNA-dependent RNA polymerases (RdRPs) and a novel ribonuclease III-like named R3B2 [67]. This silencing pathway regulates multiple genes involved in fungal physiology that include resistance to oxidative stress, suggesting an active role in regulating virulent processes. The transcriptomic analysis of mutants lacking NCRIP activity during phagocytosis revealed an NCRIP-dependent virulent response [58]. Interestingly, the expression of atf1, atf2, and many of its secondary targets are controlled by the NCRIP, indicating that this novel pathway is involved in the response to environmental signals prompted by the phagosome, regulating downstream elements like the Atf-mediated response (Figure 1). Deeper analyses revealed that the NCRIP is constitutively active during vegetative growth, and the silencing mechanism control is released during phagocytosis. The interaction of the spores with macrophages is proposed to inhibit the NCRIP, resulting in the activation of the genetic program to overcome host defense mechanisms (Figure 1) [58]. The signals and the transduction pathways that regulate the NCRIP are unknown, but typical components of transduction signal pathways as heterotrimeric G proteins are required for full virulence in M. circinelloides. Genes gpa11 and gpa12 encoding α subunits of heterotrimeric G proteins show high transcript levels in resting spores [68] and are required to survive oxidative stress, phagocytosis and virulence in DKA mice [69]. Moreover, spores produced from cultures supplemented with native human blood serum show increased germination, resistance to oxidative stress, and survival to phagocytosis by macrophages, suggesting thermolabile components in the blood serum triggering a M. circinelloides response involved in macrophage interaction [70]. It is tempting to speculate the existence of a possible functional link connecting these independent observations in which the heterotrimeric G proteins would mediate the repression of the NCRIP in response to a blood serum component, but this hypothesis is still waiting to be explored.
5. Future Directions
Pharmacological control of mucormycosis faces the problem of the innate resistance of Mucorales to most of the available antifungal drugs. The improvement in the clinical treatments for other challenging diseases and the increasing aging population are raising the number of people susceptible to Mucorales infection. Therefore, it is imperative to develop new drugs to combat mucormycosis. The interaction between Mucorales and macrophages is a critical initial point of the invasion, and its characterization may provide targets for therapeutic treatments of the disease. Advances in recent years based on the application of “omics” technologies and the incorporation of models with robust molecular genetics have revealed some of the mechanisms used by these fungi to resist or overcome the macrophage attack. However, these advances represent only the tip of the iceberg, and most of the regulatory events that take place in the fungi during the phagocytosis are unknown. Future transcriptomic and proteomic analyses of the interaction between different relevant causing agents of mucormycosis are expected to reveal the signal transduction and regulatory pathways that control the general and species-specific mechanisms involved in the response of Mucorales spores to phagocytosis. The continuous use of genetically amenable models, such as M. circinelloides, and the adaptation of CRISPR/Cas technologies to most frequent and genetically untreatable pathogenic species producing mucormycosis are supposed to allow molecular dissection of Mucorales responses to phagocytosis in the near future, providing the long-awaited targets to create new antifungals.
Author Contributions
F.E.N., L.M., E.N., and V.G. wrote different parts of the review and prepared the Figure and Table. V.G. integrated all the parts and M.I.N.-M. and C.P.-A. carefully reviewed the complete manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
F.E.N.: L.M.: E.N. and V.G. were supported by the Ministerio de Ciencia, Innovación y Universidades, Spain (PGC2018-097452-B-I00, co-financed by FEDER). F.E.N. was also supported by the Ramón and Cajal Program (RYC-2014-15844). C.P.-A. and M.I.N.-M. were supported by predoctoral fellowships from the Ministerio de Educación, Cultura y Deporte, Spain (FPU14/01983 and FPU14/01832, respectively).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Denning, D.W.; O’Driscoll, B.R.; Hogaboam, C.M.; Bowyer, P.; Niven, R.M. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef]
- Casadevall, A. Fungi and the rise of mammals. PLoS Pathog. 2012, 8, e1002808. [Google Scholar] [CrossRef]
- Greene, J.; Pak, J.; Tucci, V.; Vincent, A.; Sandin, R. Mucormycosis in immunochallenged patients. J. Emergencies Trauma Shock 2008, 1, 106. [Google Scholar] [CrossRef]
- Dannaoui, E. Antifungal resistance in mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef] [PubMed]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic short-Tailed azole resistance in mucormycetes is due to an evolutionary conserved aminoacid substitution of the lanosterol 14α-demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef] [PubMed]
- Calo, S.; Shertz-Wall, C.; Lee, S.C.; Bastidas, R.J.; Nicolás, F.E.; Granek, J.A.; Mieczkowski, P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Cardenas, M.E.; et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature 2014, 513, 555–558. [Google Scholar] [CrossRef]
- Calo, S.; Nicolás, F.E.; Lee, S.C.; Vila, A.; Cervantes, M.; Torres-Martinez, S.; Ruiz-Vazquez, R.M.; Cardenas, M.E.; Heitman, J. A non-canonical RNA degradation pathway suppresses RNAi-dependent epimutations in the human fungal pathogen Mucor circinelloides. PLoS Genet. 2017, 13, e1006686. [Google Scholar] [CrossRef]
- Chang, Z.; Billmyre, R.B.; Lee, S.C.; Heitman, J. Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides. PLoS Genet. 2019, 15, e1007957. [Google Scholar] [CrossRef]
- Nicolas, F.E.; Moxon, S.; de Haro, J.P.; Calo, S.; Grigoriev, I.V.; Torres-Martínez, S.; Moulton, V.; Ruiz-Vázquez, R.M.; Dalmay, T. Endogenous short RNAs generated by Dicer 2 and RNA-dependent RNA polymerase 1 regulate mRNAs in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010, 38, 5535–5541. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N.; Pawłowska, J.; Letcher, P.M.; Kirk, P.M.; Humber, R.A.; Schüßler, A.; Wrzosek, M.; Muszewska, A.; Okrasińska, A.; Istel, Ł.; et al. Notes for genera: Basal clades of Fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Divers. 2018, 92, 43–129. [Google Scholar] [CrossRef]
- Wagner, L.; de Hoog, S.; Alastruey-Izquierdo, A.; Voigt, K.; Kurzai, O.; Walther, G. A revised species concept for opportunistic Mucor species reveals species-specific antifungal susceptibility profiles. Antimicrob. Agents Chemother. 2019, 63, 1–8. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Outbreaks of mucorales and the species involved. Mycopathologia 2019. [Google Scholar] [CrossRef] [PubMed]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef]
- Al-Ajam, M.R.; Bizri, A.R.; Mokhbat, J.; Weedon, J.; Lutwick, L. Mucormycosis in the Eastern Mediterranean: A seasonal disease. Epidemiol. Infect. 2006, 134, 341–346. [Google Scholar] [CrossRef]
- Hoffmann, K.; Discher, S.; Voigt, K. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol. Res. 2007, 111, 1169–1183. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Chan, J.F.W.; Ngan, A.H.Y.; To, K.K.W.; Leung, S.Y.; Tsoi, H.W.; Yam, W.C.; Tai, J.W.M.; Wong, S.S.Y.; Tse, H.; et al. Outbreak of intestinal infection due to Rhizopus microsporus. J. Clin. Microbiol. 2009, 47, 2834–2843. [Google Scholar] [CrossRef]
- Schipper, M.A.A. On certain species of Mucor with a key to all accepted species. Stud. Mycol. 1978, 17, 1–52. [Google Scholar]
- Vellanki, S.; Navarro-Mendoza, M.I.; Garcia, A.B.; Murcia, L.; Perez-Arques, C.; Garre, V.; Nicolas, F.E.; Lee, S.C. Mucor circinelloides: Growth, maintenance and genetic manipulation. Curr. Protoc. Microbiol. 2018, 49, e53. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Ghuman, H.; Voelz, K. Innate and adaptive immunity to Mucorales. J. Fungi 2017, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019, 57, S245–S256. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Voelz, K. The mucormycete–host interface. Curr. Opin. Microbiol. 2017, 40, 40–45. [Google Scholar] [CrossRef]
- Bouchara, J.P.; Oumeziane, N.A.; Lissitzky, J.C.; Larcher, G.; Tronchin, G.; Chabasse, D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur. J. Cell Biol. 1996, 70, 76–83. [Google Scholar]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Chibucos, M.C.; Soliman, S.; Gebremariam, T.; Lee, H.; Daugherty, S.; Orvis, J.; Shetty, A.C.; Crabtree, J.; Hazen, T.H.; Etienne, K.A.; et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016, 7, 12218. [Google Scholar] [CrossRef]
- Watkins, T.N.; Gebremariam, T.; Swidergall, M.; Shetty, A.C.; Graf, K.T.; Alqarihi, A.; Alkhazraji, S.; Alsaadi, A.I.; Edwards, V.L.; Filler, S.G.; et al. Inhibition of EGFR signaling protects from mucormycosis. MBio 2018, 9, e01384-18. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- López-Muñoz, A.; Nicolás, F.E.; García-Moreno, D.; Pérez-Oliva, A.B.; Navarro-Mendoza, M.I.; Hernández-Oñate, M.A.; Herrera-Estrella, A.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Garre, V.; et al. An adult zebrafish model reveals that mucormycosis induces apoptosis of infected macrophages. Sci. Rep. 2018, 8, 12802. [Google Scholar] [CrossRef] [PubMed]
- Andrianaki, A.M.; Kyrmizi, I.; Thanopoulou, K.; Baldin, C.; Drakos, E.; Soliman, S.S.M.; Shetty, A.C.; McCracken, C.; Akoumianaki, T.; Stylianou, K.; et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat. Commun. 2018, 9, 3333. [Google Scholar] [CrossRef] [PubMed]
- Voelz, K.; Gratacap, R.L.; Wheeler, R.T. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Model. Mech. 2015, 8, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Kraibooj, K.; Park, H.R.; Dahse, H.M.; Skerka, C.; Voigt, K.; Figge, M.T. Virulent strain of Lichtheimia corymbifera shows increased phagocytosis by macrophages as revealed by automated microscopy image analysis. Mycoses 2014, 57, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Selsted, M.E.; Ganz, T.; Lehrer, R.I.; Diamond, R.D.; Levitz, S.M.; Selsted, M.E.; Ganz, T.; Lehrer, R.I.; Diamond, R.D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J. Infect. Dis. 1986, 154, 483–489. [Google Scholar] [CrossRef]
- Waldorf, A.R.; Ruderman, N.; Diamond, R.D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J. Clin. Invest. 1984, 74, 150–160. [Google Scholar] [CrossRef]
- Jorens, P.G.; Boelaert, J.R.; Halloy, V.; Zamora, R.; Schneider, Y.J.; Herman, A.G. Human and rat macrophages mediate fungistatic activity against Rhizopus species differently: In vitro and ex vivo studies. Infect. Immun. 1995, 63, 4489–4494. [Google Scholar] [CrossRef]
- Li, C.H.; Cervantes, M.; Springer, D.J.; Boekhout, T.; Ruiz-Vazquez, R.M.; Torres-Martinez, S.R.; Heitman, J.; Lee, S.C. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 2011, 7, e1002086. [Google Scholar] [CrossRef]
- Lee, S.C.; Li, A.; Calo, S.; Inoue, M.; Tonthat, N.K.; Bain, J.M.; Louw, J.; Shinohara, M.L.; Erwig, L.P.; Schumacher, M.A.; et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 2015, 97, 844–865. [Google Scholar] [CrossRef]
- Trieu, T.A.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Sanchis, M.; Capilla, J.; Navarro-Rodriguez, P.; Lopez-Fernandez, L.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M.; et al. RNAi-based functional genomics identifies new virulence determinants in mucormycosis. PLoS Pathog. 2017, 13, e1006150. [Google Scholar] [CrossRef]
- Pagano, L.; Ricci, P.; Tonso, A.; Nosari, A.; Cudillo, L.; Montillo, M.; Cenacchi, A.; Pacilli, L.; Fabbiano, F.; Del Favero, A. Mucormycosis in patients with haematological malignancies: A retrospective clinical study of 37 cases. Br. J. Haematol. 1997, 99, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Waldorf, A.R.; Diamond, R.D. Neutrophil chemotactic responses induced by fresh and swollen Rhizopus oryzae spores and Aspergillus fumigatus conidia. Infect.Immun. 1985, 48, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Farmakiotis, D.; Kontoyiannis, D.P. Mucormycoses. Infect. Dis. Clin. North. Am. 2016, 30, 143–163. [Google Scholar] [CrossRef]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Mucormycosis - from the pathogens to the disease. Clin. Microbiol. Infect. 2014, 20, 60–66. [Google Scholar] [CrossRef]
- Inglesfield, S.; Jasiulewicz, A.; Hopwood, M.; Tyrrell, J.; Youlden, G.; Mazon-Moya, M.; Millington, O.R.; Mostowy, S.; Jabbari, S.; Voelz, K. Robust phagocyte recruitment controls the opportunistic fungal pathogen Mucor circinelloides in innate granulomas in vivo. MBio 2018, 9, e02010-17. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Tramsen, L.; Perkhofer, S.; Lass-Flörl, C.; Hanisch, M.; Röger, F.; Klingebiel, T.; Koehl, U.; Lehrnbecher, T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology 2013, 218, 939–944. [Google Scholar] [CrossRef]
- Speth, C.; Rambach, G.; Lass-Flörl, C. Platelet immunology in fungal infections. Thromb. Haemost. 2014, 112, 632–639. [Google Scholar] [CrossRef]
- Schmidt, S.; Schneider, A.; Demir, A.; Lass-Flörl, C.; Lehrnbecher, T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses 2016, 59, 34–38. [Google Scholar] [CrossRef]
- Perkhofer, S.; Kainzner, B.; Kehrel, B.E.; Dierich, M.P.; Nussbaumer, W.; Lass-Flörl, C. Potential antifungal effects of human platelets against zygomycetes in vitro. J. Infect. Dis. 2009, 200, 1176–1179. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Forghieri, F.; Zanetti, E.; Quadrelli, C.; Candoni, A.; Maertens, J.; Rossi, G.; et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood 2011, 118, 5416–5419. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Georgiadou, S.P.; Sampsonas, F.; Chamilos, G.; Kontoyiannis, D.P. Risk factors for early mortality in haematological malignancy patients with pulmonary mucormycosis. Mycoses 2014, 57, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kyvernitakis, A.; Torres, H.A.; Jiang, Y.; Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: A propensity score analysis. Clin. Microbiol. Infect. 2016, 22, 811.e1–811.e8. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Hejazi, A.S.; Wei, S.H.; Cahalan, M.D.; Parker, I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. USA 2004, 101, 998–1003. [Google Scholar] [CrossRef]
- Chamilos, G.; Ganguly, D.; Lande, R.; Gregorio, J.; Meller, S.; Goldman, W.E.; Gilliet, M.; Kontoyiannis, D.P. Generation of IL-23 producing Dendritic Cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of TH-17 responses. PLoS ONE 2010, 5, e12955. [Google Scholar] [CrossRef]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Lax, C.; Martínez-García, P.; Heitman, J.; Nicolás, F.E.; Garre, V. Mucor circinelloides thrives inside the phagosome through an Atf-mediated germination pathway. MBio 2019, 10, e02765-18. [Google Scholar] [CrossRef]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Navarro, E.; Garre, V.; Nicolás, F.E. A non-canonical RNAi pathway controls virulence and genome stability in Mucorales. PLoS Genet. 2020. [Google Scholar] [CrossRef]
- Charlier, C.; Nielsen, K.; Daou, S.; Brigitte, M.; Chretien, F.; Dromer, F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 2009, 77, 120–127. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Lin, L.; Luo, G.; Husseiny, M.I.; Skory, C.D.; Fu, Y.; French, S.W.; Edwards, J.E.; Spellberg, B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010, 77, 587–604. [Google Scholar] [CrossRef]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.C.; Kontoyiannis, D.P.; et al. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kyrmizi, I.; Gresnigt, M.S.; Akoumianaki, T.; Samonis, G.; Sidiropoulos, P.; Boumpas, D.; Netea, M.G.; van de Veerdonk, F.L.; Kontoyiannis, D.P.; Chamilos, G. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting Dectin-1/Syk kinase signaling. J. Immunol. 2013, 191, 1287–1299. [Google Scholar] [CrossRef]
- Vellanki, S.; Billmyre, R.B.; Lorenzen, A.; Campbell, M.; Turner, B.; Huh, E.Y.; Heitman, J.; Lee, S.C. A novel resistance pathway for calcineurin inhibitors in the human-pathogenic mucorales Mucor circinelloides. MBio 2020, 11, e02949-19. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Murcia, L.; Martínez-García, P.; Lax, C.; Sanchis, M.; Capilla, J.; Nicolás, F.E.; Garre, V. Components of a new gene family of ferroxidases involved in virulence are functionally specialized in fungal dimorphism. Sci. Rep. 2018, 8, 7660. [Google Scholar] [CrossRef]
- Shiozaki, K.; Russell, P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996, 10, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Heitman, J. Drug-resistant epimutants exhibit organ-specific stability and induction during murine infections caused by the human fungal pathogen Mucor circinelloides. MBio 2019, 10, e02579-19. [Google Scholar] [CrossRef]
- Trieu, T.A.; Calo, S.; Nicolás, F.E.; Vila, A.; Moxon, S.; Dalmay, T.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M. A non-canonical RNA silencing pathway promotes mRNA degradation in basal fungi. PLoS Genet. 2015, 11, 1005168. [Google Scholar] [CrossRef]
- Valle-Maldonado, M.I.; Jácome-Galarza, I.E.; Díaz-Pérez, A.L.; Martínez-Cadena, G.; Campos-García, J.; Ramírez-Díaz, M.I.; Reyes-De la Cruz, H.; Riveros-Rosas, H.; Díaz-Pérez, C.; Meza-Carmen, V. Phylogenetic analysis of fungal heterotrimeric G protein-encoding genes and their expression during dimorphism in Mucor circinelloides. Fungal Biol. 2015, 119, 1179–1193. [Google Scholar] [CrossRef]
- Patiño-Medina, J.A.; Reyes-Mares, N.Y.; Valle-Maldonado, M.I.; Jácome-Galarza, I.E.; Pérez-Arques, C.; Nuñez-Anita, R.E.; Campos-García, J.; Anaya-Martínez, V.; Ortiz-Alvarado, R.; Ramírez-Díaz, M.I.; et al. Heterotrimeric G-alpha subunits Gpa11 and Gpa12 define a transduction pathway that control spore size and virulence in Mucor circinelloides. PLoS ONE 2019, 14, e0226682. [Google Scholar] [CrossRef]
- Patiño-Medina, J.A.; Vargas-Tejeda, D.; Valle-Maldonado, M.I.; Alejandre-Castañeda, V.; Jácome-Galarza, I.E.; Villegas-Moreno, J.; Nuñez-Anita, R.E.; Ramírez-Díaz, M.I.; Ortiz-Alvarado, R.; Meza-Carmen, V. Sporulation on blood serum increases the virulence of Mucor circinelloides. Microb. Pathog. 2019, 137, 103737. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).