Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Cohort
3.2. Isavuconazole Efficacy and Safety
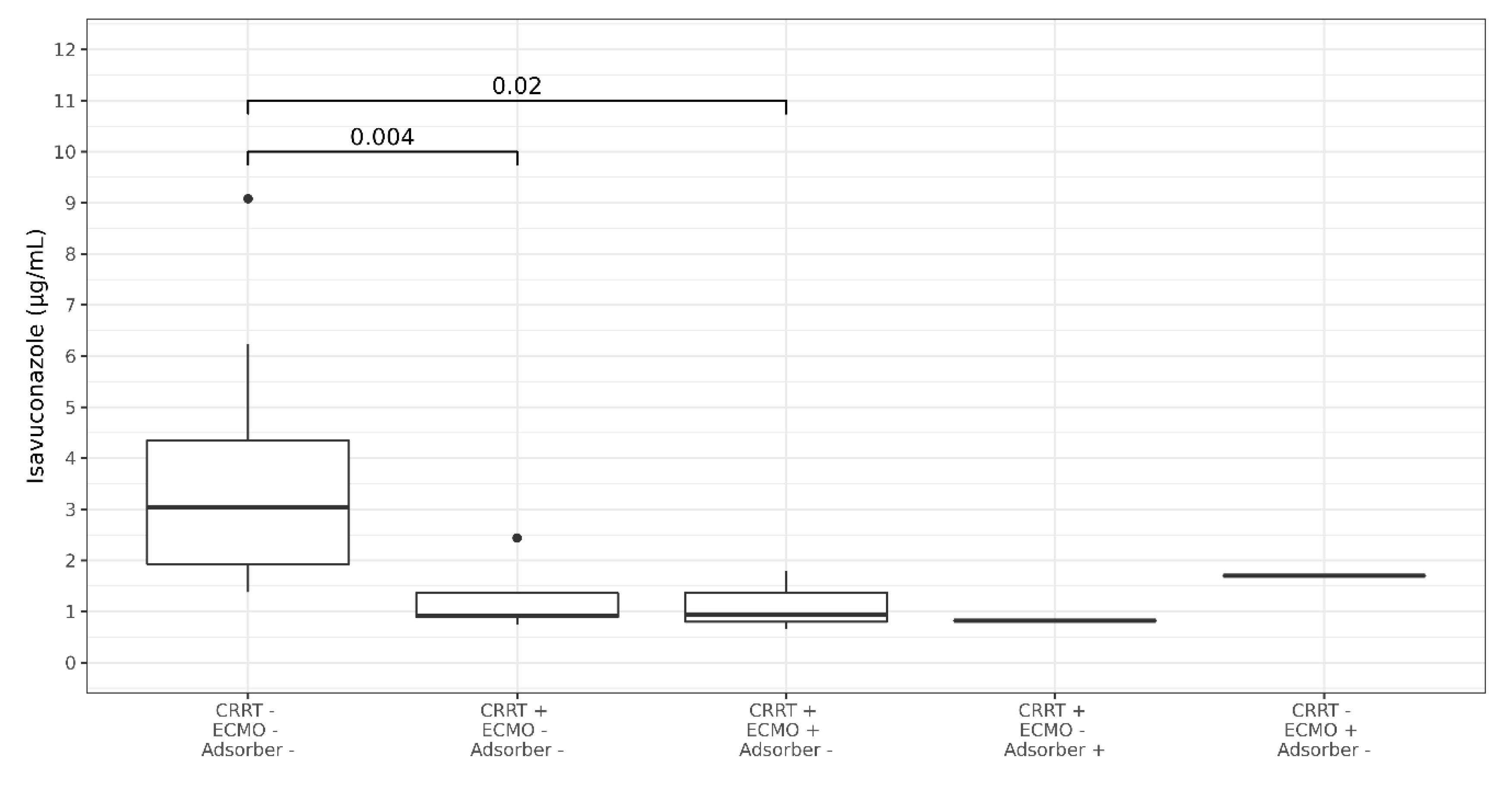
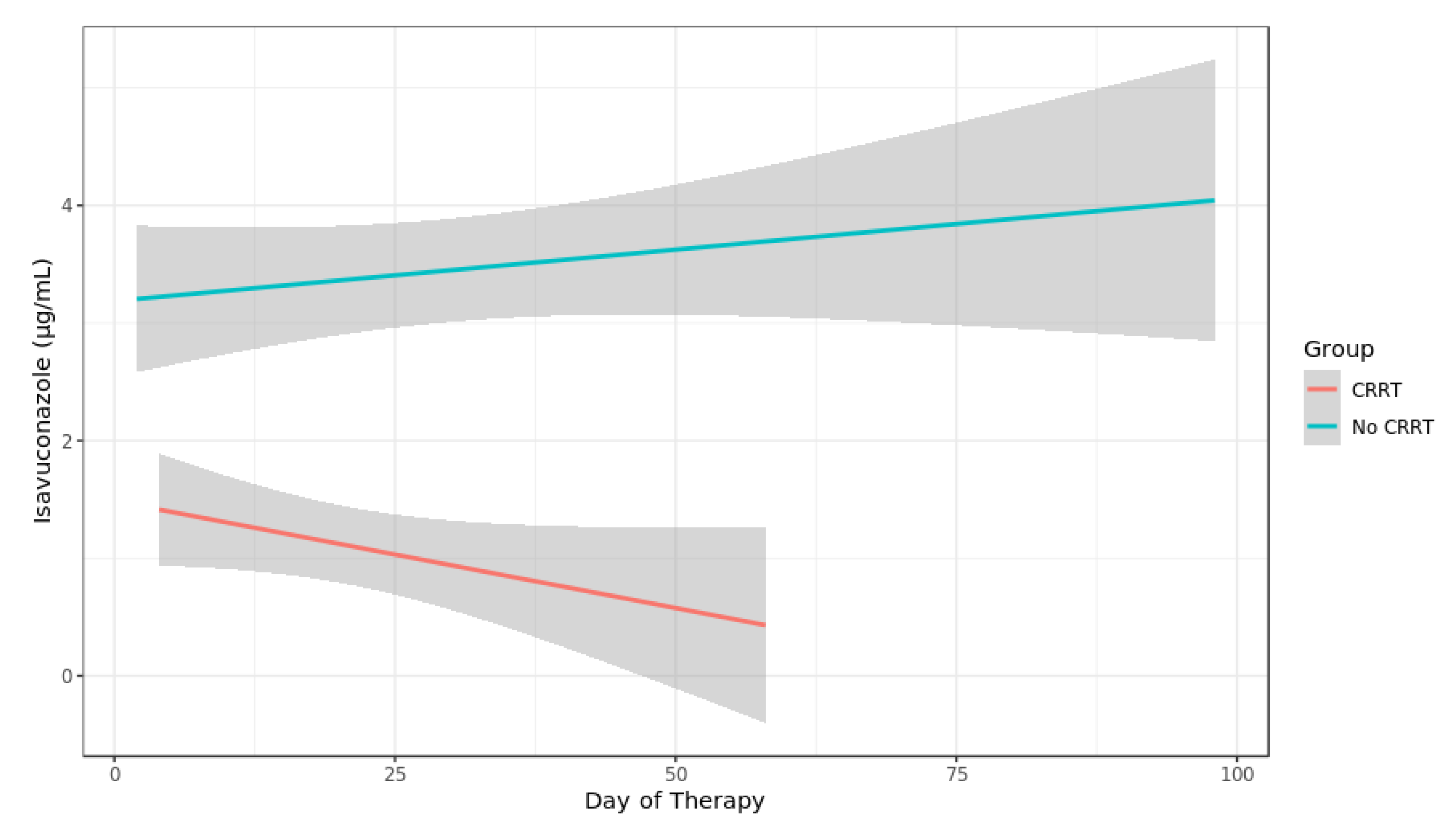
3.3. Isavuconazole TDM
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of america. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Florl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. Ecil-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- Jenks, J.D.; Salzer, H.J.; Prattes, J.; Krause, R.; Buchheidt, D.; Hoenigl, M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: Design, development, and place in therapy. Drug Des. Dev. Ther. 2018, 12, 1033–1044. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by aspergillus and other filamentous fungi (secure): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob. Agents Chemother. 2017, 61, e01034-17. [Google Scholar] [CrossRef]
- Lenczuk, D.; Zinke-Cerwenka, W.; Greinix, H.; Wolfler, A.; Prattes, J.; Zollner-Schwetz, I.; Valentin, T.; Lin, T.C.; Meinitzer, A.; Hoenigl, M.; et al. Antifungal prophylaxis with posaconazole delayed-release tablet and oral suspension in a real-life setting: Plasma levels, efficacy, and tolerability. Antimicrob. Agents Chemother. 2018, 62, e02655-17. [Google Scholar] [CrossRef]
- Jenks, J.D.; Mehta, S.R.; Hoenigl, M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med. Mycol. 2019, 57, S168–S178. [Google Scholar] [CrossRef] [PubMed]
- Kaindl, T.; Andes, D.; Engelhardt, M.; Saulay, M.; Larger, P.; Groll, A.H. Variability and exposure-response relationships of isavuconazole plasma concentrations in the phase 3 secure trial of patients with invasive mould diseases. J. Antimicrob. Chemother. 2019, 74, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole concentration in real-world practice: Consistency with results from clinical trials. Antimicrob. Agents Chemother. 2018, 62, e00585-18. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Brüggemann, R.; Padoin, C.; Maertens, J.; Marchetti, O.; Groll, A.H.; Johnson, E.M.; Arendrup, M.C. Triazole antifungal therapeutic drug monitoring. Presented at ECIL 6 Meeting, Sophia Antipolis, France, 11–12 September 2015; Available online: http://www.ecil-leukaemia.com/telechargements2015/ECIL6-Triazole-TDM-07-12-2015-Lewis-R-et-al.pdf (accessed on 20 June 2020).
- Slavin, M.A.; Thursky, K.A. Isavuconazole: A role for the newest broad-spectrum triazole. Lancet 2016, 387, 726–728. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the european organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (eortc/msg) consensus group. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 46, 1813–1821. [Google Scholar]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Segal, B.H.; Herbrecht, R.; Stevens, D.A.; Ostrosky-Zeichner, L.; Sobel, J.; Viscoli, C.; Walsh, T.J.; Maertens, J.; Patterson, T.F.; Perfect, J.R.; et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses study group and european organization for research and treatment of cancer consensus criteria. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Strenger, V.; Buzina, W.; Valentin, T.; Koidl, C.; Wolfler, A.; Seeber, K.; Valentin, A.; Strohmeier, A.T.; Zollner-Schwetz, I.; et al. European organization for the research and treatment of cancer/mycoses study group (eortc/msg) host factors and invasive fungal infections in patients with haematological malignancies. J. Antimicrob. Chemother. 2012, 67, 2029–2033. [Google Scholar] [CrossRef]
- Cornely, O.A.; Hoenigl, M.; Lass-Florl, C.; Chen, S.C.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R., 3rd. Defining breakthrough invasive fungal infection-position paper of the mycoses study group education and research consortium and the european confederation of medical mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Pea, F.; Krause, R.; Müller, C.; Hennart, B.; Richardson, M.; Meinitzer, A.; Wiesen, M.H.J.; Wiktorowicz, T.; Spickermann, J.; Henriksen, A.S. Interlaboratory analysis of isavuconazole plasma concentration assays among european laboratories. Ther. Drug Monit. 2019, 41, 657–664. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Spriet, I.; Annaert, P.; Meersseman, P.; Hermans, G.; Meersseman, W.; Verbesselt, R.; Willems, L. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J. Antimicrob. Chemother. 2009, 63, 767–770. [Google Scholar] [CrossRef]
- Watt, K.M.; Cohen-Wolkowiez, M.; Williams, D.C.; Bonadonna, D.K.; Cheifetz, I.M.; Thakker, D.; Benjamin, D.K., Jr.; Brouwer, K.L.R. Antifungal extraction by the extracorporeal membrane oxygenation circuit. J. Extra-Corporeal Technol. 2017, 49, 150–159. [Google Scholar]
- Lahmer, T.; Batres Baires, G.; Heilmaier, M.; Schmid, R.M.; Sorgel, F.; Kinzig, M.; Huber, W.; Mayr, U.; Rasch, S. Influence of sustained low-efficiency dialysis (sled) treatment on isavuconazole plasma levels in critically ill patients. Antimicrob. Agents Chemother. 2019, 63, e01162-19. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.L.; Desai, A.V.; Lu, Q.; Townsend, R.W.; Akhtar, S.; Bonate, P.; Hope, W.W. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the vital study). Antimicrob. Agents Chemother. 2016, 60, 4568–4576. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R., 3rd; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Rouzaud, C.; Jullien, V.; Herbrecht, A.; Palmier, B.; Lapusan, S.; Morgand, M.; Guery, R.; Dureault, A.; Danion, F.; Puget, S.; et al. Isavuconazole diffusion in infected human brain. Antimicrob. Agents Chemother. 2019, 63, e02474-18. [Google Scholar] [CrossRef]
| 32 ISA Patients | |
| Age, median (IQR), years | 60 years (46–69) |
| Female sex, No. (%) | 9 (28%) |
| Weight, median (IQR), kg | 75 (65–84) |
| Body Mass Index (BMI), median (IQR), kg/m2 | 24.6 (23.3–28.5) |
| <18.5: underweight, No. (%) | 0 (0%) |
| 18.5 to <25: normal, No. (%) | 19 (59%) |
| 25 to <30: overweight, No. (%) | 7 (22%) |
| ≥30: obese, No. (%) | 6 (19%) |
| Invasive fungal infection (IFI), EORTC (33 ISA courses *) | |
| Possible, No. (%) | 9 (27%) |
| Probable, No. (%) | 10 (30%) * |
| Proven, No. (%) | 14 (42%) |
| Underlying diseases | |
| Hematological disease ** | 14 (44%) |
| Solid cancer | 2 (6%) |
| Solid organ transplantation | 4 (13%) |
| Collagenosis/autoimmune diseases | 2 (6%) |
| Type 2 diabetes | 2 (6%) |
| Respiratory tract diseases | 3 (9%) |
| Bacterial infections | 3 (9%) |
| Coronary heart disease | 1 (3%) |
| Trauma associated osteomyelitis | 1 (3%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurl, C.; Waller, M.; Schwameis, F.; Muhr, T.; Bauer, N.; Zollner-Schwetz, I.; Valentin, T.; Meinitzer, A.; Ullrich, E.; Wunsch, S.; et al. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. J. Fungi 2020, 6, 90. https://doi.org/10.3390/jof6020090
Zurl C, Waller M, Schwameis F, Muhr T, Bauer N, Zollner-Schwetz I, Valentin T, Meinitzer A, Ullrich E, Wunsch S, et al. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. Journal of Fungi. 2020; 6(2):90. https://doi.org/10.3390/jof6020090
Chicago/Turabian StyleZurl, Christoph, Maximilian Waller, Franz Schwameis, Tina Muhr, Norbert Bauer, Ines Zollner-Schwetz, Thomas Valentin, Andreas Meinitzer, Elisabeth Ullrich, Stefanie Wunsch, and et al. 2020. "Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations" Journal of Fungi 6, no. 2: 90. https://doi.org/10.3390/jof6020090
APA StyleZurl, C., Waller, M., Schwameis, F., Muhr, T., Bauer, N., Zollner-Schwetz, I., Valentin, T., Meinitzer, A., Ullrich, E., Wunsch, S., Hoenigl, M., Grinschgl, Y., Prattes, J., Oulhaj, A., & Krause, R. (2020). Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. Journal of Fungi, 6(2), 90. https://doi.org/10.3390/jof6020090






