Mangrove-Associated Fungi: A Novel Source of Potential Anticancer Compounds
Abstract
1. Introduction
2. Bioactive Compounds in Mangrove Plants
2.1. Compounds Produced by Coelomycetes
2.2. Compounds Produced by Ascomycetes
2.3. Compounds Produced by Hyphomycetes
2.4. Compounds Produced by Basidiomycetes
2.5. Compounds Produced by Zygomycetes
3. Methods Used for the Activation of Silent Biosynthetic Genes
3.1. The Co-Culture Strategy
3.2. Epigenetic Modification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gopal, B.; Chauhan, M. Biodiversity and its conservation in the Sundarban mangrove ecosystem. Aquat. Sci. 2006, 68, 338–354. [Google Scholar] [CrossRef]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fuangal diversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Sridhar, K.R. Mangrove fungi in India. Curr. Sci. 2004, 86, 1586–1587. [Google Scholar]
- Sun, H.H.; Mao, W.J.; Chen, Y.; Guo, S.D.; Li, H.Y.; Qi, X.H.; Chen, Y.L.; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Balagurunathan, R.; Radhakrishnan, M. Exploiting the less explored-microbial endophytes. Adv. Biotechnol. 2007, 6, 20–23. [Google Scholar]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Bioactive natural products derived from mangrove associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Thatoi, H.; Behera, B.C.; Mishra, R.R. Ecological role and biotechnological potential of mangrove fungi: A review. Mycology 2013, 4, 54–71. [Google Scholar]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-cultivation—A powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.M.; Gillaspy, A.F.; Gipson, M.; Henrikson, J.C.; Hoover, A.R.; Jackson, L.; Najar, F.Z.; Wagele, H.; Cichewicz, R.H. Chemical induction of silent pathway transcription in Aspergillus niger. J. Ind. Microbiol. Biotechnol. 2009, 36, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, J.C.; Hoover, A.R.; Joyner, P.M.; Cichewicz, R.H. A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org. Biomol. Chem. 2009, 7, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Long, D.M. Endophytic microbes embody pharmaceutical potential. Am. Soc. Microbiol. News 1998, 64, 263–268. [Google Scholar]
- Xu, J.; Ebada, S.S.; Proksch, P. Pestalotiopsis a highly creative genus: Chemistry and bioactivity of secondary metabolites. Fungal Divers. 2010, 44, 15–31. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Recent advances in the discovery of bioactive metabolites from Pestalotiopsis. Phytochem. Rev. 2017, 16, 883–920. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Deng, Q.; Zheng, D.; Yang, X.; Xu, J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G0/G1 cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dai, H.; Makhloufi, G.; Heering, C.; Janiak, C.; Hartmann, R.; Mándi, A.; Kurtán, T.; Müller, W.E.; Kassack, M.U.; et al. Cytotoxic 14-membered macrolides from a mangrove-derived endophytic fungus Pestalotiopsis microspora. J. Nat. Prod. 2016, 79, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, C.F.P.; Daletos, G.; Liu, Z.; Lin, W.; Proksch, P. Polyketides from the mangrove-derived fungal endophyte Pestalotiopsis clavispora. Tetrahedron Lett. 2016, 57, 2078–2083. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; Ma, W.; Fang, W.; Chen, Z.; Yang, B.; Liu, Y. A new aromatic amine from fungus Pestalotiopsis vaccinia. Phytochem. Lett. 2014, 7, 35–37. [Google Scholar] [CrossRef]
- Kong, F.; Wang, Y.; Liu, P.; Dong, T.; Zhu, W. Thiodiketopiperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiu, Z.; Liu, X.; Huang, B.; Hu, L.; Liu, J.; Zhou, Z.; Tang, X.; Wu, X. Cytochalasin H isolated from mangrove-derived endophytic fungus induces apoptosis and inhibits migration in lung cancer cells. Oncol. Rep. 2018, 39, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.F.; Zhang, M.; Dai, J.G.; Pedpradab, P.; Wang, W.J.; Wu, J. Cytochalasins from mangrove endophytic fungi Phomopsis sp. xy21 and xy22. Phytochem. Lett. 2016, 17, 162–166. [Google Scholar] [CrossRef]
- Song, X.; Zhou, X.; Li, X.; Zheng, C.; Huang, G.; Yu, Z.; Song, X.; Chen, G. Secondary metabolites of a Bruguiera sexangula var. Rhynchopetala-derived fungus Phomopsis longicolla HL-2232. Youji Huaxue 2015, 35, 2102–2107. [Google Scholar]
- Ding, B.; Yuan, J.; Huang, X.; Wen, W.; Zhu, X.; Liu, Y.; Li, H.; Lu, Y.; He, L.; Tan, H.; et al. New dimeric members of the phomoxanthone family: Phomolactonexanthones A, B and deacetylphomoxanthone C isolated from the fungus Phomopsis sp. Mar. Drugs 2013, 11, 4961–4972. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, J.; Lei, F.; She, Z.; Lin, Y. A new xanthone O-glycoside from the mangrove endophytic fungus Phomopsis sp. Chem. Nat. Compd. 2013, 49, 27–30. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A-F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yu, J.; Chen, S.; Ding, M.; Huang, X.; Yuan, J.; She, Z. Alkaloids from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. Bioorg. Med. Chem. Lett. 2017, 27, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, B.; Du, L.; Shen, Y. Cytotoxic polyketides with an oxygen-bridged cyclooctadiene core skeleton from the mangrove endophytic fungus Phomosis sp. A818. Molecules 2017, 22, 1547. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.C.; Chen, Q.; Liu, K.; Mo, P.L.; Zhu, J.W.; Zhuang, M.Q.; Shen, Y.M.; Yu, C.D. Mycoepoxydiene inhibits antigen-stimulated activation of mast cells and suppresses IgE-mediated anaphylaxis in mice. Int. Immunopharmacol. 2013, 17, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, W.; Wu, L.; Zhu, T.; Gu, Q.; Li, D.; Che, Q. Saroclazines A-C, thio-diketopiperazines from mangrove-derived fungi Sarocladium kiliense HDN11-84. Arch. Pharm. Res. 2018, 41, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Stuhldreier, F.; Kurtan, T.; Mandi, A.; Arumugam, S.; Lin, W.; Stork, B.; Wesselborg, S.; Weber, H.; Henrich, B.; et al. Daldinone derivatives from the mangrove-derived endophytic fungus Annulohypoxylon sp. RSC Adv. 2017, 7, 5381–5393. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, A.; Chen, H.; Wang, M.; Ding, G.; Sun, L.; Li, L.; Dai, M. Anthraquinones from the saline-alkali plant endophytic fungus Eurotium rubrum. J. Antibiot. 2017, 70, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wu, A.A.; Zhang, L.; Hu, Z.; Huang, H.; Xu, Q.; Deng, X. New 12,8-Eudesmanolides from Eutypella sp. 1–15. J. Antibiot. 2017, 70, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Siridechakorn, I.; Yue, Z.; Mittraphab, Y.; Lei, X.; Pudhom, K. Identification of spirobisnaphthalene derivatives with anti-tumor activities from the endophytic fungus Rhytidhysteron rufulum AS21B. Bioorg. Med. Chem. 2017, 25, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, J.; Wang, Z.; Khan, D.; Niaz, S.I.; Zhu, Y.; Lin, Y.; Li, J.; Liu, L. New lasiodiplodins from mangrove endophytic fungus Lasiodiplodia sp. 318. Nat. Prod. Res. 2017, 31, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Yuan, J.; Lu, Y.; Zhu, X.; Lin, Y.; Liu, L. Lasiodiplodins from mangrove endophytic fungus Lasiodiplodia sp. 318#. Nat. Prod. Res. 2016, 30, 755–760. [Google Scholar] [PubMed]
- Chen, S.; Chen, D.; Cai, R.; Cui, H.; Long, Y.; Lu, Y.; Li, C.; She, Z. Cytotoxic and antibacterial preussomerins from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. J. Nat. Prod. 2016, 79, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Chokpaiboon, S.; Choodej, S.; Boonyuen, N.; Teerawatananond, T.; Pudhom, K. Highly oxygenated chromones from mangrove-derived endophytic fungus Rhytidhysteron rufulum. Phytochemstry 2016, 122, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, X.; Feng, H.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Campyridones A-D, pyridone alkaloids from a mangrove endophytic fungus Campylocarpon sp. HDN13-307. Tetrahedron 2016, 72, 5679–5683. [Google Scholar] [CrossRef]
- Moussa, M.; Ebrahim, W.; El-Neketi, M.; Mandi, A.; Kurtan, T.; Hartmann, R.; Lin, W.; Liu, Z.; Proksch, P. Tetrahydroanthraquinone derivatives from the mangrove-derived endophytic fungus Stemphylium globuliferum. Tetrahedron Lett. 2016, 57, 4074–4078. [Google Scholar] [CrossRef]
- Liu, Y.; Marmann, A.; Abdel-Aziz, M.S.; Wang, C.Y.; Müller, W.E.G.; Lin, W.H.; Mandi, A.; Kurtan, T.; Daletos, G.; Proksch, P. Tetrahydroanthraquinone derivatives from the endophytic fungus Stemphylium globuliferum. Eur. J. Org. Chem. 2015, 2015, 2646–2653. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Pretsch, A.; Pescitelli, G.; Kurtan, T.; Proksch, P. New anthracene derivatives–structure elucidation and antimicrobial activity. Eur. J. Org. Chem. 2012, 1351–1359. [Google Scholar] [CrossRef]
- Mishra, P.D.; Verekar, S.A.; Deshmukh, S.K.; Joshi, K.S.; Fiebig, H.H.; Kelter, G. Altersolanol A: A selective cytotoxic anthraquinone froma Phomopsis sp. Lett. Appl. Microbiol. 2015, 60, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Muller, W.E.G.; Totzke, F.; Zirrgiebel, U.; Schachtele, C.; Kubbutat, M.H.G.; Lin, W.; et al. Bioactive metabolites from endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J. Nat. Prod. 2009, 72, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Mack, F.; Debbab, A.; Aly, A.H.; Dicato, M.; Proksch, P.; Diederich, M. Anticancer effect of altersolanol A, a metabolite produced by the endophytic fungus Stemphylium globuliferum, mediated by its proapoptotic and anti-invasive potential via the inhibition of NF-kB activity. Bioorg. Med. Chem. 2013, 21, 3850–3858. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Chinthanom, P.; Rachtawee, P.; Srichomthong, K.; Srikitikulchai, P.; Kongsaeree, P.; Prabpai, S. Cytotoxic hydroanthraquinones from the mangrove-derived fungus Paradictyoarthrinium diffractum BCC 8704. J. Antibiot. 2015, 68, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, T.; Mao, Z.G.; Lei, N.; Wang, Z.M.; Hu, B.; Chen, Z.Y.; She, Z.G.; Zhu, Y.H.; Wang, H.J. The marine metabolite SZ-685C induces apoptosis in primary human nonfunctioning pituitary adenoma cells by inhibition of the Akt pathway in vitro. Mar. Drugs 2015, 13, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Xiao, W.W.; Jiang, X.B.; Wang, J.W.; Mao, Z.G.; Lei, N.; Fan, X.; Song, B.B.; Liao, C.X.; Wang, H.J.; et al. A novel marine drug, SZ-685C, induces apoptosis of MMQ pituitary tumor cells by downregulating miR-200c. Curr. Med. Chem. 2013, 20, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Du, X.P.; Su, W.J. Two new polyketides from mangrove endophytic fungus Dothiorella sp. Chem. Nat. Compd. 2014, 50, 214–216. [Google Scholar] [CrossRef]
- Pudhom, K.; Teerawatananond, T.; Chookpaiboon, S. Spirobisnaphthalenes from the mangrove-derived fungus Rhytidhysteron sp. AS21B. Mar. Drugs 2014, 12, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wei, Q.; Chen, G.; Cai, J.; She, Z. Chemical constituents from the mangrove endophytic fungus Sporothrix sp. Chem. Nat. Compd. 2013, 49, 137–140. [Google Scholar] [CrossRef]
- Yan, H.J.; Li, X.M.; Li, C.S.; Wang, B.G. Alkaloid and anthraquinone derivatives produced by the marine-derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2012, 95, 163–168. [Google Scholar] [CrossRef]
- Ebrahim, W.; Kjer, J.; El Amrani, M.; Wray, V.; Lin, W.; Ebel, R.; Lai, D.; Proksch, P. Pullularins E and F, two new peptides from the endophytic fungus Bionectria ochroleuca isolated from the mangrove plant Sonneratia caseolaris. Mar. Drugs 2012, 10, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, S.; Liu, H.; Huang, X.; Liu, Y.; Tao, Y.; She, Z. Cytotoxic isocoumarin derivatives from the mangrove endophytic fungus Aspergillus sp. HN15-5D. Arch. Pharm. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.M.; Li, C.S.; Wang, B.G. Nigerasterols A and B, antiproliferative sterols from the mangrove-derived endophytic fungus Aspergillus niger MA-132. Helv. Chim. Acta 2013, 96, 1055–1061. [Google Scholar] [CrossRef]
- An, C.Y.; Li, X.M.; Luo, H.; Li, C.S.; Wang, M.H.; Xu, G.M.; Wang, B.G. 4-Phenyl-3,4-dihydroquinolone derivatives from Aspergillus nidulans MA-143, an endophytic fungus isolated from the mangrove plant Rhizophorastylosa. J. Nat. Prod. 2013, 76, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary metabolites of a mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Huang, C.; Wu, Q.; Pang, J.; Lin, Y. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013, 27, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, P.; Liao, G.; Zeng, Y.; Cai, C.; Kong, F.; Guo, Z.; Dai, H.; Mei, W.; Qiu, L.; et al. New eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus Penicillium sp. J-54. Mar. Drugs. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Liao, H.X.; Mei, R.Q.; Huang, G.L.; Yang, L.J.; Zhou, X.M.; Shao, T.M.; Chen, G.Y.; Wang, C.Y. Two new benzophenones and one new natural amide alkaloid isolated from a mangrove-derived Fungus Penicillium citrinum. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, Y.Y.; Zhang, T.Y.; Zhang, M.Y.; Zhu, W.W.; Gullen, E.A.; Wang, Z.J.; Cheng, Y.C.; Zhang, Y.X. New and bioactive natural products from an endophyte of Panax notoginseng. RSC Adv. 2017, 7, 38100–38109. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, D.; Liang, F.; Wu, Z.; She, Z.; Li, C. Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia 2017, 123, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, Z.; Feng, H.; Gan, Q.; Che, Q.; Zhu, T.; Gu, Q.; Han, B.; Li, D. Trichodermamides D-F, heterocyclic dipeptides with a highly functionalized 1,2-oxazadecaline core isolated from the endophytic fungus Penicillium janthinellum HDN13-309. RSC Adv. 2017, 7, 48019–48024. [Google Scholar] [CrossRef]
- Huang, S.; Chen, H.; Li, W.; Zhu, X.; Ding, W.; Li, C. Bioactive chaetoglobosins from the mangrove endophytic fungus Penicillium chrysogenum. Mar. Drugs 2016, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Wang, C.Y.; Mandi, A.; Li, X.M.; Hu, X.Y.; Kassack, M.U.; Kurtan, T.; Wang, B.G. Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endophytic fungus Penicillium brocae MA-231. Org. Lett. 2016, 18, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Li, X.M.; Lv, C.T.; Huang, C.G.; Wang, B.G. Brocazines A-F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicenniam arina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Darsih, C.; Prachyawarakorn, V.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic metabolites from the endophytic fungus Penicillium chermesinum: Discovery of a cysteine-targeted Michael acceptor as a pharmacophore for fragment-based drug discovery, bioconjugation and click reactions. RSC Adv. 2015, 5, 70595–70603. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; Peng, J.; Zhu, T.; Gu, Q.; Li, D. Penicitols A-C and Penixanacid A from the mangrove-derived Penicillium chrysogenum HDN11-24. J. Nat. Prod. 2015, 78, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, Y.; Jiang, L.L.; Shi, X.M. Antiproliferative metabolites from the endophytic fungus Penicillium sp. FJ-1 isolated from a mangrove Avicennia marina. Phytochem. Lett. 2014, 10, 272–275. [Google Scholar]
- Zhou, Z.F.; Kurtan, T.; Yang, X.H.; Mandi, A.; Geng, M.Y.; Ye, B.P.; Taglialatela-Scafati, O.; Guo, Y.W. Penibruguieramine A, a novel pyrrolizidine alkaloid from the endophytic fungus Penicillium sp. GD6 associated with Chinese mangrove Bruguiera gymnorrhiza. Org. Lett. 2014, 16, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Lin, Y.; Yuan, J.; Lu, Y.; Zhu, X.; Li, J.; Li, M.; Lin, Y.; He, J.; et al. Meroterpenes and azaphilones from marine mangrove endophytic fungus Penicillium 303#. Fitoterapia 2014, 97, 241–246. [Google Scholar] [PubMed]
- Yang, J.; Huang, R.; Qiu, S.X.; She, Z.; Lin, Y. A new isobenzofuranone from the mangrove endophytic fungus Penicillium sp. (ZH58). Nat. Prod. Res. 2013, 27, 1902–1905. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Li, X.M.; Lv, C.T.; Li, C.S.; Xu, G.M.; Huang, C.G.; Wang, B.G. Sulfur-containing cytotoxic curvularin macrolides from Penicillium sumatrense MA-92, a fungus obtained from the rhizosphere of the mangrove Lumnitzera racemose. J. Nat. Prod. 2013, 76, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, J.; Cai, X.; She, Z.; Lin, Y. A new furanocoumarin from the mangrove endophytic fungus Penicillium sp. ZH16. Nat. Prod. Res. 2012, 26, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niaz, S.I.; Khan, D.; Wang, Z.; Zhu, Y.; Zhou, H.; Lin, Y.; Li, J.; Liu, L. Induction of diverse bioactive secondary metabolites from the mangrove endophytic fungus Trichoderma sp. (Strain 307) by Co-Cultivation with Acinetobacter johnsonii (Strain B2). Mar. Drugs 2017, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niaz, S.I.; Wang, Z.; Zhu, Y.; Lin, Y.; Li, J.; Liu, L. α-Glucosidase inhibitory and cytotoxic botryorhodines from mangrove endophytic fungus Trichoderma sp. 307. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.M.; Zhao, J.L.; Li, N.; Chen, R.D.; Xie, K.B.; Zhang, W.J.; Feng, K.P.; Yan, Z.; Wang, N.; et al. Two new diterpenoids from the endophytic fungus Trichoderma sp. Xy24 isolated from mangrove plant Xylocarpus granatum. Chin. Chem. Lett. 2016, 27, 957–960. [Google Scholar] [CrossRef]
- Shang, Z.; Li, X.M.; Li, C.S.; Wang, B.G. Diverse secondary metabolites produced by marine-derived fungus Nigrospora sp. MA75 on various vulture media. Chem. Biodivers. 2012, 9, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.W.; Lin, Y.C.; She, Z.G.; Lin, M.T.; Chen, P.X.; Zhang, J.Y. Anticancer activity and mechanism investigation of Beauvericin isolated from secondary metabolites of the mangrove endophytic fungi. Anticancer Agents Med. Chem. 2015, 15, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lai, Z.; Huang, W.; Ling, H.; Lin, M.; Tang, S.; Liu, Y.; Tao, Y. Apicidin inhibited proliferation and invasion and induced apoptosis via mitochondrial pathway in non-small cell lung cancer glc-82 cells. Anticancer Agents Med. Chem. 2017, 17, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Guo, Z.Y.; Yang, R.Y.; She, Z.G.; Lin, Y.C. Alkaloid metabolites of mangrove endophytic fungus ZZF42 from the South China Sea. Zhong Yao Cai 2007, 30, 939–941. [Google Scholar] [PubMed]
- Ding, L.; Dahse, H.M.; Hertweck, C. Cytotoxic alkaloids from Fusarium incarnatum associated with the mangrove tree Aegiceras corniculatum. J. Nat. Prod. 2012, 75, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, C.F.P.; Daletos, G.; Hamacher, A.; Kassack, M.U.; Lin, W.; Mandi, A.; Kurtan, T.; Proksch, P. Absolute configuration and antitumor activity of torrubiellin B. Tetrahedron Lett. 2015, 56, 4430–4433. [Google Scholar] [CrossRef]
- Hammerschmidt, L.; Debbab, A.; Ngoc, T.D.; Wray, V.; Hemphil, C.P.; Lin, W.H.; Broetz-Oesterhelt, H.; Kassack, M.U.; Proksch, P.; Aly, A.H. Polyketides from the mangrove-derived endophytic fungus Acremonium strictum. Tetrahedron Lett. 2014, 55, 3463–3468. [Google Scholar] [CrossRef]
- Zeng, Y.B.; Gu, H.G.; Zuo, W.J.; Zhang, L.L.; Bai, H.J.; Guo, Z.K.; Proksch, P.; Mei, W.L.; Dai, H.F. Two new sesquiterpenoids from endophytic fungus J3 isolated from Mangrove Plant Ceriopstagal. Arch. Pharm. Res. 2015, 38, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Qiu, S.X.; She, Z.G.; Lin, Y.C. Metabolites of mangrove endophytic fungus ZH-3 from South China Sea. Huagong Jishu Yu Kaifa 2014, 43, 1–3. [Google Scholar]
- Yang, J.X.; Qiu, S.X.; She, Z.G.; Lin, Y.C. Metabolites of mangrove endophytic fungus 5094 from the South China Sea. Shizhen Guoyi Guoyao 2013, 24, 1059–1061. [Google Scholar]
- Zhu, F.; Chen, G.Y.; Wu, J.S.; Pan, J.H. Structure revision and cytotoxic activity of marinamide and its methyl ester, novel alkaloids produced by co-cultures of two marine-derived mangrove endophytic fungi. Nat. Prod. Res. 2013, 27, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Qiu, S.X.; She, Z.G.; Lin, Y.C. Metabolites of mangrove endophytic fungus Gx-3a from the South China Sea. Guangxi Kexue 2013, 20, 168–170. [Google Scholar]
- Yang, J.X.; Qiu, S.X.; She, Z.G.; Lin, Y.C. Metabolites of mangrove endophytic fungus SK7RN3G1 from South China Sea. Zhongguo Shiyan Fangjixue Zazhi 2012, 18, 95–98. [Google Scholar]
- Choodej, S.; Teerawatananond, T.; Mitsunaga, T.; Pudhom, K. Chamigrane sesquiterpenes from a basidiomycetous endophytic fungus XG8D associated with Thai mangrove Xylocarpus granatum. Mar. Drugs 2016, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic sesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Phytochemstry 2016, 122, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Tricyclic and spirobicyclicnorsesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Eur. J. Org. Chem. 2014, 19, 3976–3980. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Williams, K.; Proksch, P.; Ji, N.Y.; Wang, B.G. Rhizovarins A-F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189. J. Nat. Prod. 2016, 79, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Sanglier, J.J.; DiTullio, D.; Braccili, S.; Bonner, P.; Waters, J.; Hughes, D.; Zhang, L. Diversifying microbial natural products for drug discovery. Appl. Microbiol. Biotechnol. 2003, 62, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites–strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Cichewicz, R.H. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat. Prod. Rep. 2010, 27, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Shwab, E.K.; Bok, J.W.; Tribus, M.; Galehr, J.; Graessle, S.; Keller, N.P. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot. Cell 2007, 6, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Ola, A.R.B.; Thomy, D.; Lai, D.; Brötz-Oesterhelt, H.; Proksch, P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J. Nat. Prod. 2013, 76, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Chen, G.; Chen, X.; Huang, M.; Wan, X. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 2011, 47, 767–769. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Shao, C.; Ding, W.; She, Z.; Lin, Y. A new xanthone derivative from the co-culture broth of two marine fungi (strain No. E33 and K38). Chem. Nat. Compd. 2011, 47, 382–384. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A–D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kwon, H.C.; Lee, C.-H.; Yang, H.O. Glionitrin A, an antibiotic-antitumor metabolite derived from competitive interaction between abandoned mine microbes. J. Nat. Prod. 2009, 72, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; Henrikson, J.C.; Hoover, A.R.; Lee, A.E.; Cichewicz, R.H. Epigenetic remodeling of the fungal secondary metabolome. Org. Biomol. Chem. 2008, 6, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Chung, Y.M.; Sakurai, H.; Ozeki, T.; Chang, F.R.; Yamashita, K.; Oshima, Y. Tenuipyrone, a novel skeletal polyketide from the entomopathogenic fungus, Isaria tenuipes, cultivated in the presence of epigenetic modifiers. Org. Lett. 2012, 14, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Filho, J.G.S.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. Chemical epigenetics alters the secondary metabolite composition of guttate excreted by an atlantic-florest-soil-derived Penicillium citreonigrum. J. Nat. Prod. 2010, 73, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Ul-Hassan, S.R.; Strobel, G.A.; Booth, E.; Knighton, B.; Floerchinger, C.; Sear, J. Modulation of volatile organic compound formation in the mycodiesel-producing endophyte Hypoxylon sp. CI-4. Microbiology 2012, 158, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Beau, J.; Mahid, N.; Burda, W.N.; Harrington, L.; Shaw, L.N.; Mutka, T.; Kyle, D.E.; Barisic, B.; Olphen, A.; Baker, B.J. Epigenetic tailoring for the production of anti-infective cytosporones from the marine fungus Leucostoma persoonii. Mar. Drugs 2012, 10, 762–774. [Google Scholar] [CrossRef] [PubMed]
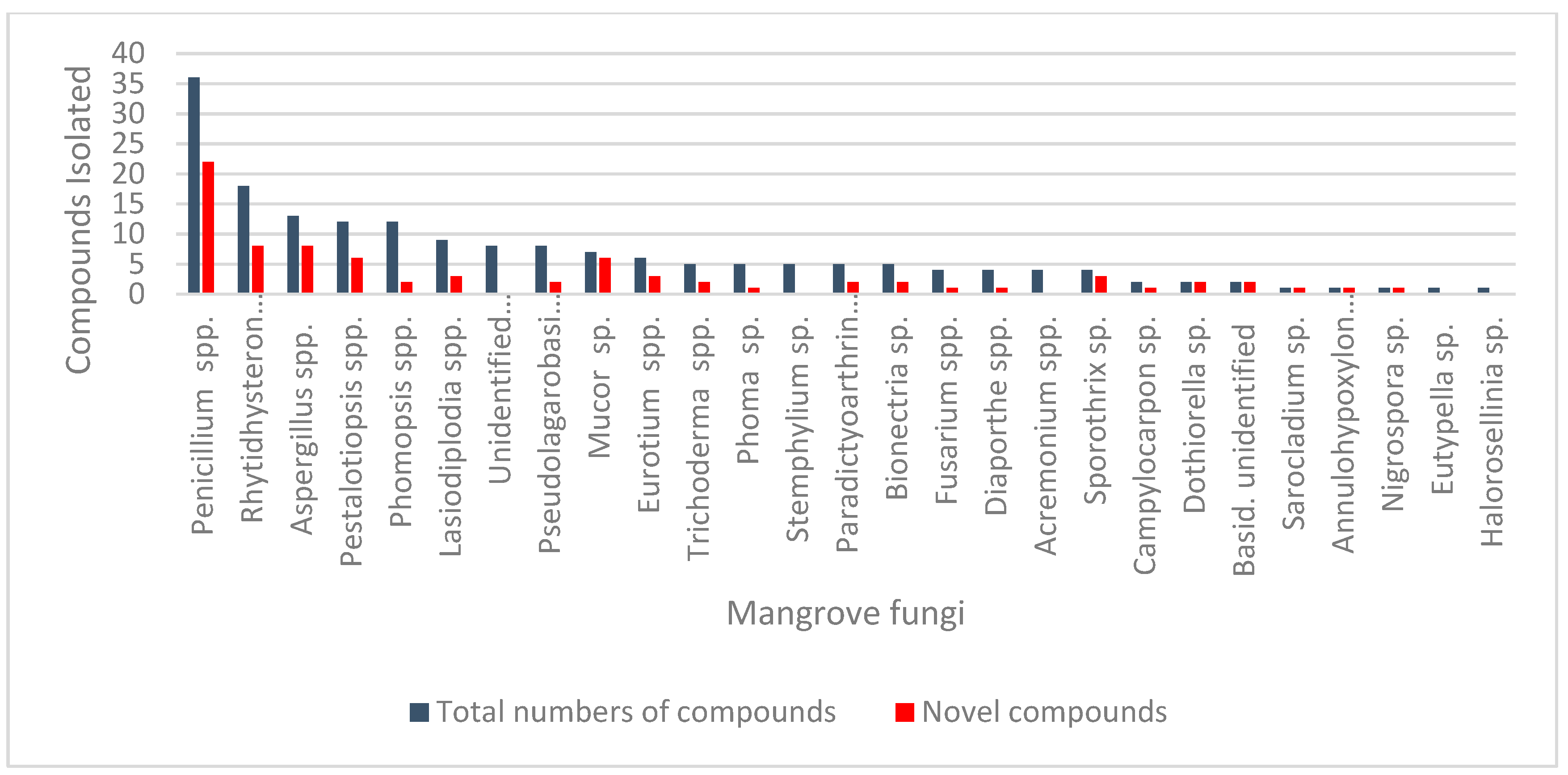
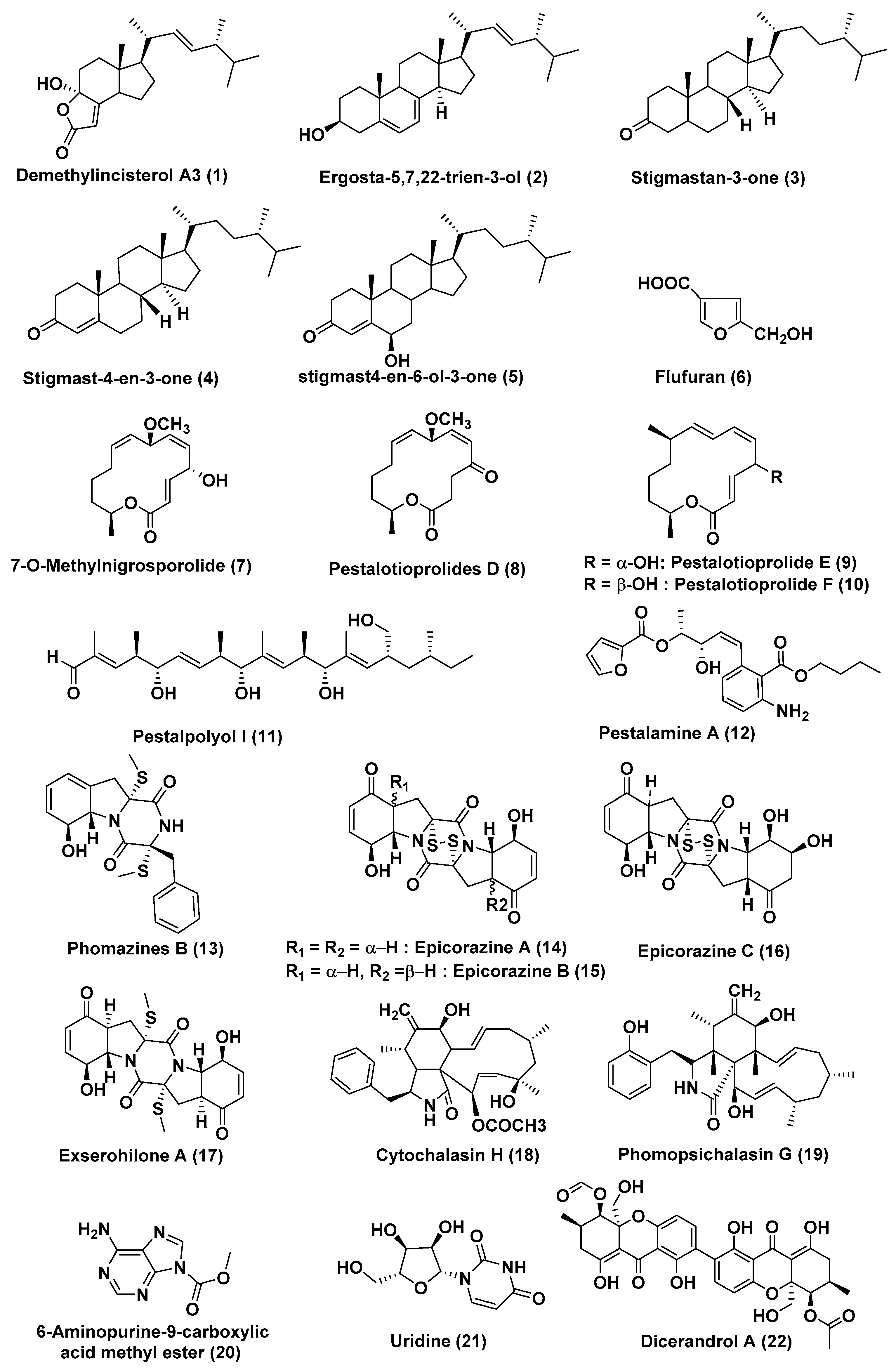
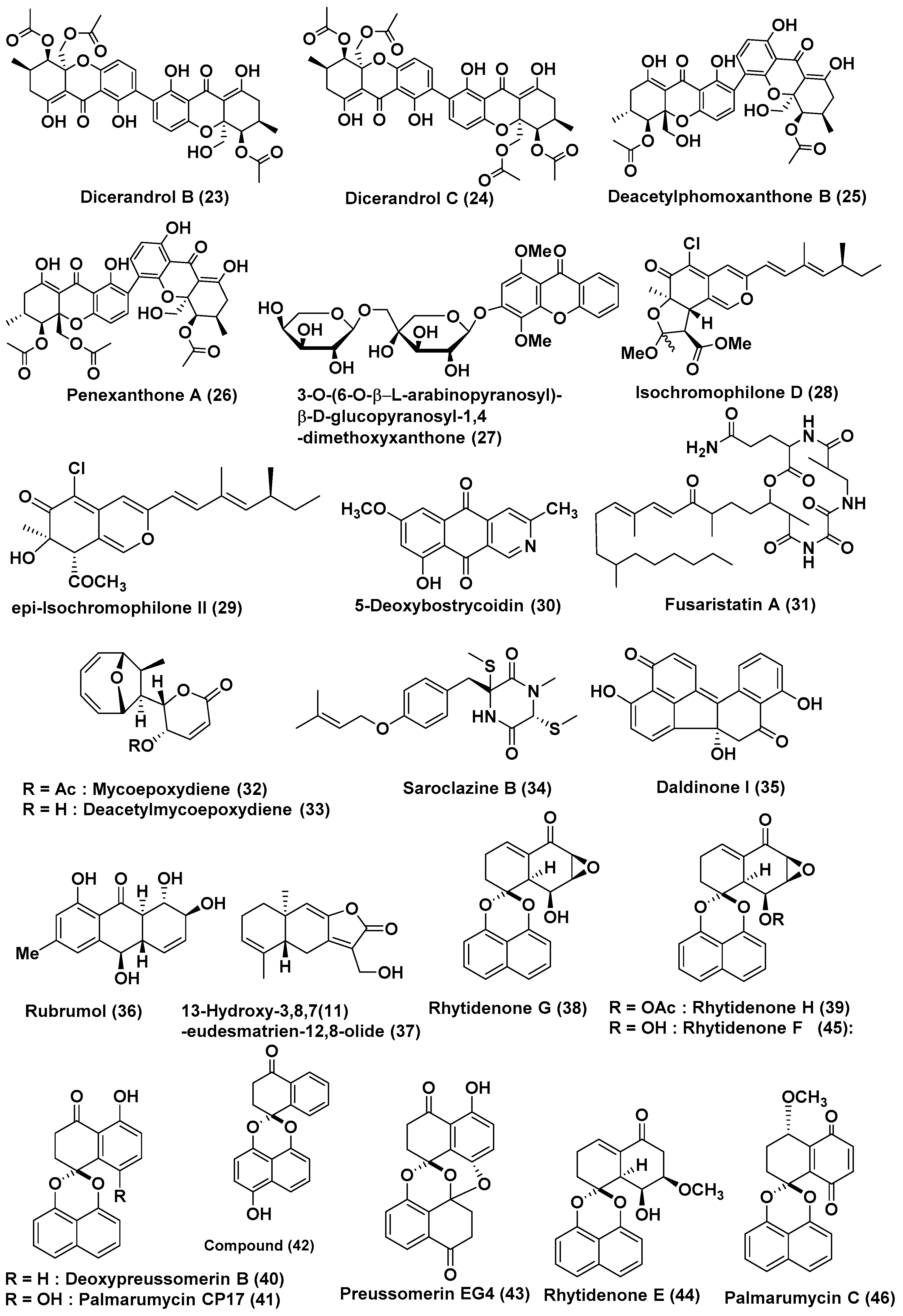
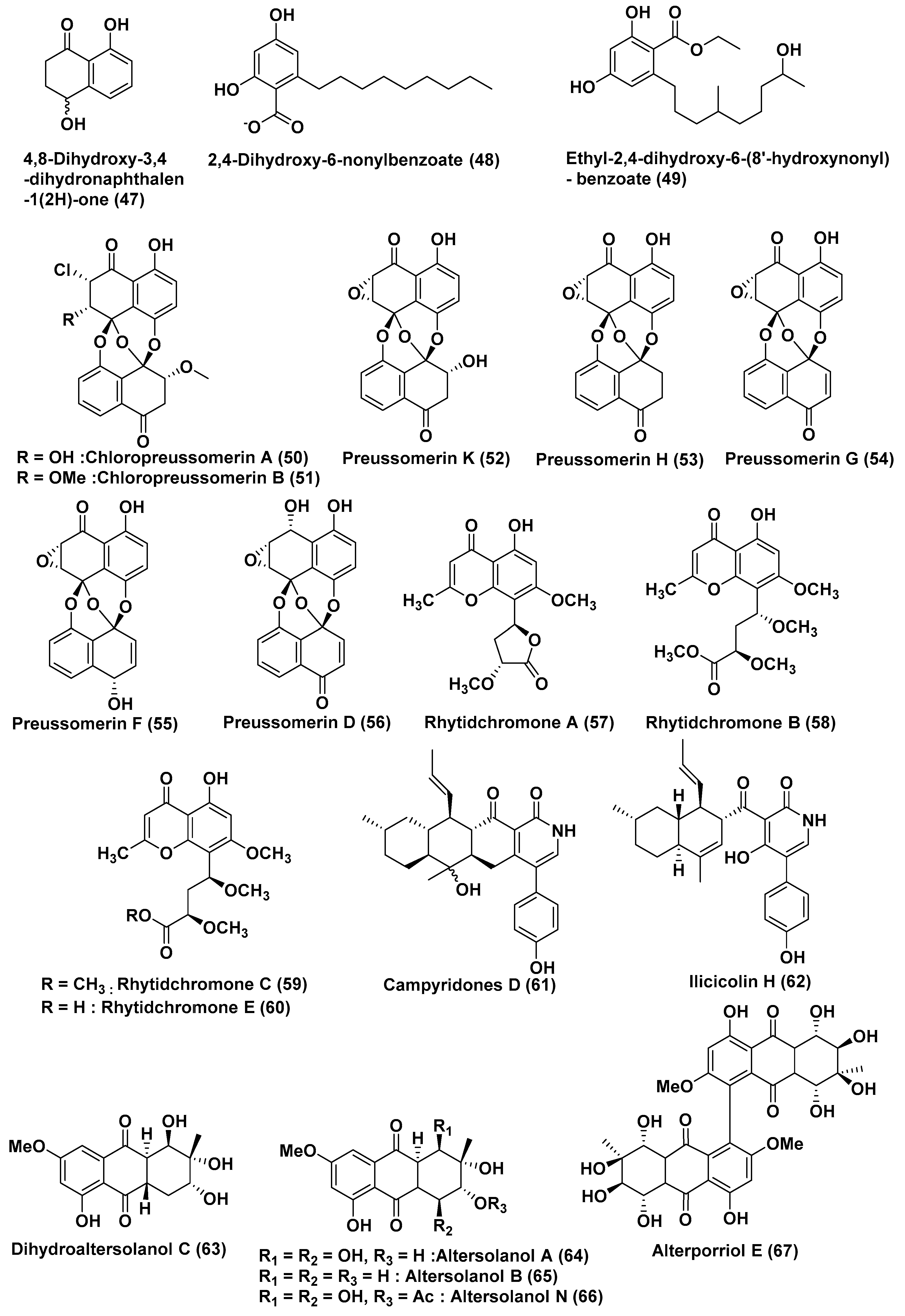
| Sr. No. | Fungus | Host Plant(s) | Plant Part or Tissue Locality of Host Plants | Compounds Isolated | Cell Line | IC50/EC50/Inhibition | Refs. |
|---|---|---|---|---|---|---|---|
| Compounds Produced by Coelomycetes | |||||||
| 1 | Pestalotiopsis sp. | Rhizophora mucronata | Not reported | Demethylincisterol A3 (1) | HeLa, A549 and HepG | In the range of 0.17 to 14.16 nM | [16] |
| Ergosta-5,7,22-trien-3-ol (2), stigmastan-3-one (3), stigmast-4-en-3-one (4), stigmast4-en-6-ol-3-one (5), flufuran (6) | HeLa, A549 and HepG | In the range of 11.44–102.11 µM | |||||
| 2 | Pestalotiopsis microspore | Drepanocarpus lunatus | Cameroon | Compound (7) Pestalotioprolide D–F (8–10) | L5178Y | 0.7, 5.6, 3.4, and 3.9 µM | [17] |
| Pestalotioprolide E (9) | A2780 | 1.2 µM | |||||
| 3 | Pestalotiopsis clavispora | Rhizophora harrisonii | Port Harcourt, Nigeria | Pestalpolyol I (11) | L5178Y | 4.10 µM | [18] |
| 4 | Pestalotiopsis vaccinia | Kandelia candel | China | Pestalamine A (12) | MCF-7, HeLa, and HepG2 | 40.3, 22.0, and 32.8 µM | [19] |
| 5 | Phoma sp. OUCMDZ-1847 | Fruit sample of Kandelia candel | Wenchang, Hainan Province, China | Phomazines B (13), epicorazine A (14), epicorazine B (15), epicorazine C (16), exserohilone A (17) | HL-60, HCT-116, K562, MGC-803, and A549 Cells | In the range 0.05 to 8.5 µM | [20] |
| 6 | Phomopsis sp. | Zhanjiang, China | Cytochalasin H (18) | A549 cells | Arrested A549 cells at the G2/M phase, inhibited the migration ability of A549 cells in a dose-dependent manner | [21] | |
| 7 | Phomopsis spp. xy21 and xy22 | Leaves Xylocarpus granatum | Trang Province, Thailand | Phomopsichalasin G (19) | HCT-8, HCT-8/T, A549, MDA-MB-231, and A2780 cancer Cells | 7.5, 8.6, 6.4, 3.4, and 7.1 µM | [22] |
| 8 | Phomopsis longicolla HL-2232 | Bruguiera sexangula var. rhynchopetala | 6-aminopurine-9-carboxylic acid Me ester (20), uridine (21) | B16F10, A549, HL-60 and MCF-7 Cells | 14.9 and 8.6 µM | [23] | |
| 9 | Phomopsis sp. HNY29-2B | Branch of Acanthus llicifolius | South China Sea, Hainan province, China | Dicerandrol A (22) | MDA-MB-435, HCT-116, Calu-3 and Huh7 Cells | 3.03, 2.64, 1.76 and 4.19 µM | [24] |
| Dicerandrol B (23), Penexanthone A (26) | MDA-MB-435, HCT-116 and Calu-3 | <10 µM | |||||
| Dicerandrol C (24) | MDA-MB-435, HCT-116, Calu-3, MCF-10A Cells | 44.10, 42.63, 36.52, and 33.05 µM | |||||
| diacetyl phomoxanthone B (25) | MDA-MB-435, HCT-116, Calu-3, Huh7 Cells | 14.40, 7.12, 4.14 and 29.20 µM | |||||
| 10 | Phomopsis sp. (ZH76) | Excoecaria agallocha | Dong Sai, South China Sea coast | 3-O-(6-O-α-l-arabinopyranosyl)-β-d-glucopyranosyl-1,4-dimethoxyxanthone (27) | HEp-2 and HepG2 | 9 and 16 µM | [25] |
| 11 | Diaporthe sp. SCSIO 41011 | Rhizophora stylosa | Sanya city, Hainan Province, China | Isochromophilone D (28) | 786-O cells | 8.9 µM | [26] |
| epi-Isochromophilone II (29) | ACHN, OS-RC-2, and 786-O cells, | In the range of 3.0 to 4.4 µM, Sorafenib (3.4 to 7.0 µM) | |||||
| 12 | Diaporthe phaseolorum SKS019 | Branches of Acanthus ilicifolius | Shankou, Guangxi province, China | 5-deoxybostrycoidin (30) | MDA-MB-435 and NCI-H460 cancer cells | 5.32 and 6.57 µM | [27] |
| Fusaristatin A (31) | MDA-MB-435 cancer cells | 8.15 µM | |||||
| 13 | Phomosis sp. A818 | Foliage of Kandelia candel | Fujian Province, China | Mycoepoxydiene (32), deacetylmycoepoxydiene (33) | MDA-MB-435 | 7.85 and 14.61 µM, | [28] |
| 14 | Phomosis sp. A818 | Foliage of Kandelia candel | Fujian Province, China | Mycoepoxydiene (32) | Suppress antigen-stimulated degranulation and cytokine production in mast cells and IgE-mediated passive cutaneous anaphylaxis in mice | [29] | |
| Compounds Produced by Ascomycetes | |||||||
| 15 | Sarocladium kiliense HDN11-84 | rhizosphere soil of Thespesia populnea, | Guangxi Province, China | Saroclazine B (34) | HeLa Cells | 4.2 µM | [30] |
| 16 | Annulohypoxylon sp. | Rhizophora racemosa | Cameroon | Daldinone I (35) | Ramos and Jurkat J16 | 6.6 and 14.1 µM, Potently blocks autophagy, a potential pro-survival pathway for cancer cells | [31] |
| 17 | Eurotium rubrum | Suaeda salsa | “BoHai” seaside, China | Rubrumol (36) | A549, MDA-MB-231, PANC-1 and HepG2 | Cytotoxic | [32] |
| Topo I | Relaxation activity The band backward shifting and trailing of rubrumol (36) was observed at 100, 50, 10, 5 and 1 µM | ||||||
| 18 | Eutypella sp. 1–15 | Soil of mangrove rhizosphere in Jimei, Fujian Province, China | Not reported | 13-Hydroxy-3,8,7(11)-eudesmatrien-12,8-olide (37) | JEKO-1 and HepG2 | 8.4 and 28.5 µM | [33] |
| 19 | Rhytidhysteron rufulum AS21B | Leaves of Azima sarmentosa | Samutsakhon province, Thailand | Rhytidenones G (38), H (39)), deoxypreussomerin B (40), palmarumycin CP17 (41), 1-oxo-1,4-dihydronapthalene-4-spiro-20-naptho[400-hydroxy-100,800-de][10,30]-dioxine (42), preussomerin EG4 (43), rhytidenone E (44), rhytidenone F (45), palmarumycin C5 (46), and 4,8-dihydroxy-3,4-dihydronaphthalen-1(2H)-one (47) | Ramos lymphoma | 17.98, 0.018, 18.00, 33.1, 15, 82.9, 0.461, 0.048, 31.7 and 23.1 µM, (Ibrutinib 28.7 µM) | [34] |
| Compounds (38), (39), (44), (45), and (47) | H1975 Cell | 7.3, 0.252, 10.24, 1.17 and 50 µM (afatinib 1.97 µM) | |||||
| 20 | Lasiodiplodia sp. 318# | Excoecaria agallocha | Guangdong Province, China | 2,4-Dihydroxy-6-nonylbenzoate (48) | MMQ and GH3 Cells | 5.2 and 13.0 µM | [35] |
| 21 | Lasiodiplodia sp. 318# | Excoecaria agallocha | Guangdong Province, China | Ethyl-2,4-dihydroxy-6-(80-hydroxynonyl)-benzoate (49) | MDA-MB-435, HepG2, HCT-116, A549 and leukaemia THP1 Cells | 0.13, 12.50, 11.92, 13.31 and 39.74 µM | [36] |
| 22 | Lasiodiplodia theobromae ZJ-HQ1 | Acanthus ilicifolius | Guangdong Province, China | Chloropreussomerins A and B (50, 51) Preussomerin D (56) | A549 and MCF-7 | In the range of 5.9–8.9 µM | [37] |
| Preussomerin K (52), Preussomerin H (53), Preussomerin G (54), Preussomerin F (55), | A549, HepG2, MCF-7 | In the range of 2.5–9.4 µM | |||||
| 23 | Rhytidhysteron rufulum BG2-Y | Leaves of Bruguiera gymnorrhiza | Pak Nam Pran, Prachuab Kiri Khan Province, Thailand | Rhytidchromone A (57), B (58), C (59), and E (60) | Kato-3 Cells | In the range of 16.0–23.3 µM | [38] |
| MCF-7 cells | 19.3–17.7 µM | ||||||
| 24 | Campylocarpon sp. HDN13-307 | Root of Sonneratia caseolaris | China | Campyridone D (61), and ilicicolin H (62) | HeLa | 8.8 and 4.7 µM | [39] |
| 25 | Stemphylium globuliferum | Avicennia marina | Hurghada, Egypt | Dihydroaltersolanol C (63), Altersolanol A (64), Altersolanol B (65), Alterporriol E (67) | L5178Y | 3.4, 2.53, 3.78 and 6.9 µM | [40,41,42] |
| Altersolanol N (66) | L5178Y | Low micromolar range (% growth-1.4) | |||||
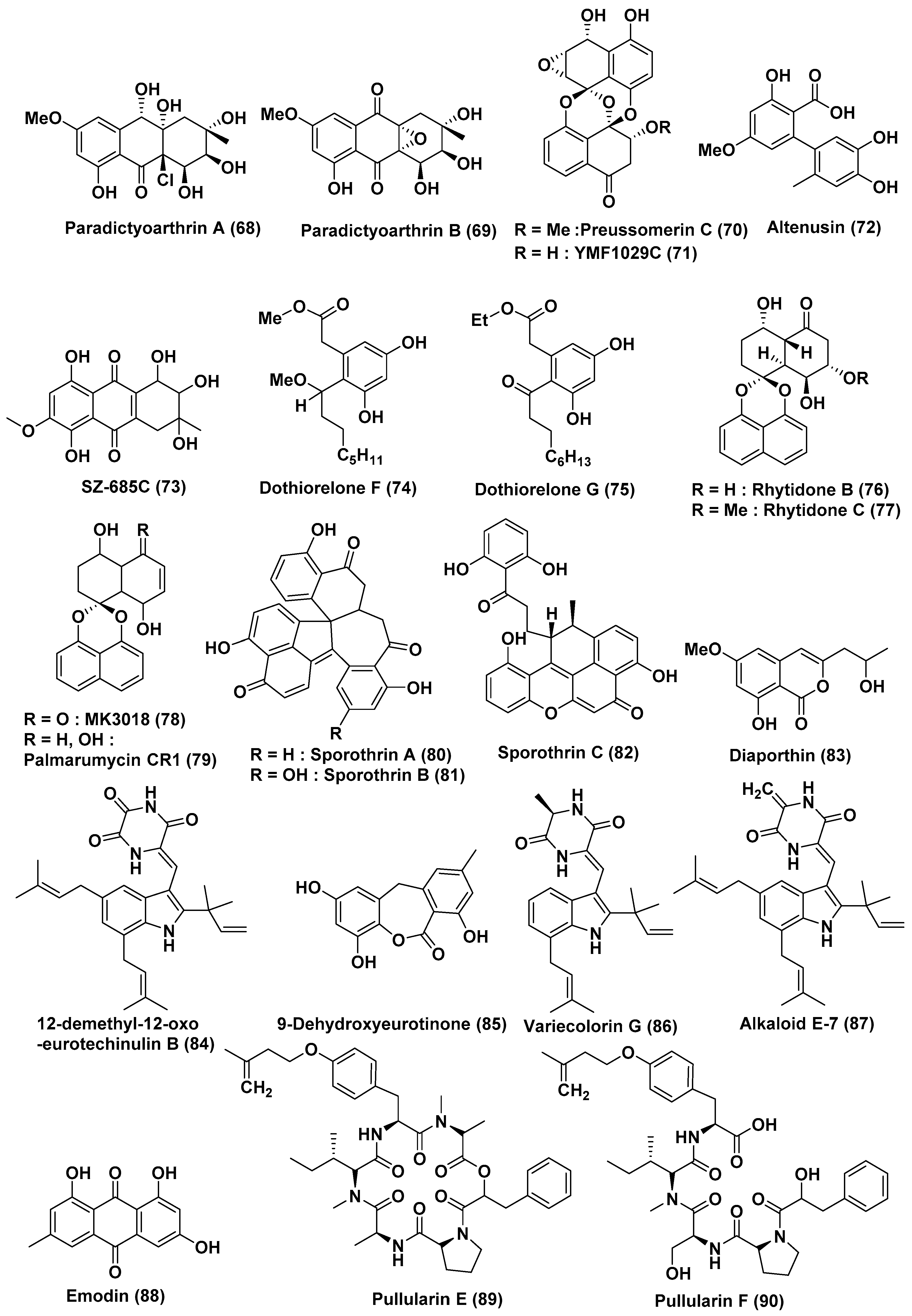
| 26 | Paradictyoarthrinium diffractum BCC 8704 | Associated with mangrove wood | Laem SonNational Park, Ranong Province, Thailand | Paradictyoarthrins A (68) | KB, MCF-7, NCI-H187, Vero Cells, KB, MCF-7, NCI-H187, Vero Cells | In the range of 23–31 µg/mL | [46] |
| Paradictyoarthrin B (69) | KB, MCF-7, NCI-H187, Vero Cells | 3.1, 3.8, 9.5, and 5.6 µg/mL | |||||
| Preussomerin C (70), ymf 1029C (71) and altenusin (72) | KB, MCF-7, NCI-H187, Vero Cells | Moderate to poor activity | |||||
| ymf 1029C (71) | NCI-H187 cells | 5.0 µg/mL | |||||
| 27 | Halorosellinia sp. (No. 1403) | -- | South China Sea | SZ-685C (73) | NFPA, MMQ and RPC cells | 18.76, 14.51, and 56.09 µM | [47] |
| 28 | Dothiorella sp. | Aegiceras corniculatum | Fujian Province, China | Dothiorelone F (74), Dothiorelone G (75) | Raji cancer | 2 µg/mL | [49] |
| 29 | Rhytidhysteron sp. | Leaves of Azima sarmentosa | Samutsakhon province, Thailand | Rhytidones B–C (76, 77), MK3018 (78), palmarumycin CR1 (79) | MCF-7 and CaSki Cells | In the range of 14.47 and 25.59 µM | [50] |
| Rhytidones B (76) | CaSki | 22.81 µM | |||||
| 30 | Sporothrix sp. | Bark, Kandelia candel | South China Sea | Sporothrin A (80) | Inhibition of AChE in vitro | 1.05 µM | [51] |
| sporothrin B (81), sporothrin C (82), diaporthin (83) | HepG2 | 20, 23, and 23 µg/mL | |||||
| 31 | Eurotium rubrum | Semi-mangrove plant Hibiscus tiliaceus | Hainan Island, China | 12-demethyl-12-oxo-eurotechinulin B (84), 9-dehydroxyeurotinone (85), variecolorin G (86), alkaloid E-7 (87), and emodin (88) | HepG2, MCF-7, SW1990, HepG2, NCI-H460, SMMC7721, HeLa, and Du145 | In the range of 15–30 µg/mL | [52] |
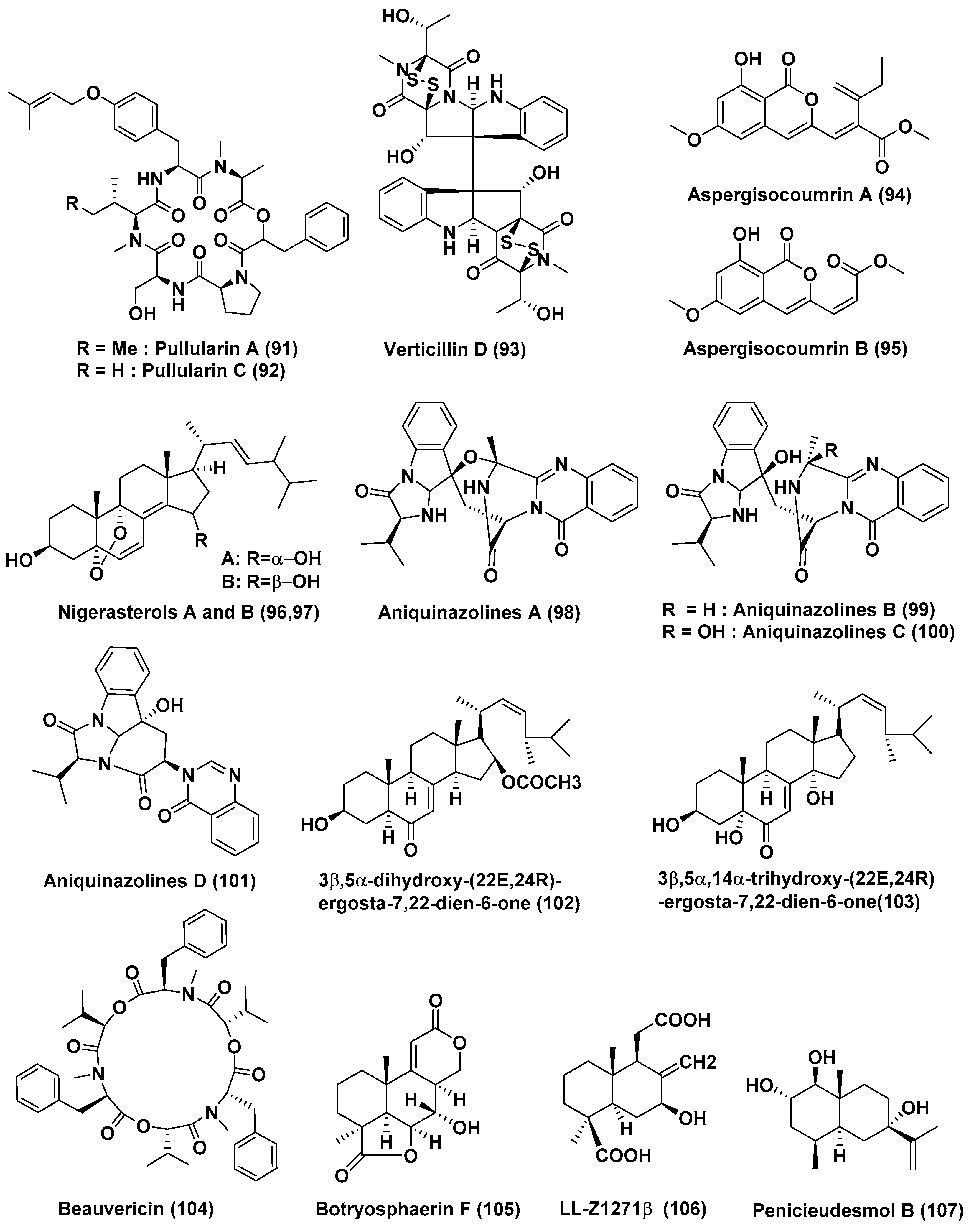
| 32 | Bionectria ochroleuca | Inner leaf tissues of the plant Sonneratia caseolaris | Hainan island, China | Pullularins E (89), F (90), pullularins A (91), and C (92) | L5178Y | EC50 values In the range of 0.1 and 6.7 µg/mL | [53] |
| verticillin D (93) | L5178Y | <0.1 µg/mL | |||||
| 33 | Aspergillus sp. HN15-5D | Leaves, Acanthus ilicifolius | Hainan Island, China | Aspergisocoumrins A–B (94–95) | MDA-MB-435 | 5.08 and 4.98 µM | [54] |
| 34 | Aspergillus niger MA-132 | Avicennia marina | Hainan, China | Nigerasterol A (96) Nigerasterol B (97) | HL60 | 0.30 µM, 1.50 µM | [55] |
| Nigerasterol A (96) Nigerasterol B (97) | A549 | 1.82 and 5.41 µM | |||||
| 35 | Aspergillus nidulans MA-143 | Leaves, Rhizophora stylosa | Aniquinazolines A–D (98–101) | Brine shrimp | LD50 1.27, 2.11, 4.95 and 3.42 µM, (Colchicine LD50 88.4 µM | [56] | |
| 36 | Aspergillus terreus (No. GX7-3B) | Branch of Bruguiera gymnoihiza (Linn.) | South China Sea | 3β,5α-dihydroxy-(22E,24R)-ergosta-7,22-dien-6-one (102), Beauvericin (104) | MCF-7, A549, HeLa and KB | 4.98 and 2.02, 1.95 and 0.82, 0.68 and 1.14, 1.50 and 1.10 µM | [57] |
| 3β,5α,14α-trihydroxy-(22E,24R)-ergosta-7, 22-dien-6-one (103) | MCF-7, A549, HeLa and KB | 25.4, 27.1, 24.4, 19.4 µM | |||||
| 37 | Aspergillus terreus (No. GX7-3B) | Branch of Bruguiera gymnoihiza (Linn.) | South China Sea | Botryosphaerin F (105) and LL-Z1271β (106) | MCF-7 and HL-60 | 4.49 and 3.43 µM | [58] |
| HL-60 | 0.6 µM | ||||||
| 38 | Penicillium sp. J-54 | Leaves, Ceriops tagal | Hainan province, China | Penicieudesmol B (107), | K-562 | 90.1 µM, (paclitaxel, 9.5 µM) | [59] |
| 39 | Penicillium citrinum HL-5126 | Bruguiera sexangula var. rhynchopetala | South China Sea | Penibenzophenone B (108) | A549 Cells | 15.7 µg/mL | [60] |
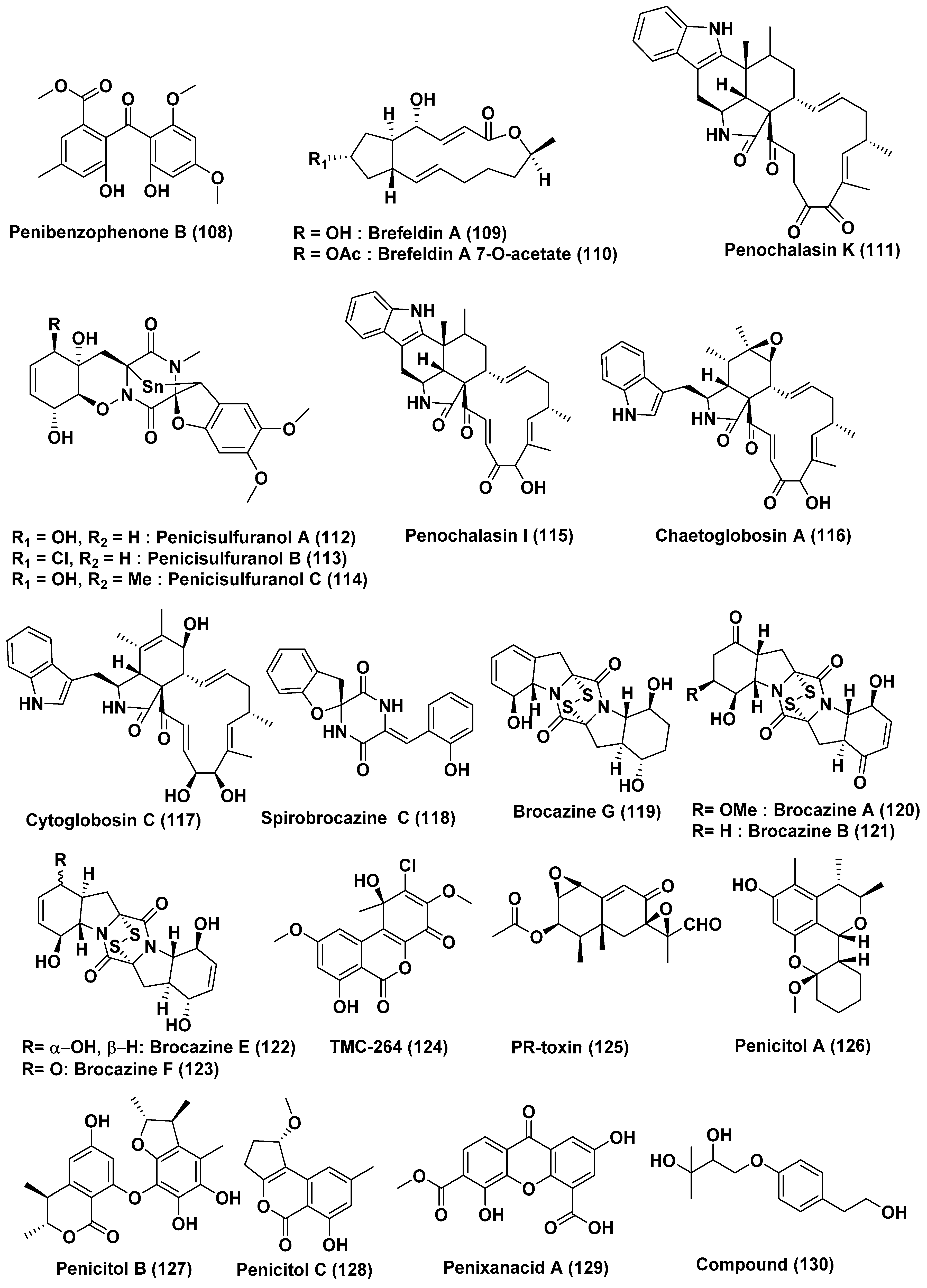
| 40 | Penicillium sp. | Panax notoginseng | Wenshan, Yunnan province, China | Brefeldin A (109), Brefeldin A 7-O-acetate (110) | 293, HepG2, Huh7 and KB cell line | LD50 value from 0.024 to 0.62 µM. Both the compounds arrested HepG2 cells at the S phase | [61] |
| 41 | Penicillium chrysogenum V11 | Vein of Myoporum bontioides | Leizhou Peninsula, China | Penochalasin K (111) | MDA-MB-435, SGC-7901 and A549 cells | <10 µM | [62] |
| 42 | Penicillium janthinellum HDN13-309 | Sonneratia caseolaris | Hainan Province, China | Penicisulfuranols A–C (112–114) | HeLa and HL-60 Cells | In the range of 0.1 to 3.9 µM | [63] |
| 43 | Penicillium chrysogenum V11 | Not reported | Not reported | Penochalasin I (115), chaetoglobosins A (116), and cytoglobosin C (117) | MDA-MB-435 and SGC-7901 cells | <10 µM | [64] |
| Compounds (116), and (117) | SGC-7901 and A549 cells | <10 μM | |||||
| 44 | Penicillium brocae MA-231 | Mangrove plant Avicennia marina | Hainan Island, China | Spirobrocazine C (118) | A2780 | 59 µM | [65] |
| Brocazine G (119) | A2780 and A2780 CisR | 664 nM, 661 nM (cisplatin 1.67 and 12.63 µM) | |||||
| 45 | Penicillium brocae MA-231 | Mangrove plant Avicennia marina | Hainan Island, China | Brocazines A (120), B (121), E (122), F (123) | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, SW480, and U251 | from 0.89 to 9.0 µM | [66] |
| Compounds (120) and (121) | SW480 tumor cell line | 2.0 and 1.2 µM | |||||
| Compound (123) | DU145 and NCI-H460 Cells, | 1.7 and 0.89 µM | |||||
| 46 | Penicillium chermesinum strain HLit-ROR2 | Heritiera littoralis, | Samut Sakhon province, Thailand | TMC-264 (124) | T47D and MDA-MB231 | 1.08 and 2.81 µM (doxorubicin 1.55 and 2.24 µM) | [67] |
| HepG2 | 3.27 µM (Etoposide, 35.66 µM) | ||||||
| MOLT-3 | 1.36 µM | ||||||
| T47D | 1.08 µM | ||||||
| PR-toxin (125) | HuCCA-1, HeLa, T47D, and MDA-MB231 | 0.81–2.19 µM (doxorubicin, 0.26–2.24 µM) | |||||
| HL-60 cell line | 0.06 µM (doxorubicin, 1.21 µM) | ||||||
| MOLT-3 and HL-60 | 0.09 µM, 0.06 µM | ||||||
| 47 | Penicillium chrysogenum HND11-24 | The rhizosphere soil of the mangrove plant Acanthus ilicifolius | China | Penicitols A (126) | HeLa, BEL-7402, HEK-293, HCT-116, and A549 Cells | 4.6−10.5 µM | [68] |
| Penicitols B (127) | 3.4−9.6 µM | ||||||
| Penicitols C (128) and Penixanacid A (129) | In the range of 10–40.5 µM | ||||||
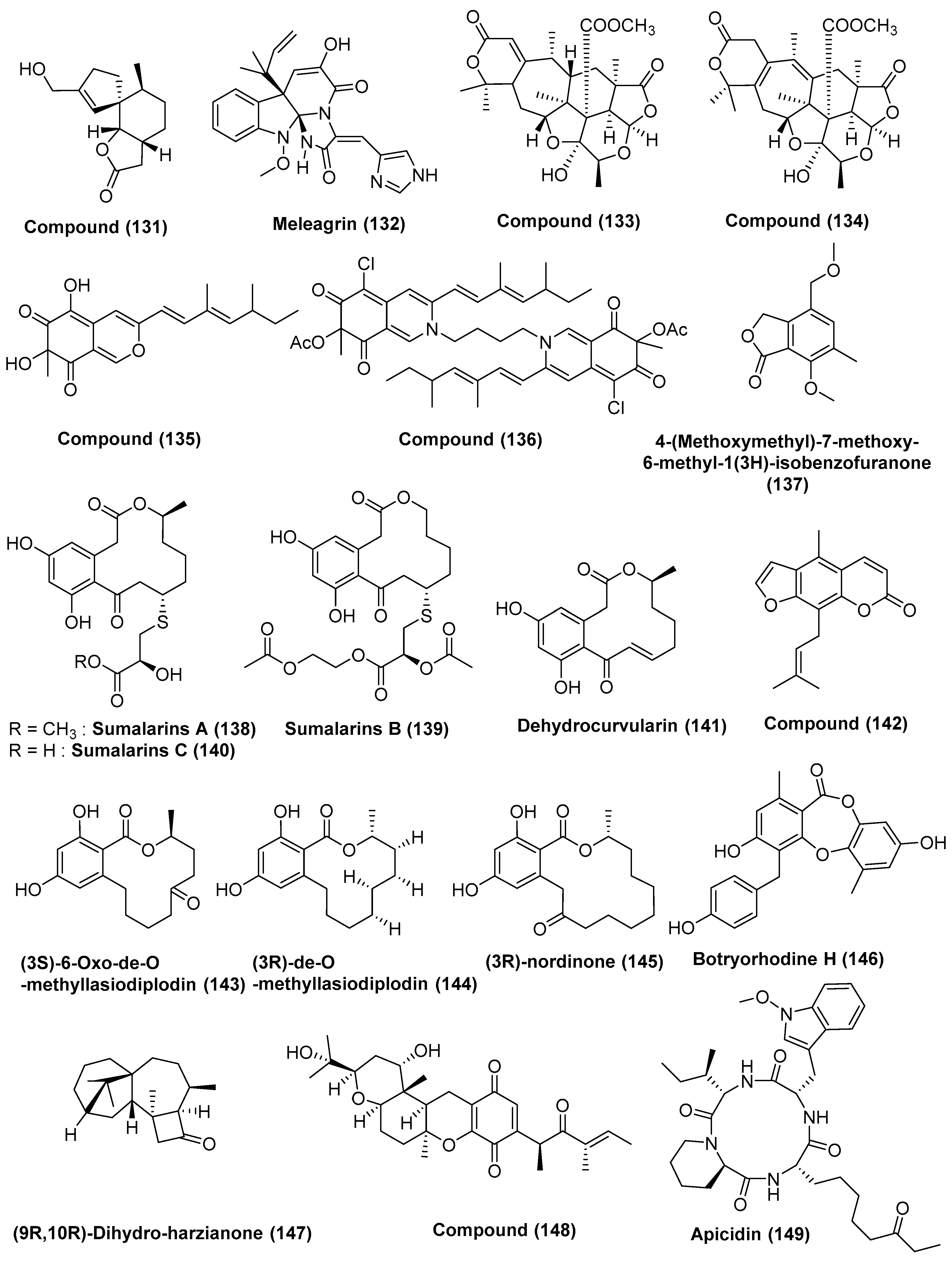
| 48 | Penicillium sp. FJ-1 | Avicennia marina | Fujian, China | Compound (130) | Tca8113 and MG-63 cells | 26 and 35 µM (Taxol, 46 and 10 nM) | [69] |
| Compounds (131) | Tca8113 and WRL-68 | 10 and 58 µM | |||||
| Compounds (131) | MG-63 cells | 55 nM | |||||
| 49 | Penicillium sp. GD6, | Bruguiera gymnorrhiza | Zhanjiang, China | Meleagrin (132) | HL60 and A549 | 9.7 and 8.3 µM | [70] |
| 50 | Penicillium 303# | Sea water | Guangdong Province, China | 5S, 7R, 9S, 10S, 11R, 12S, 13R, 22R, and 23R. (133), 7R, 9S, 10S, 11R, 12S, 13R, 22R, and 23R (134) | MDA-MB-435, HepG2, HCT-116, and A549 | In the range of 11.9–37.82 µg/mL | [71] |
| Compounds (135) | MDA-MB-435 | 7.13 | |||||
| Compound (136) | HepG2 and HCT-116 | 39.64 and 27.80 µM | |||||
| 51 | Penicillium sp. ZH58 | Leaves, Avicennia sp. | Dong Sai, Hainan of the South China Sea coast | 4-(methoxymethyl)-7-methoxy-6-methyl-1(3H)-isobenzofuranone (137) | KB and KBV200 cells | 6 and 10 µg/mL | [72] |
| 52 | Penicillium sumatrense MA-92 | Rhizosphere, Lumnitzera racemose | WenChang in Hainan Island, China | Sumalarins A–C (138, 139, 140), and dehydrocurvularin (141) | Du145, HeLa, Huh 7, MCF-7, NCI-H460, SGC-7901, and SW1990 Cells | In the range of 3.8 to 10 µM | [73] |
| 53 | Penicillium sp. ZH16 | Avicennia sp. | South China Sea | 5-methyl-8-(3-methylbut-2-enyl) furanocoumarin (142) | KB and KBV200 | 5 and 10 µg/mL | [74] |
| 54 | Trichoderma sp. 307 | Stem bark, Clerodendrum inerme | Guangdong Province, China | (3S)-6-oxo-de-O-methyllasiodiplodin (143) | GH3 and MMQ Cells RPC | 21.42 and 13.59 µM, 142.8 µM | [75] |
| Co cultured with Acinetobacter johnsonii B2 | (3R)-de-O-methyllasiodiplodin (144) | 6.44 and 6.58 µM, 6.94 µM | |||||
| (3R)-nordinone (145) | 12.33 and 10.13 µM, 100.03 µM. | ||||||
| 55 | Trichoderma sp. 307 co-culturing with Acinetobacter johnsonii B2 | Stem bark of Clerodendrum inerme | Guangdong Province, China | Botryorhodine H (146) | MMQ GH3 Cells | 3.09 and 3.64 µM | [76] |
| 56 | Trichoderma sp. Xy24 | Leaves, stems and peels of Xylocarpus granatum | Hainan province, China | (9R,10R)-dihydro-harzianone (147) | HeLa and MCF-7 Cells | 30.1 µM and 30.7 µM | [77] |
| 57 | Nigrospora sp. MA75 | Pongamia pinnata | Guangxi Zhuang Autonomous Region of China | 2,3-didehydro-19α-hydroxy-14-epicochlioquinone B (148) | MCF-7, SW1990, and SMMC7721 | 4, 5, and 7 µg/mL | [78] |
| 58 | Fusarium sp. (No. DZ27) | Bark of Kandelia candel | Dongzhai mangrove, Hainan, China | Beauvericin (104) | KB and KBv200 cells | 5.76 and 5.34 µM | [79] |
| 59 | Fusarium sp. | Leaf of mangrove Kandelia candel | Dongzhai Harbor of Hainan Island, China | Apicidin (149) | GLC-82 cells | 6.94 µM | [80] |
| 60 | Unidentified fungus ZZF42 | South China Sea | Not reported | Apicidin (149) | KB and KBv200 | 0.78 µg/mL | [81] |
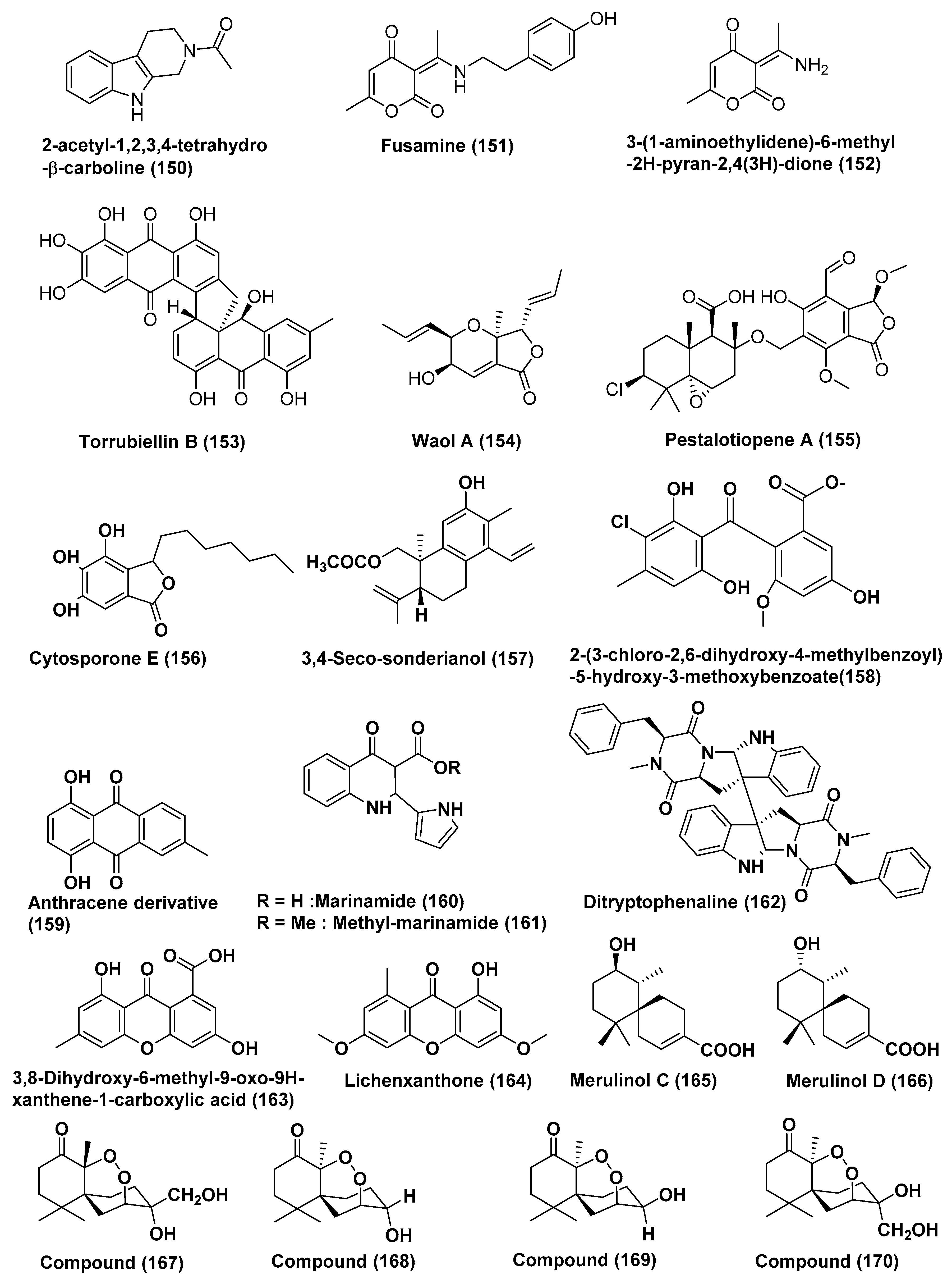
| 61 | Fusarium incarnatum (HKI0504) | Aegiceras corniculatum | Not reported | 2-acetyl-1,2,3,4-tetrahydro-β-carboline (150) | HUVEC and K-562 | GI50 41.1 and 33.3 | [82] |
| HeLa cell | CC50 23.8 µM | ||||||
| Fusarium incarnatum (HKI0504) | Aegiceras corniculatum | Not reported | Fusamine (151) | HUVEC and K-562 | GI50 37.3 and 37.6 | [82] | |
| HeLa cell | CC50 23.3 µM | ||||||
| Fusarium incarnatum (HKI0504) | Aegiceras corniculatum | Not reported | 3-(1-aminoethylidene)-6-methyl-2H-pyran-2,4(3H)-dione (152) | HUVEC and K-562 | GI50 41.1 and 33.3 | [82] | |
| HeLa cell | CC50 23.8 µM | ||||||
| 62 | Acremonium sp. | leaves of Sonneratia caseolaris c | Dong Zhai Gang Mangrove Garden, Hainan, China | Torrubiellin B (153) | Cisplatin sensitive Cal27, Kyse510, HCC38, A2780, MDA-MB-231 | In the range of 0.3 to 1.5 µM | [83] |
| Cisplatin resistant, Cal27, Kyse510, HCC38, A2780, MDA-MB-231 | In the range of 0.2 to 2.6 µM | ||||||
| 63 | Acremonium strictum | Rhizophora apiculata | Island of Cat Ba, Vietnam | Waol A (154), Pestalotiopene A (155) Cytosporone E (156) | Cisplatin-sensitive, A2780 | 27.1, 76.2, and 8.3 µM | [84] |
| Cisplatin-Resistant A2780 | 12.6, 30.1, and 19.0 µM | ||||||
| 64 | Endophytic fungus J3 | Ceriops tagal | Hainan province, China | 3,4-seco-sonderianol (157) | K562, SGC-7901, and BEL-7402 Cells | 9.2, 15.7, and 25.4 µg/mL | [85] |
| 65 | Endophytic fungus No. ZH-3 | Not reported | South China Sea | 2-(3-chloro-2, 6-dihydroxy-4-methylbenzoyl)-5-hydroxy-3-methoxybenzoate (158) | HepG2 cell line | 25 µg/mL | [86] |
| 66 | Endophytic fungus No. 5094 | Not reported | South China Sea | Anthracene derivative (159) | KB and KBv200 | LD50 values of 5.5 and 10.2 µM | [87] |
| 67 | Co-cultures of two mangrove endophytic fungi (strains Nos. 1924 and 3893) | Not reported | Marinamide (160) | HepG2, 95-D, MGC832 and HeLa Cells | 7.0, 0.4, 91 nM and 0.529 µM | [88] | |
| Methyl marinamide (161) | HepG2, 95-D, MGC832 and HeLa Cells | 2.52, 1.54 13, 0.110 µM | |||||
| 68 | Endophytic fungus No·Gx-3a | Not reported | South China sea | Ditryptophenaline (162) | KB, KBv200 | 8.0 and 12.0 µM | [89] |
| 69 | Mangrove endophytic fungus No·SK7RN3G1 | Not reported | South China Sea | 3,8-dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (163), Lichenxanthone (164), | HepG2 cell line | 20 and 25 µg/mL | [90] |
| Compounds Produced by Basidiomycetes | |||||||
| 70 | Basidiomycetous fungus XG8D | leaves of Xylocarpus granatum | Samutsakorn province, Thailand | Merulinols C and D (165, 166) | KATO-3 cells | 35.0 and 25.3 µM | [91] |
| 71 | Pseudolagarobasidium acaciicola, | Bruguiera gymnorrhiza | Samut Sakhon province, Thailand | Compound (167) | HuCCA-1, A549, MOLT-3, HepG2, MDA-MB231, T47D | 0.28–37.46 µM | [92] |
| MRC-5 | IC50 17.92 µM | ||||||
| HL-60 cell line | IC50 0.28 µM | ||||||
| Compound (168) | A549, MOLT-3, HepG2, HL-60, MDA-MB231, T47D, HeLa cancer cell, MRC-5 | 12.09–170.08 µM | |||||
| Compound (169) | HuCCA-1, A549, MOLT-3, HepG2, HL-60, MDA-MB231, T47D, HeLa cancer cell | 15.20–76.97 µM | |||||
| Compound (170) | HuCCA-1, A549, MOLT-3, HepG2, MDA-MB231, T47D, HeLa cancer cell | 18.31–154.51 µM | |||||
| HL-60 | 18.31 µM | ||||||
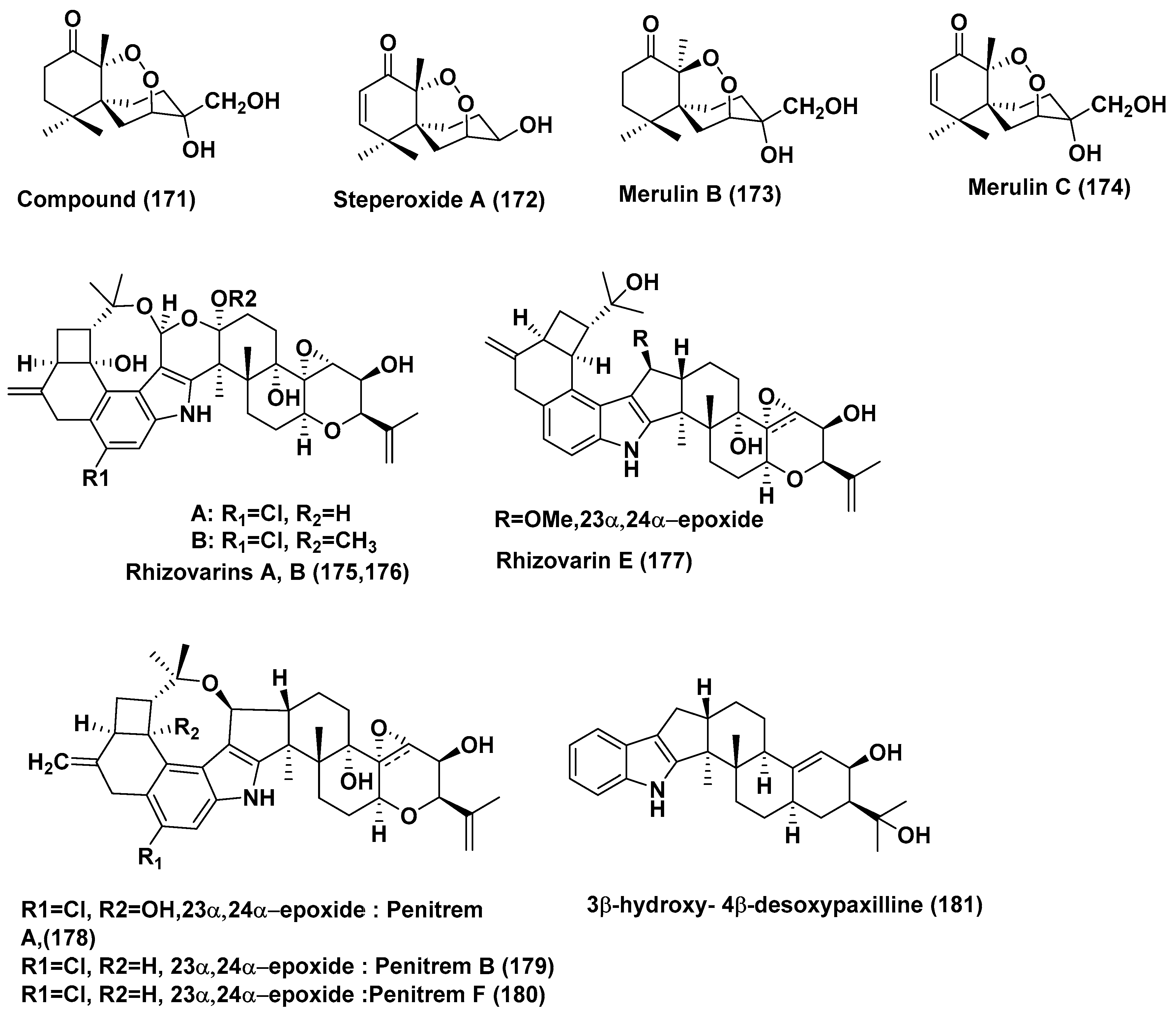
| 72 | Pseudolagarobasidium acaciicola | Bruguiera gymnorrhiza | Not reported | Endoperoxide (171), Steperoxide A (172) | MOLT-3, HuCCA-1, A549, HepG2, HL-60, MDA-MB-231, T47D, and HeLa cancer Cells | In the range of 0.68–3.71 and 0.67–5.25 µg/mL | [93] |
| Merulin B (173) | MOLT-3, A549, HepG2, HL-60, MDA-MB-231 and T47D Cells | In the range of 11.94–49.08 µg/mL | |||||
| Merulin C (174) | HL60 cancer cells | 0.08 µg/mL | |||||
| MOLT-3, HuCCA-1, A549, HepG2, MDA-MB-231, T47D, and HeLa Cells | In the range of 0.19–3.75 µg/mL | ||||||
| 73 | Mucor irregularis QEN-189 | Rhizophora stylosa | Hainan Island, China | Rhizovarins A, B, E (175, 176, 177) Penitrems A, C, F (178, 179, 180) and 3β-hydroxy-4β-desoxypaxilline (181) | A-549 | 11.5, 6.3, 9.2, 8.4, 8.0, 8.2 and 4.6 µM | [94] |
| Rhizovarins A, B, (175, 176), Penitrems A, C, F (178, 179, 180) and 3β-hydroxy-4β-desoxypaxilline (181) | HL-60 | 9.6, 5.0, 7.0, 4.7, 3.3 and 2.6 µM | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, S.K.; Gupta, M.K.; Prakash, V.; Reddy, M.S. Mangrove-Associated Fungi: A Novel Source of Potential Anticancer Compounds. J. Fungi 2018, 4, 101. https://doi.org/10.3390/jof4030101
Deshmukh SK, Gupta MK, Prakash V, Reddy MS. Mangrove-Associated Fungi: A Novel Source of Potential Anticancer Compounds. Journal of Fungi. 2018; 4(3):101. https://doi.org/10.3390/jof4030101
Chicago/Turabian StyleDeshmukh, Sunil K., Manish K. Gupta, Ved Prakash, and M. Sudhakara Reddy. 2018. "Mangrove-Associated Fungi: A Novel Source of Potential Anticancer Compounds" Journal of Fungi 4, no. 3: 101. https://doi.org/10.3390/jof4030101
APA StyleDeshmukh, S. K., Gupta, M. K., Prakash, V., & Reddy, M. S. (2018). Mangrove-Associated Fungi: A Novel Source of Potential Anticancer Compounds. Journal of Fungi, 4(3), 101. https://doi.org/10.3390/jof4030101






