Impact of Biofilm Formation by Vaginal Candida albicans and Candida glabrata Isolates and Their Antifungal Resistance: A Comprehensive Study in Ecuadorian Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Candida Isolates and Growth Conditions
2.2. Evaluation of the Antifungal Resistance of Candida Planktonic Cells
2.2.1. CANDIDA EU Commercial Kit
2.2.2. Minimum Inhibitory Concentration (MIC)
2.3. Biofilm Formation
2.4. Quantification of Biofilm Formation
2.4.1. Phosphate-Buffered Saline Suspension
2.4.2. Crystal Violet Staining
2.5. Biofilm Classification
2.6. Antibiofilm Activity
2.6.1. Biofilm Inhibition Assays
2.6.2. Biofilm Eradication Assays
2.7. Biomass Evaluation
2.8. Colony-Forming Unit Counting Assays
2.9. Statistical Analysis
3. Results
3.1. Diagnosis of Samples in the Study
3.2. Evaluation of the Antifungal Resistance of Candida Planktonic Cells
3.3. Biofilm Formation
3.4. Biofilm Inhibition and Eradication Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| MIC | Minimum inhibitory concentration |
| MBIC | Minimum biofilm inhibitory concentration |
| MBEC | Minimum biofilm eradication concentration |
| VVC | Vulvovaginal candidiasis |
| AV | Aerobic vaginitis |
| BV | Bacterial vaginosis |
| CV | Crystal violet |
| PBS | Phosphate-buffered saline |
References
- Kıvanç, M.; Er, S. Biofilm Formation of Candida spp. Isolated from the Vagina and Antibiofilm Activities of Lactic Acid Bacteria on the These Candida Isolates. Afr. Health Sci. 2020, 20, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of Non-Candida albicans Candida Species: Quantification, Structure and Matrix Composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef]
- Lopes, J.P.; Stylianou, M.; Nilsson, G.; Urban, C.F. Opportunistic Pathogen Candida albicans Elicits a Temporal Response in Primary Human Mast Cells. Sci. Rep. 2015, 5, 12287. [Google Scholar] [CrossRef]
- Jacobsen, I.D. The Role of Host and Fungal Factors in the Commensal-to-Pathogen Transition of Candida albicans. Curr. Clin. Microbiol. Rep. 2023, 10, 55–65. [Google Scholar] [CrossRef]
- Muderris, T.; Kaya, S.; Ormen, B.; Aksoy Gokmen, A.; Varer Akpinar, C.; Yurtsever Gul, S. Mortality and Risk Factor Analysis for Candida Blood Stream Infection: A Three-Year Retrospective Study. J. Mycol. Med. 2020, 30, 101008. [Google Scholar] [CrossRef]
- Alkharashi, N.; Aljohani, S.; Layqah, L.; Masuadi, E.; Baharoon, W.; Al-Jahdali, H.; Baharoon, S. Candida Bloodstream Infection: Changing Pattern of Occurrence and Antifungal Susceptibility over 10 Years in a Tertiary Care Saudi Hospital. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 2015692. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.C.d.O.; Vasconcelos, C.C.; Lopes, A.J.O.; Cartágenes, M.d.S.d.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, Y.; Chen, L.; Cheng, Y.; Jin, P.; Zhang, W.; Zheng, L.; Liu, J.; Zhou, T.; Xu, Z.; et al. Candida Causes Recurrent Vulvovaginal Candidiasis by Forming Morphologically Disparate Biofilms on the Human Vaginal Epithelium. Biofilm 2023, 6, 100162. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging Opportunistic Yeast Infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Brandt, M.E.; Lockhart, S.R. Recent Taxonomic Developments with Candida and Other Opportunistic Yeasts. Curr. Fungal Infect. Rep. 2012, 6, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Amann, V.; Kissmann, A.-K.; Firacative, C.; Rosenau, F. Biofilm-Associated Candidiasis: Pathogenesis, Prevalence, Challenges and Therapeutic Options. Pharmaceuticals 2025, 18, 460. [Google Scholar] [CrossRef]
- Munusamy, K.; Vadivelu, J.; Tay, S.T. A Study on Candida Biofilm Growth Characteristics and Its Susceptibility to Aureobasidin A. Rev. Iberoam. Micol. 2018, 35, 68–72. [Google Scholar] [CrossRef]
- Rybak, J.M.; Barker, K.S.; Muñoz, J.F.; Parker, J.E.; Ahmad, S.; Mokaddas, E.; Abdullah, A.; Elhagracy, R.S.; Kelly, S.L.; Cuomo, C.A.; et al. In vivo Emergence of High-Level Resistance during Treatment Reveals the First Identified Mechanism of Amphotericin B Resistance in Candida auris. Clin. Microbiol. Infect. 2022, 28, 838–843. [Google Scholar] [CrossRef]
- Denning, D.W. Renaming Candida glabrata—A Case of Taxonomic Purity over Clinical and Public Health Pragmatism. PLoS Pathog. 2024, 20, e1012055. [Google Scholar] [CrossRef]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal Susceptibility of Candida albicans in Biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Vizuete, K.; Debut, A.; Tejera, E.; Machado, A. Evaluation of the Biofilm Life Cycle between Candida albicans and Candida tropicalis. Front. Cell. Infect. Microbiol. 2022, 12, 953168. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- de Barros, P.P.; Rossoni, R.D.; de Souza, C.M.; Scorzoni, L.; Fenley, J.D.C.; Junqueira, J.C. Candida Biofilms: An Update on Developmental Mechanisms and Therapeutic Challenges. Mycopathologia 2020, 185, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Rodrigues, C.F.; Araújo, D.; Rodrigues, M.E.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Soll, D.R.; Daniels, K.J. Plasticity of Candida albicans Biofilms. Microbiol. Mol. Biol. Rev. 2016, 80, 565–595. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- Hasan, F.; Xess, I.; Wang, X.; Jain, N.; Fries, B.C. Biofilm Formation in Clinical Candida Isolates and Its Association with Virulence. Microbes. Infect. 2009, 11, 753–761. [Google Scholar] [CrossRef]
- Salinas, A.M.; Osorio, V.G.; Endara, P.F.; Salazar, E.R.; Vasco, G.P.; Vivero, S.G.; Machado, A. Bacterial Identification of the Vaginal Microbiota in Ecuadorian Pregnant Teenagers: An Exploratory Analysis. PeerJ 2018, 6, e4317. [Google Scholar] [CrossRef]
- Pacha-Herrera, D.; Vasco, G.; Cruz-betancourt, C.; Galarza, J.M.; Barragán, V.; Machado, A. Vaginal Microbiota Evaluation and Lactobacilli Quantification by QPCR in Pregnant and Non-Pregnant Women: A Pilot Study. Front. Cell. Infect. Microbiol. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Pacha-Herrera, D.; Erazo-Garcia, M.P.; Cueva, D.F.; Orellana, M.; Borja-Serrano, P.; Arboleda, C.; Tejera, E.; Machado, A. Clustering Analysis of the Multi-Microbial Consortium by Lactobacillus Species Against Vaginal Dysbiosis Among Ecuadorian Women. Front. Cell. Infect. Microbiol. 2022, 12, 863208. [Google Scholar] [CrossRef]
- Salinas, A.M.; Osorio, V.G.; Herrera, D.P.; Vivanco, J.S.; Trueba, A.F.; Machado, A. Vaginal Microbiota Evaluation and Prevalence of Key Pathogens in Ecuadorian Women: An Epidemiologic Analysis. Sci. Rep. 2020, 10, 18358. [Google Scholar] [CrossRef] [PubMed]
- EUCAST European Committee on Antimicrobial Susceptibility Testing—Clinical Breakpoints for Fungi (Candida and Aspergillus Species). Available online: https://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals (accessed on 2 February 2024).
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef]
- Fernandez-Soto, P.; Celi, D.; Tejera, E.; Alvarez-Suarez, J.M.; Machado, A. Cinnamomum sp. and Pelargonium odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants. Molecules 2023, 28, 693. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.M.; Karatela, S.; Sunay, S.; Adiri, Y. Candida Surveillance Study Investigators Antifungal Susceptibility Testing of Candida Isolates from the Candida Surveillance Study. J. Clin. Microbiol. 2010, 48, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Turan, H.; Demirbilek, M. Biofilm-Forming Capacity of Blood–Borne Candida albicans Strains and Effects of Antifungal Agents. Rev. Argent Microbiol. 2018, 50, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Mera, F.S.; Atiencia-Carrera, M.B.; Villacrés-Granda, I.; Proaño, A.A.; Debut, A.; Vizuete, K.; Herrero-Bayo, L.; Gonzalez-Paramás, A.M.; Giampieri, F.; Abreu-Naranjo, R.; et al. Evaluation of the Polyphenolic Profile of Native Ecuadorian Stingless Bee Honeys (Tribe: Meliponini) and Their Antibiofilm Activity on Susceptible and Multidrug-Resistant Pathogens: An Exploratory Analysis. Curr. Res. Food Sci. 2023, 7, 100543. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Arevalo, A.V.; Rodriguez, D.L.; Nobile, C.J. In vitro Culturing and Screening of Candida albicans Biofilms. Curr. Protoc. Microbiol. 2018, 50, e60. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. In vitro Growth and Analysis of Candida Biofilms. Nat. Protoc. 2008, 3, 1909–1924. [Google Scholar] [CrossRef]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining Conditions for Biofilm Inhibition and Eradication Assays for Gram-Positive Clinical Reference Strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC Versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Liu, J.; Yang, C.; Wang, Q.; Su, M.; Wei, M.; Gu, L. Antibiofilm Activity of Essential Fatty Acids Against Candida albicans from Vulvovaginal Candidiasis and Bloodstream Infections. Infect. Drug Resist. 2022, 15, 4181–4193. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, S.S. Optimization of Single Plate-Serial Dilution Spotting (SP-SDS) with Sample Anchoring as an Assured Method for Bacterial and Yeast CFU Enumeration and Single Colony Isolation from Diverse Samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; IBM: New York, NY, USA, 2021. [Google Scholar]
- Tejera, E.; Machado, A.; Rebelo, I.; Nieto-Villar, J. Fractal Protein Structure Revisited: Topological, Kinetic and Thermodynamic Relationships. Phys. A Stat. Mech. Its Appl. 2009, 388, 4600–4608. [Google Scholar] [CrossRef]
- Machado, A.; Tejera, E.; Cruz-Monteagudo, M.; Rebelo, I. Application of Desirability-Based Multi(Bi)-Objective Optimization in the Design of Selective Arylpiperazine Derivates for the 5-HT1A Serotonin Receptor. Eur. J. Med. Chem. 2009, 44, 5045–5054. [Google Scholar] [CrossRef]
- Alomeir, N.; Zeng, Y.; Fadaak, A.; Wu, T.T.; Malmstrom, H.; Xiao, J. Effect of Nystatin on Candida albicans—Streptococcus mutans Duo-Species Biofilms. Arch. Oral Biol. 2023, 145, 105582. [Google Scholar] [CrossRef]
- Firacative, C.; Escandón, P. Antifungal Susceptibility of Clinical Cryptococcus gattii Isolates from Colombia Varies among Molecular Types. Med. Mycol. 2021, 59, 1122–1125. [Google Scholar] [CrossRef]
- Feldman, M.; Sionov, R.V.; Mechoulam, R.; Steinberg, D. Anti-Biofilm Activity of Cannabidiol against Candida albicans. Microorganisms 2021, 9, 441. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, V.; Ballal, M. Distribution of Candida Species in Different Clinical Samples and Their Virulence: Biofilm Formation, Proteinase and Phospholipase Production: A Study on Hospitalized Patients in Southern India. J. Glob. Infect. Dis. 2011, 3, 4–8. [Google Scholar] [CrossRef]
- Hetta, H.F.; Melhem, T.; Aljohani, H.M.; Salama, A.; Ahmed, R.; Elfadil, H.; Alanazi, F.E.; Ramadan, Y.N.; Battah, B.; Rottura, M.; et al. Beyond Conventional Antifungals: Combating Resistance Through Novel Therapeutic Pathways. Pharmaceuticals 2025, 18, 364. [Google Scholar] [CrossRef] [PubMed]
- Marak, M.B.; Dhanashree, B. Antifungal Susceptibility and Biofilm Production of Candida spp. Isolated from Clinical Samples. Int. J. Microbiol. 2018, 10, 7495218. [Google Scholar] [CrossRef]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the Vaginal Microbiome and Candida Colonization in Women of Reproductive Age. Am. J. Obstet. Gynecol. 2020, 222, e1–e471. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arias, R.J.; Guachi-Álvarez, B.O.; Montalvo-Vivero, D.E.; Machado, A. Lactobacilli Displacement and Candida albicans Inhibition on Initial Adhesion Assays: A Probiotic Analysis. BMC Res. Notes 2022, 15, 239. [Google Scholar] [CrossRef]
- McKloud, E.; Delaney, C.; Sherry, L.; Kean, R.; Williams, S.; Metcalfe, R.; Thomas, R.; Richardson, R.; Gerasimidis, K.; Nile, C.J.; et al. Recurrent Vulvovaginal Candidiasis: A Dynamic Interkingdom Biofilm Disease of Candida and Lactobacillus. mSystems 2021, 6, 622–643. [Google Scholar] [CrossRef]
- Abdullahi Nasir, I.; Uchenna, E.; Onyia, J.; Ifunanya, A.L. Prevalence of Vulvovaginal Candidiasis among Nonpregnant Women Attending a Tertiary Health Care Facility in Abuja, Nigeria. Res. Rep. Trop. Med. 2015, 6, 37. [Google Scholar] [CrossRef]
- Sajjan, A.C.; MahalakshmiMD, V.V.; Hajare, D. Prevalence and Antifungal Susceptibility of Candida Species Isolated From Patients Attending Tertiary Care Hospital. IOSR J. Dent. Med. Sci. (IOSR-JDMS) 2014, 13, 44–49. [Google Scholar] [CrossRef]
- Puri, K.; Madan, A.; Bajaj, K. Incidence of Various Causes of Vaginal Discharge among Sexually Active Females in Age Group 20–40 Years. Indian J. Dermatol. Venereol. Leprol. 2003, 69, 122–125. [Google Scholar]
- Hellberg, D.; Zdolsek, B.; Nilsson, S.; Mårdh, P.A. Sexual Behavior of Women with Repeated Episodes of Vulvovaginal Candidiasis. Eur. J. Epidemiol. 1995, 11, 575–579. [Google Scholar] [CrossRef]
- Quindós, G.; Marcos-Arias, C.; San-Millán, R.; Mateo, E.; Eraso, E. The Continuous Changes in the Aetiology and Epidemiology of Invasive Candidiasis: From Familiar Candida albicans to Multiresistant Candida auris. Int. Microbiol. 2018, 21, 107–119. [Google Scholar] [CrossRef]
- Kobayashi, T.; Marra, A.R.; Schweizer, M.L.; Eyck, P.T.; Wu, C.; Alzunitan, M.; Salinas, J.L.; Siegel, M.; Farmakiotis, D.; Auwaerter, P.G.; et al. Impact of Infectious Disease Consultation in Patients with Candidemia: A Retrospective Study, Systematic Literature Review, and Meta-Analysis. Open Forum. Infect. Dis. 2020, 7, ofaa270. [Google Scholar] [CrossRef] [PubMed]
- Sarpong, A.K.; Odoi, H.; Boakye, Y.D.; Boamah, V.E.; Agyare, C. Resistant, C. albicans Implicated in Recurrent Vulvovaginal Candidiasis (RVVC) among Women in a Tertiary Healthcare Facility in Kumasi, Ghana. BMC Womens Health 2024, 24, 412. [Google Scholar] [CrossRef] [PubMed]
- Danby, C.S.; Boikov, D.; Rautemaa-Richardson, R.; Sobel, J.D. Effect of PH on in vitro Susceptibility of Candida glabrata and Candida albicans to 11 Antifungal Agents and Implications for Clinical Use. Antimicrob. Agents Chemother. 2012, 56, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Ahmad, S.; Al-Obaid, I.; Al-Sweih, N.A.; Joseph, L.; Farhat, D. Emergence of Resistance to Amphotericin B and Triazoles in Candida glabrata Vaginal Isolates in a Case of Recurrent Vaginitis. J. Chemother. 2008, 20, 488–491. [Google Scholar] [CrossRef]
- Shu, Y.; Shi, Y.; Yang, Y.; Dong, Z.; Yi, Q.; Shi, H. Progress of Triazole Antifungal Agent Posaconazole in Individualized Therapy. J. Clin. Pharm. Ther. 2022, 47, 1966–1981. [Google Scholar] [CrossRef]
- Fisher, B.T.; Zaoutis, T.E.; Xiao, R.; Wattier, R.L.; Castagnola, E.; Pana, Z.D.; Fullenkamp, A.; Boge, C.L.K.; Ross, R.K.; Yildirim, I.; et al. Comparative Effectiveness of Echinocandins vs Triazoles or Amphotericin B Formulations as Initial Directed Therapy for Invasive Candidiasis in Children and Adolescents. J. Pediatric Infect. Dis. Soc. 2021, 10, 994–1003. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Cantón, E.; Pemán, J. Antifungal Resistance among Less Prevalent Candida Non-albicans and Other Yeasts versus Established and under Development Agents: A Literature Review. J. Fungi. 2021, 7, 24. [Google Scholar] [CrossRef]
- Fothergill, A.W.; Sutton, D.A.; McCarthy, D.I.; Wiederhold, N.P. Impact of New Antifungal Breakpoints on Antifungal Resistance in Candida Species. J. Clin. Microbiol. 2014, 52, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Mesquida, A.; Vicente, T.; Reigadas, E.; Palomo, M.; Sánchez-Carrillo, C.; Muñoz, P.; Guinea, J.; Escribano, P. In vitro Activity of Ibrexafungerp and Comparators against Candida albicans Genotypes from Vaginal Samples and Blood Cultures. Clin. Microbiol. Infect. 2021, 27, 915.e5–915.e8. [Google Scholar] [CrossRef]
- Martínez-Herrera, E.; Frías-De-León, M.G.; Hernández-Castro, R.; García-Salazar, E.; Arenas, R.; Ocharan-Hernández, E.; Rodríguez-Cerdeira, C. Antifungal Resistance in Clinical Isolates of Candida glabrata in Ibero-America. J. Fungi 2021, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Galia, L.; Pezzani, M.D.; Compri, M.; Callegari, A.; Rajendran, N.B.; Carrara, E.; Tacconelli, E. Surveillance of Antifungal Resistance in Candidemia Fails to Inform Antifungal Stewardship in European Countries. J. Fungi 2022, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; El Sissy, C.; Bachelier-Bassi, S.; Scemla, A.; Quesne, G.; Sitterlé, E.; Legendre, C.; Lortholary, O.; Bougnoux, M.E. Acquired Flucytosine Resistance during Combination Therapy with Caspofungin and Flucytosine for Candida glabrata Cystitis. Antimicrob. Agents Chemother. 2016, 60, 662–665. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Kim, G.-Y.; Kim, J.K. Trends in Candida albicans Bloodstream Infections and Antifungal Resistance in a Tertiary Care Hospital in South Korea (2013–2023). Mycobiology 2025, 53, 236–242. [Google Scholar] [CrossRef]
- Shafiei, M.; Peyton, L.; Hashemzadeh, M.; Foroumadi, A. History of the Development of Antifungal Azoles: A Review on Structures, SAR, and Mechanism of Action. Bioorg. Chem. 2020, 104, 104240. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Mallick, U.; Sahu, B.K.; Hegde, R.; Jena, P.; Turuk, J.; Sahu, M.C.; Panda, S.K. Antifungal Resistance in Vaginal Candidiasis Among Reproductive-Age Women: A Review. Curr. Pharm. Biotechnol. 2025, 26. Epub ahead of print. [Google Scholar] [CrossRef]
- Garcia-Effron, G. Rezafungin—Mechanisms of Action, Susceptibility and Resistance: Similarities and Differences with the Other Echinocandins. J. Fungi 2020, 6, 262. [Google Scholar] [CrossRef]
- Kritikos, A.; Neofytos, D.; Khanna, N.; Schreiber, P.W.; Boggian, K.; Bille, J.; Schrenzel, J.; Mühlethaler, K.; Zbinden, R.; Bruderer, T.; et al. Accuracy of Sensititre YeastOne Echinocandins Epidemiological Cut-off Values for Identification of FKS Mutant Candida albicans and Candida glabrata: A Ten Year National Survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin. Microbiol. Infect. 2018, 24, 1214.e1–1214.e4. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Fernández-Figueras, M.T. Tirbanibulina: Revisión de Su Mecanismo de Acción Novedoso y de Cómo Encaja En El Tratamiento de La Queratosis Actínica. Actas Dermosifiliogr. 2022, 113, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, L.; Gothwal, M.; Mukkoli, M.M.; Bari, V.K. Antifungal Drug Resistance in Candida: A Special Emphasis on Amphotericin B. APMIS 2024, 132, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes-Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Czajka, K.M.; Venkataraman, K.; Brabant-Kirwan, D.; Santi, S.A.; Verschoor, C.; Appanna, V.D.; Singh, R.; Saunders, D.P.; Tharmalingam, S. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells 2023, 12, 2655. [Google Scholar] [CrossRef]
- Zarei Mahmoudabadi, A.; Zarrin, M.; Kiasat, N. Biofilm Formation and Susceptibility to Amphotericin B and Fluconazole in Candida albicans. Jundishapur. J. Microbiol. 2014, 7, e17105. [Google Scholar] [CrossRef]
- Romera, D.; Aguilera-Correa, J.J.; Gadea, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. Candida auris: A Comparison between Planktonic and Biofilm Susceptibility to Antifungal Drugs. J. Med. Microbiol. 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Gómez-Perosanz, M.; Escribano, P.; Zaragoza, O.; Bouza, E.; Guinea, J. Biofilm Production and Antibiofilm Activity of Echinocandins and Liposomal Amphotericin B in Echinocandin-Resistant Yeast Species. Antimicrob. Agents Chemother. 2016, 60, 3579–3586. [Google Scholar] [CrossRef]
- Vila, T.; Ishida, K.; Seabra, S.H.; Rozental, S. Miltefosine Inhibits Candida albicans and Non-albicans Candida spp. Biofilms and Impairs the Dispersion of Infectious Cells. Int. J. Antimicrob. Agents 2016, 48, 512–520. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, Z.; Lou, Z.; Wang, H. Evaluation of Anti-Biofilm Capability of Cordycepin Against Candida albicans. Infect. Drug Resist. 2021, 14, 435–448. [Google Scholar] [CrossRef]
- Fernandes, L.; Fortes, B.N.; Lincopan, N.; Ishida, K. Caspofungin and Polymyxin B Reduce the Cell Viability and Total Biomass of Mixed Biofilms of Carbapenem-Resistant Pseudomonas aeruginosa and Candida spp. Front. Microbiol. 2020, 11, 573263. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Susceptibility of Candida glabrata Biofilms to Echinocandins: Alterations in the Matrix Composition. Biofouling 2018, 34, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, L.; Dittmer, S.; Weisner, A.-K.; Buer, J.; Rath, P.-M.; Steinmann, J. Antibiofilm Activity of Antifungal Drugs, Including the Novel Drug Olorofim, against Lomentospora prolificans. J. Antimicrob. Chemother. 2020, 75, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Król, J.; Nawrot, U.; Bartoszewicz, M. Activity of Base Analogues (5-Fluorouracil, 5-Flucytosine) against Planktonic Cells and Mature Biofilm of Candida Yeast. Effect of Combination with Folinic Acid. J. Mycol. Med. 2019, 29, 147–153. [Google Scholar] [CrossRef]
- Mathé, L.; Van Dijck, P. Recent Insights into Candida albicans Biofilm Resistance Mechanisms. Curr. Genet. 2013, 59, 251–264. [Google Scholar] [CrossRef]
- Pereira, R.; Dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, Regulation and Resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal Drug-Resistance Mechanisms in Candida Biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef]
- Fox, E.P.; Nobile, C.J. A Sticky Situation: Untangling the Transcriptional Network Controlling Biofilm Development in Candida albicans. Transcription 2012, 3, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and Regulation of Single-and Multi-Species Candida albicans Biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef]
- Kantroo, H.A.; Mubarak, M.M.; Ahmad, Z. Exploring Therapeutic Strategies for Candidiasis: From Current Treatments to Future Perspectives. Bioorg. Chem. 2025, 164, 108797. [Google Scholar] [CrossRef] [PubMed]
- Satora, M.; Grunwald, A.; Zaremba, B.; Frankowska, K.; Żak, K.; Tarkowski, R.; Kułak, K. Treatment of Vulvovaginal Candidiasis—An Overview of Guidelines and the Latest Treatment Methods. J. Clin. Med. 2023, 12, 5376. [Google Scholar] [CrossRef] [PubMed]
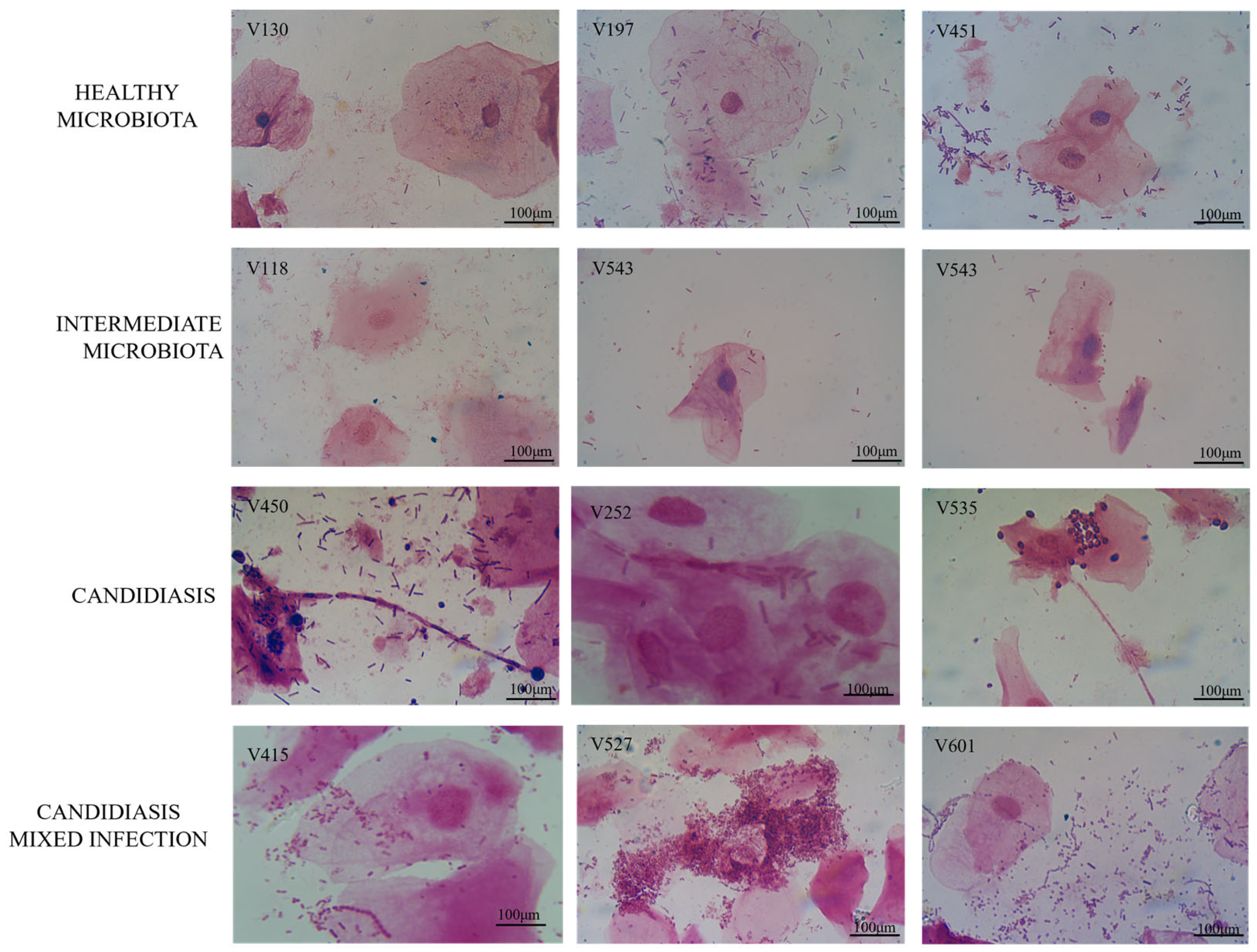
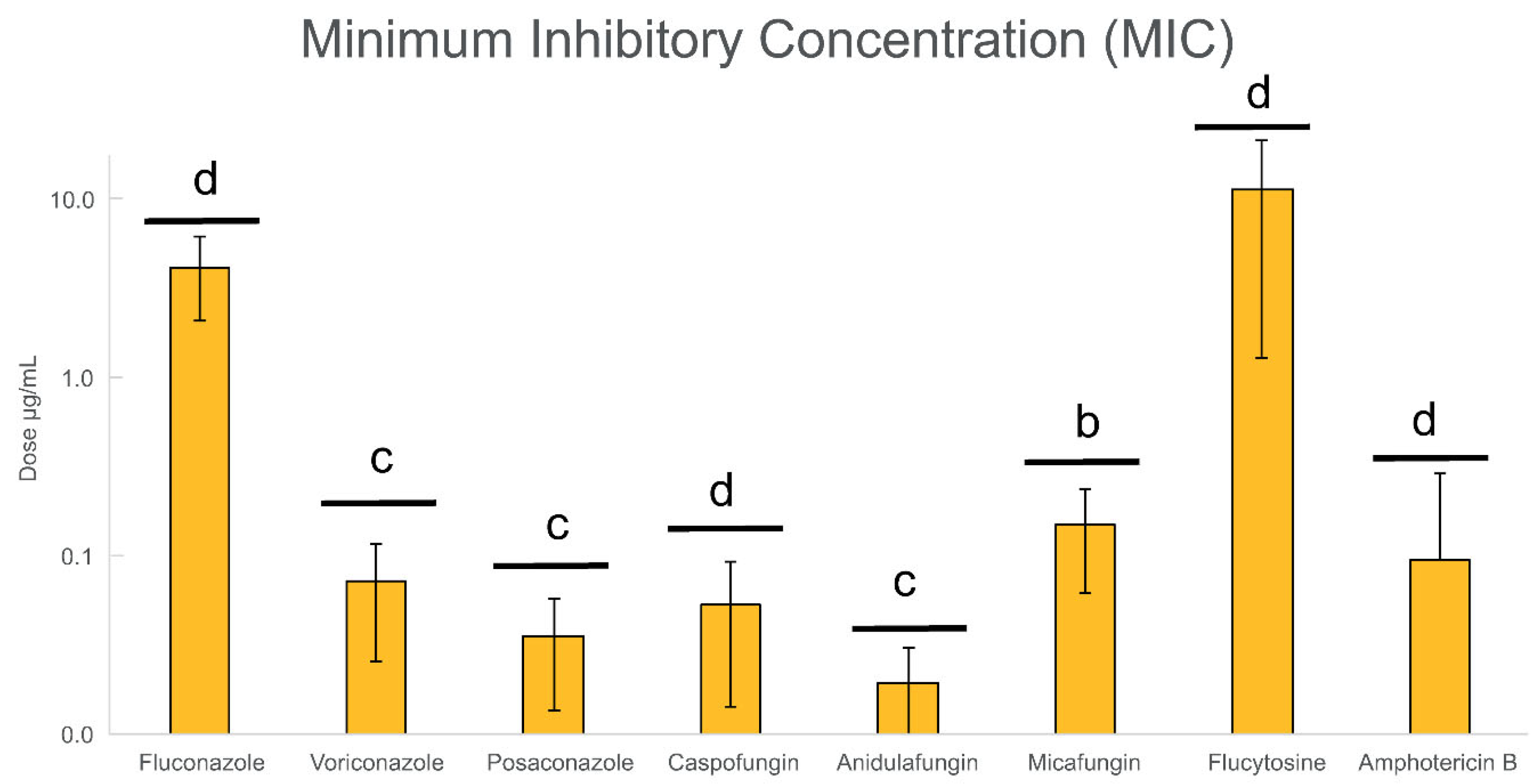

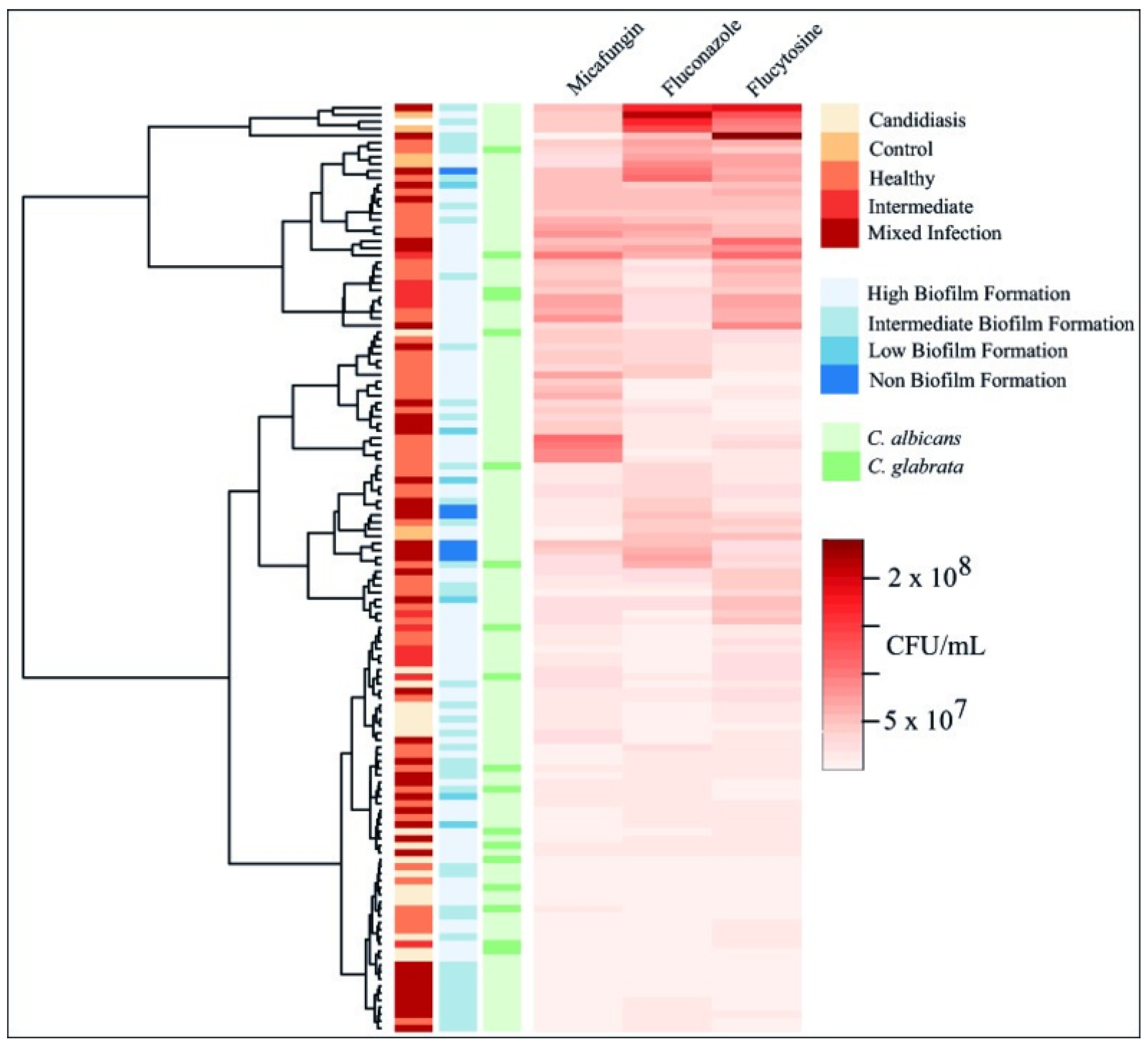


| Isolates | Sample | P vs. B * | Fluconazole | Micafungin | Flucytosine | |
|---|---|---|---|---|---|---|
| Healthy Microbiota | ||||||
| C. albicans | V130 | Planktonic | Classification | Sensitive | Resistant | Resistant |
| MIC (µg/mL) | 4 | - | - | |||
| Biofilm | MBIC90 (µg/mL) | 128 | 4 | 256 | ||
| MBEC90 (µg/mL) | 128 | 4 | 256 | |||
| C. albicans | V134 | Planktonic | Classification | Sensitive | Sensitive | Sensitive |
| MIC (µg/mL) | 1 | 0.03 | 4 | |||
| Biofilm | MBIC90 (µg/mL) | 4 | 0.50 | 128 | ||
| MBEC90 (µg/mL) | 8 | 0.50 | 128 | |||
| C. albicans | V196 | Planktonic | Classification | Sensitive | Resistant | Resistant |
| MIC (µg/mL) | 4 | - | - | |||
| Biofilm | MBIC90 (µg/mL) | 16 | 1 | 256 | ||
| MBEC90 (µg/mL) | 16 | 1 | 256 | |||
| C. albicans | V197 | Planktonic | Classification | Resistant | Sensitive | Resistant |
| MIC (µg/mL) | - | 0.03 | - | |||
| Biofilm | MBIC90 (µg/mL) | 64 | 0.50 | 64 | ||
| MBEC90 (µg/mL) | 64 | 0.50 | 128 | |||
| C. albicans | V202 | Planktonic | Classification | Sensitive | Resistant | Sensitive |
| MIC (µg/mL) | 4 | - | 16 | |||
| Biofilm | MBIC90 (µg/mL) | 16 | - | 128 | ||
| MBEC90 (µg/mL) | 16 | - | 32 | |||
| C. albicans | V251 | Planktonic | Classification | Sensitive | Sensitive | Resistant |
| MIC (µg/mL) | 4 | 0.25 | - | |||
| Biofilm | MBIC90 (µg/mL) | 64 | 1 | 256 | ||
| Biofilm | MBEC90 (µg/mL) | 64 | 2 | 512 | ||
| C. albicans | V448 | Planktonic | Classification | Resistant | Sensitive | Resistant |
| MIC (µg/mL) | - | 0.25 | - | |||
| Biofilm | MBIC90 (µg/mL) | 128 | 2 | - | ||
| Biofilm | MBEC90 (µg/mL) | 128 | 2 | - | ||
| C. albicans | V451 | Planktonic | Classification | Sensitive | Resistant | Resistant |
| MIC (µg/mL) | 2 | - | - | |||
| Biofilm | MBIC90 (µg/mL) | 64 | 1 | 256 | ||
| Biofilm | MBEC90 (µg/mL) | 64 | 1 | 256 | ||
| C. albicans | V580 | Planktonic | Classification | Resistant | Resistant | Resistant |
| MIC (µg/mL) | - | - | - | |||
| Biofilm | MBIC90 (µg/mL) | - | - | 256 | ||
| MBEC90 (µg/mL) | - | - | 32 | |||
| Intermediate Microbiota | ||||||
| C. albicans-E. coli | V118 | Planktonic | Classification | Sensitive | Sensitive | Sensitive |
| MIC (µg/mL) | 2 | 0.25 | 16 | |||
| Biofilm | MBIC90 (µg/mL) | 8 | 1 | 256 | ||
| MBEC90 (µg/mL) | 16 | 1 | 512 | |||
| C. glabrata-Gram-positive coccus | V543 | Planktonic | Classification | Sensitive | Sensitive | Resistant |
| MIC (µg/mL) | 8 | 0.06 | - | |||
| Biofilm | MBIC90 (µg/mL) | 32 | 1 | 128 | ||
| MBEC90 (µg/mL) | 32 | 2 | 128 | |||
| Candidiasis | ||||||
| C. albicans | V161 | Planktonic | Classification | Resistant | Resistant | Resistant |
| MIC (µg/mL) | - | - | - | |||
| Biofilm | MBIC90 (µg/mL) | - | - | - | ||
| MBEC90 (µg/mL) | - | - | - | |||
| C. albicans | V218 | Planktonic | Classification | Sensitive | Sensitive | Sensitive |
| MIC (µg/mL) | 4 | 0.25 | 8 | |||
| Biofilm | MBIC90 (µg/mL) | - | - | 512 | ||
| MBEC90 (µg/mL) | - | 2 | 512 | |||
| C. albicans | V252 | Planktonic | Classification | Sensitive | Sensitive | Resistant |
| MIC (µg/mL) | 4 | 0.12 | - | |||
| Biofilm | MBIC90 (µg/mL) | 64 | 0.50 | 128 | ||
| MBEC90 (µg/mL) | 64 | 4 | 256 | |||
| C. albicans | V449 | Planktonic | Classification | Sensitive | Sensitive | Sensitive |
| MIC (µg/mL) | 8 | 0.12 | 16 | |||
| Biofilm | MBIC90 (µg/mL) | 64 | 2 | 512 | ||
| MBEC90 (µg/mL) | - | 4 | - | |||
| C. albicans | V450 | Planktonic | Classification | Sensitive | Sensitive | Resistant |
| MIC (µg/mL) | 4 | 0.25 | - | |||
| Biofilm | MBIC90 (µg/mL) | 8 | 0.50 | 128 | ||
| MBEC90 (µg/mL) | 4 | 0.12 | 128 | |||
| C. albicans | V535 | Planktonic | Classification | Sensitive | Resistant | Sensitive |
| MIC (µg/mL) | 4 | - | 32 | |||
| Biofilm | MBIC90 (µg/mL) | 64 | 0.25 | 128 | ||
| Biofilm | MBEC90 (µg/mL) | 128 | 0.50 | 256 | ||
| C. albicans | V540 | Planktonic | Classification | Sensitive | Resistant | Sensitive |
| MIC (µg/mL) | 4 | - | 32 | |||
| Biofilm | MBIC90 (µg/mL) | 128 | 0.50 | 512 | ||
| MBEC90 (µg/mL) | 128 | 1 | 512 | |||
| Mixed Infection | ||||||
| C. albicans C.-AV | V415 | Planktonic | Classification | Sensitive | Sensitive | Sensitive |
| MIC (µg/mL) | 8 | 0.12 | 16 | |||
| Biofilm | MBIC90 (µg/mL) | 128 | 4 | - | ||
| MBEC90 (µg/mL) | 128 | - | 128 | |||
| C. albicans C.-AV | V527 | Planktonic | Classification | Sensitive | Sensitive | Sensitive |
| MIC (µg/mL) | 2 | 0.12 | 16 | |||
| Biofilm | MBIC90 (µg/mL) | 128 | - | - | ||
| MBEC90 (µg/mL) | 16 | 4 | 128 | |||
| C. glabrata C.-BV | V601 | Planktonic | Classification | Resistant | Sensitive | Resistant |
| MIC (µg/mL) | - | 0.03 | - | |||
| Biofilm | MBIC90 (µg/mL) | 32 | 0.50 | 256 | ||
| Biofilm | MBEC90 (µg/mL) | 16 | 2 | 256 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedeño-Pinargote, A.C.; Jara-Medina, N.R.; Pineda-Cabrera, C.C.; Cueva, D.F.; Erazo-Garcia, M.P.; Tejera, E.; Machado, A. Impact of Biofilm Formation by Vaginal Candida albicans and Candida glabrata Isolates and Their Antifungal Resistance: A Comprehensive Study in Ecuadorian Women. J. Fungi 2025, 11, 620. https://doi.org/10.3390/jof11090620
Cedeño-Pinargote AC, Jara-Medina NR, Pineda-Cabrera CC, Cueva DF, Erazo-Garcia MP, Tejera E, Machado A. Impact of Biofilm Formation by Vaginal Candida albicans and Candida glabrata Isolates and Their Antifungal Resistance: A Comprehensive Study in Ecuadorian Women. Journal of Fungi. 2025; 11(9):620. https://doi.org/10.3390/jof11090620
Chicago/Turabian StyleCedeño-Pinargote, Ariana Cecibel, Nicolás Renato Jara-Medina, Carlos C. Pineda-Cabrera, Darío F. Cueva, María P. Erazo-Garcia, Eduardo Tejera, and António Machado. 2025. "Impact of Biofilm Formation by Vaginal Candida albicans and Candida glabrata Isolates and Their Antifungal Resistance: A Comprehensive Study in Ecuadorian Women" Journal of Fungi 11, no. 9: 620. https://doi.org/10.3390/jof11090620
APA StyleCedeño-Pinargote, A. C., Jara-Medina, N. R., Pineda-Cabrera, C. C., Cueva, D. F., Erazo-Garcia, M. P., Tejera, E., & Machado, A. (2025). Impact of Biofilm Formation by Vaginal Candida albicans and Candida glabrata Isolates and Their Antifungal Resistance: A Comprehensive Study in Ecuadorian Women. Journal of Fungi, 11(9), 620. https://doi.org/10.3390/jof11090620







