Tracking of Tobacco Mosaic Virus in Taxonomically Different Plant Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. TMV-GFP-1056 Recombinant Vector and Plant Materials
2.2. Fungal Isolates and Culture Conditions
2.3. RNA Extraction and Subculturing Conditions
2.4. Optical and Confocal Microscopy
2.5. Virus Detection and Analysis
2.6. Pathogenicity Assays
2.7. Statistical Analyses
3. Results
3.1. TMV Enters and Replicates in Mycelia of Botrytis and Verticillium spp., but Not in Fusarium and Monilinia spp.
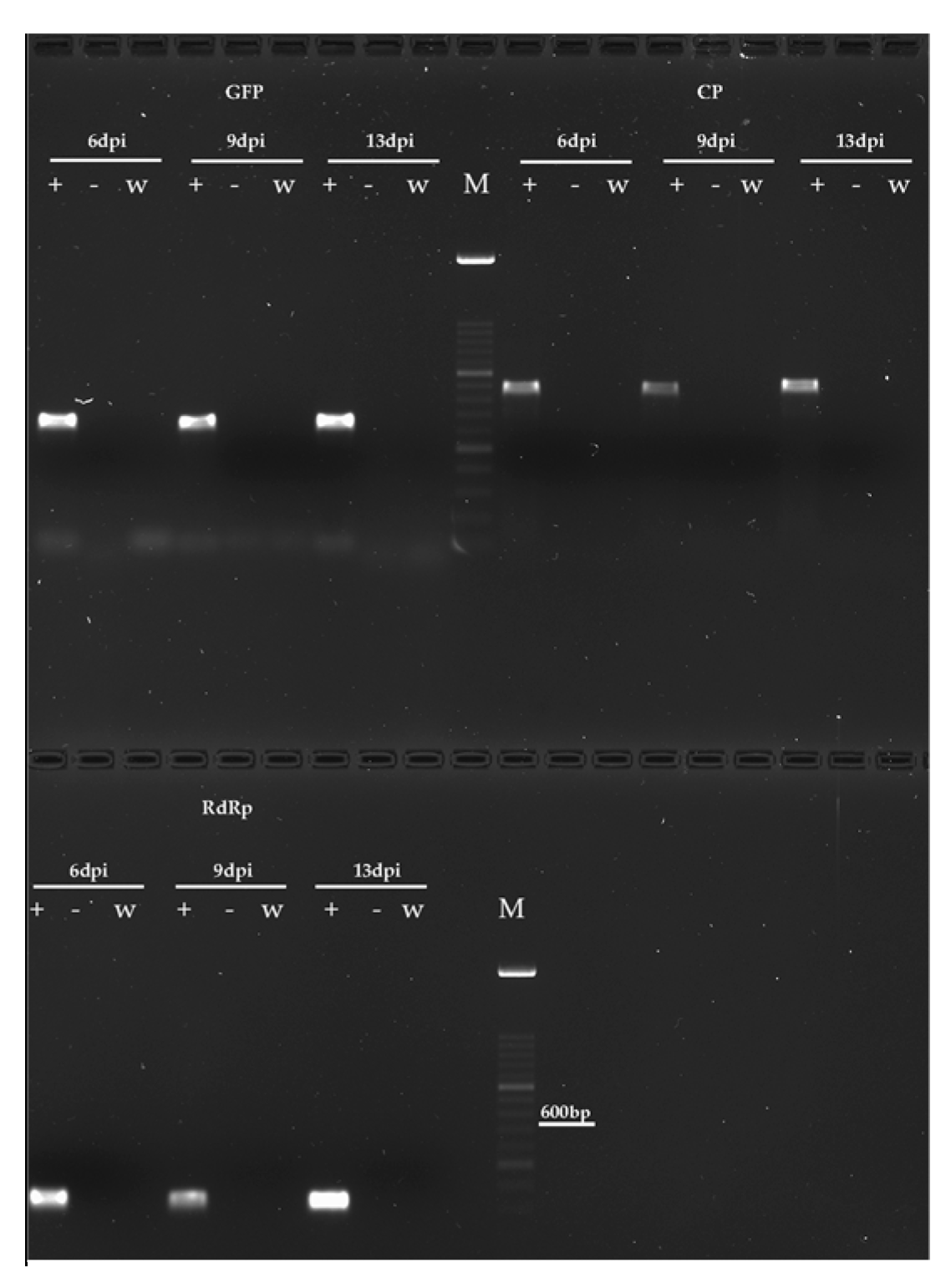
3.2. Temporal Dynamics of TMV RNA Accumulation in Mycelia of Verticillium and Botrytis spp.
3.3. Cross-Kingdom Viral Infection Activates RNA Interference Machinery and siRNA Biogenesis in Verticillium and Botrytis spp.
3.4. TMV Infection Persists in Plated Subcultures
3.5. Pathogenicity, Morphology, and Growth Rate of Verticillium and Botrytis spp. Remain Unaffected by TMV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 Plant Viruses in Molecular Plant Pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Stobbe, A.H.; Roossinck, M.J. Plant Virus Metagenomics: What We Know and Why We Need to Know More. Front. Plant Sci. 2014, 5, 150. [Google Scholar] [CrossRef]
- Roossinck, M.J. Viruses in the Phytobiome. Curr. Opin. Virol. 2019, 37, 72–76. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Shi, M.; Holmes, E.C. Using Metagenomics to Characterize an Expanding Virosphere. Cell 2018, 172, 1168–1172. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect Vector-Mediated Transmission of Plant Viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Huot, O.B.; Martin, K.M.; Kondo, H.; Dietzgen, R.G. Plant Rhabdoviruses—Their Origins and Vector Interactions. Curr. Opin. Virol. 2018, 33, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Andika, I.B.; Kondo, H.; Sun, L. Interplays between Soil-Borne Plant Viruses and RNA Silencing-Mediated Antiviral Defense in Roots. Front. Microbiol. 2016, 7, 1458. [Google Scholar] [CrossRef]
- Bragard, C.; Caciagli, P.; Lemaire, O.; Lopez-Moya, J.J.; MacFarlane, S.; Peters, D.; Susi, P.; Torrance, L. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 2013, 51, 177–201. [Google Scholar] [CrossRef]
- Casper, S.J.; Holt, C.A. Expression of the green fluorescent protein-encoding gene from a tobacco mosaic virus-based vector. Gene 1996, 173, 69–73. [Google Scholar] [CrossRef]
- Galbraith, D.A.; Fuller, Z.L.; Ray, A.M.; Brockmann, A.; Frazier, M.; Gikungu, M.W.; Losyev, O. Investigating the viral ecology of global bee communities with high-throughput metagenomics. Sci. Rep. 2018, 8, 8879. [Google Scholar] [CrossRef]
- Dacheux, L.; Cervantes-Gonzalez, M.; Guigon, G.; Thiberge, J.M.; Vandenbogaert, M.; Maufrais, C.; Valérie Caro, V.; Bourhy, H. A preliminary study of viral metagenomics of French bat species in contact with humans: Identification of neew mammalian viruses. PLoS ONE 2014, 9, e87194. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Q.; Andika, I.B.; Kondo, H.; Wang, Y. Viruses Shuttle between Fungi and Plants. Trends Microbiol. 2024, 32, 620–621. [Google Scholar] [CrossRef]
- Cao, X.; Liu, J.; Pang, J.; Kondo, H.; Chi, S.; Zhang, J.; Sun, L.; Andika, I.B. Common but Nonpersistent Acquisitions of Plant Viruses by Plant-Associated Fungi. Viruses 2022, 14, 2279. [Google Scholar] [CrossRef]
- Bian, R.; Andika, I.; Pang, T.; Lian, Z.; Wei, S.; Niu, E.; Wu, Y.; Kondō, H.; Liu, X.; Sun, L. Facilitative and Synergistic Interactions between Fungal and Plant Viruses. Proc. Natl. Acad. Sci. USA 2020, 117, 3779–3788. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.X.; Schnabel, G.; Hu, M.; Zhang, R.; Chen, X.; Zhao, Y.; Xie, B. Global Distribution and Management of Peach Diseases. Phytopathol. Res. 2022, 4, 30. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen Profile Update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Maciąg, T.; Meller, M.; Frąc, M.; Cybulska, J.; Oszust, K. Microbial Consortia for Plant Protection against Diseases: More than the Sum of Its Parts. Int. J. Mol. Sci. 2023, 24, 12227. [Google Scholar] [CrossRef]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The Destructive Fungal Pathogen Botrytis cinerea-Insights from Genes Studied with Mutant Analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Miccolis Angelini, R.M.; Landi, L.; Raguseo, C.; Pollastro, S.; Faretra, F.; Romanazzi, G. Tracking of Diversity and Evolution in the Brown Rot Fungi Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Microbiol. 2022, 13, 854852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, M.; Schmidt, U.; Büttner, C.; Bandte, M. Electrolytic disinfection of irrigation water for intensive crop production in greenhouses as demonstrated on tomatoes (solanum lycopersicum mill). Horticulturae 2022, 8, 414. [Google Scholar] [CrossRef]
- Sun, A.; Jiao, X.; Ren, P.; Yu, D.; Li, F.; Chen, Q.; Bi, L.; He, J.-Z.; Hu, H. Organic fertilization regimes suppress fungal plant pathogens through modulating the resident bacterial and protistan communities. J. Sustain. Agric. Environ. 2022, 1, 43–53. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, Y.; Qi, J.; Gao, H.; Li, X.; Tian, Q.; Qian, X.; Wei, G.; Jiao, S. The climate-driven distribution and response to global change of soil-borne pathogens in agroecosystems. Glob. Ecol. Biogeogr. 2023, 32, 766–779. [Google Scholar] [CrossRef]
- Mascia, T.; Vučurović, A.; Minutillo, S.A.; Nigro, F.; Labarile, R.; Savoia, M.A.; Palukaitis, P.; Gallitelli, D. Infection of Colletotrichum acutatum and Phytophthora infestans by taxonomically different plant viruses. Eur. J. Plant Pathol. 2019, 153, 1001–1017. [Google Scholar] [CrossRef]
- Mascia, T.; Labarile, R.; Doohan, F.; Gallitelli, D. Tobacco Mosaic Virus Infection Triggers an RNAi-Based Response in Phytophthora infestans. Sci. Rep. 2019, 9, 2657. [Google Scholar] [CrossRef]
- Mascia, T.; Nigro, F.; Abdallah, A.; Ferrara, M.; De Stradis, A.; Faedda, R.; Minafra, A.; Gallitelli, D. Gene Silencing and Gene Expression in Phytopathogenic Fungi Using a Plant Virus Vector. Proc. Natl. Acad. Sci. USA 2014, 111, 4291–4296. [Google Scholar] [CrossRef] [PubMed]
- Brants, D.H. Tobacco Mosaic Virus in Pythium Spec. Neth. J. Plant Pathol. 1969, 75, 296–299. [Google Scholar] [CrossRef]
- Nienhaus, F.; Mack, C. Infection of Pythium arrhenomanes In Vitro with Tobacco Mosaic Virus and Tobacco Necrosis Virus/Infektionsversuche an Pythium arrhenomanes in Flüssigkeitskultur mit Tabakmosaikvirus und Tabaknekrosevirus. Z. Pflanzkrankh. Pflanzenschutz 1974, 81, 728–731. Available online: https://www.jstor.org/stable/43213848 (accessed on 15 June 2025).
- Dawson, W.O.; Lehto, K. Plant Virology. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar] [CrossRef]
- Zaitlin, M.; Palukaitis, P. Advances in understanding plant viruses and virus diseases. Annu. Rev. Phytopathol. 2000, 38, 117–143. [Google Scholar] [CrossRef]
- Hull, R. Plant Virology, 4th ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Goelet, P.; Lomonossoff, G.P.; Butler, P.J.G.; Akam, M.; Gait, M.J.; Karn, J. Nucleotide Sequence of Tobacco Mosaic Virus RNA. Proc. Natl. Acad. Sci. USA 1982, 79, 5818–5822. [Google Scholar] [CrossRef]
- Palukaitis, P.; Zaitlin, M. Replicase-Mediated Resistance to Plant Virus Disease. Adv. Virus Res. 1997, 48, 349–377. [Google Scholar] [CrossRef]
- Kagale, S.; Li, S.; Wilson, N.; Cloutier, S.; You, F.M.; Pasha, A.; Ratashak, J.; Wang, Z.; Sharpe, A.G.; Kianian, S.F.; et al. TMV-Gate Vectors: Gateway Compatible Tobacco Mosaic Virus Based Expression Vectors for Functional Analysis of Proteins. Sci. Rep. 2012, 2, 874. [Google Scholar] [CrossRef]
- Park, M.; Baek, E.; Yoon, J.Y.; Palukaitis, P. The use of a tobacco mosaic virus-based expression vector system in chrysanthemum. Plant Pathol. J. 2017, 33, 429. [Google Scholar] [CrossRef]
- Shivprasad, S.; Pogue, G.P.; Lewandowski, D.J.; Hidalgo, J.; Donson, J.; Grill, L.K.; Dawson, W.O. Heterologous Sequences Greatly Affect Foreign Gene Expression in Tobacco Mosaic Virus-Based Vectors. Virology 1999, 255, 312–323. [Google Scholar] [CrossRef]
- Rabindran, S.; Dawson, W.O. Assessment of Recombinants That Arise from the Use of a TMV-Based Transient Expression Vector. Virology 2001, 284, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; González, M.; González, C.; Brito, N. Efficiency of different strategies for gene silencing in Botrytis cinerea. Appl. Microbiol. Biotechnol. 2014, 98, 9413–9424. [Google Scholar] [CrossRef]
- Jeseničnik, T.; Štajner, N.; Radišek, S.; Jakše, J. RNA interference core components identified and characterised in Verticillium nonalfalfae, a vascular wilt pathogenic plant fungus of hops. Sci. Rep. 2019, 9, 8651. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.; Huang, H.D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA Silencing in Plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, J.H.; Zhao, J.H.; Liu, T.; Chen, Y.Y.; Wang, C.H.; Liu, X.H.; Wang, M.Q.; Zhang, Y.; Wang, Q.; et al. A Fungal Effector Suppresses the Nuclear Export of AGO1–miRNA Complex to Promote Infection in Plants. Proc. Natl. Acad. Sci. USA 2022, 119, e2114583119. [Google Scholar] [CrossRef]
- Cogoni, C.; Macino, G. Posttranscriptional Gene Silencing in Neurospora by a RecQ DNA Helicase. Science 1999, 286, 2342–2344. [Google Scholar] [CrossRef]
- Fulci, V.; Macino, G. Quelling: Post-Transcriptional Gene Silencing Guided by Small RNAs in Neurospora crassa. Curr. Opin. Microbiol. 2007, 10, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhao, J.H.; Zhao, P.; Zhang, T.; Wang, S.; Guo, H.S. A Fungal milRNA Mediates Epigenetic Repression of a Virulence Gene in Verticillium dahliae. Phil. Trans. R. Soc. B 2019, 374, 20180309. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, N.; Jiang, Q.; Sun, X.; Wang, Y.; Zhang, H.; Hu, Z. Exploring MicroRNA-Like Small RNAs in the Filamentous Fungus Fusarium oxysporum. PLoS ONE 2014, 9, e104956. [Google Scholar] [CrossRef]
- Jo, S.M.; Ayukawa, Y.; Yun, S.H.; Komatsu, K.; Arie, T. A putative RNA silencing component protein FoQde-2 is involved in virulence of the tomato wilt fungus Fusarium oxysporum f. sp. lycopersici. J. Gen. Plant Pathol. 2018, 84, 395–398. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Abate, D.; Rotolo, C.; Gerin, D.; Pollastro, S.; Faretra, F. De Novo Assembly and Comparative Transcriptome Analysis of Monilinia fructicola, Monilinia laxa and Monilinia fructigena, the Causal Agents of Brown Rot on Stone Fruits. BMC Genom. 2018, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Canto, T.; Palukaitis, P. Novel N Gene-Associated, Temperature-Independent Resistance to the Movement of Tobacco Mosaic Virus Vectors Neutralized by a Cucumber Mosaic Virus RNA1 Transgene. J. Virol. 2002, 76, 12908–12916. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Nigro, F.; Gallone, P.; Romanazzi, G.; Schena, L.; Ippolito, A.; Salerno, M.G. Incidence of Verticillium Wilt on Olive in Apulia and Genetic Diversity of Verticillium dahliae Isolates from Infected Trees. J. Plant Pathol. 2005, 87, 13–23. Available online: https://www.jstor.org/stable/41998203 (accessed on 8 June 2025).
- Tan, K.K.; Epton, H.A.S. Effect of light on the growth and sporulation of Botrytis cinerea. Trans. Br. Mycol. Soc. 1973, 61, 145–157. [Google Scholar] [CrossRef]
- Ershova, N.; Kamarova, K.; Sheshukova, E.; Antimonova, A.; Komarova, T. A Novel Cellular Factor of Nicotiana benthamiana Susceptibility to Tobamovirus Infection. Front. Plant Sci. 2023, 14, 1224958. [Google Scholar] [CrossRef]
- Ren, H.; Wu, X.; Lyu, Y.; Zhou, H.; Xie, X.; Zhang, X.; Yang, H. Selection of reliable reference genes for gene expression studies in Botrytis cinerea. J. Microbiol. Methods 2017, 142, 71–75. [Google Scholar] [CrossRef]
- Atallah, Z.K.; Bae, J.; Jansky, S.H.; Rouse, D.I.; Stevenson, W.R. Multiplex Real-Time Quantitative PCR to Detect and Quantify Verticillium dahliae Colonization in Potato Lines That Differ in Response to Verticillium Wilt. Phytopathology 2007, 97, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Medrano, J.F. Real-Time PCR for mRNA Quantitation. BioTechniques 2005, 39, 75–85. [Google Scholar] [CrossRef]
- Minutillo, S.A.; Mascia, T.; Gallitelli, D. A DNA Probe Mix for the Multiplex Detection of Ten Artichoke Viruses. Eur. J. Plant Pathol. 2012, 134, 459–465. [Google Scholar] [CrossRef]
- Skepper, J.N.; Powell, J.M. Immunogold Staining of Epoxy Resin Sections for Transmission Electron Microscopy (TEM). Cold Spring Harb. Protoc. 2008, 2008, pdb.prot5015. [Google Scholar] [CrossRef]
- Bletsos, F.; Thanassoulopoulos, C.; Roupakias, D. Effect of Grafting on Growth, Yield, and Verticillium Wilt of Eggplant. HortScience 2003, 38, 183–186. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Blanco-López, M.A. Recovery of Young Olive Trees from Verticillium dahliae. Eur. J. Plant Pathol. 2005, 113, 367–375. [Google Scholar] [CrossRef]
- Liu, S.; Liu, R.; Lv, J.; Feng, Z.; Wei, F.; Zhao, L.; Zhang, Y.; Zhu, H.; Feng, H. The glycoside hydrolase 28 member VdEPG1 is a virulence factor of Verticillium dahliae and interacts with the jasmonic acid pathway-related gene GhOPR9. Mol. Plant Pathol. 2023, 24, 1238–1255. [Google Scholar] [CrossRef]
- Ninkuu, V.; Liu, Z.; Liu, H.; Li, C.; Zhou, Y.; Zhao, Q.; Qin, A.; Li, M.; Gao, P.; Yan, L.; et al. Genome sequencing of a novel Verticillium dahliae strain (huangweibingjun). Sci. Rep. 2025, 15, 15143. [Google Scholar] [CrossRef]
- Valero-Jiménez, C.A.; Veloso, J.; Staats, M.; van Kan, J.A.L. Comparative genomics of plant pathogenic Botrytis species with distinct host specificity. BMC Genom. 2019, 20, 203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Gao, Y.; Liang, Y.; Dong, Y.; Yang, X.; Qiu, D. Verticillium dahliae PevD1, an Alt a 1-like protein, targets cotton PR5-like protein and promotes fungal infection. J. Exp. Bot. 2019, 70, 613–626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Csorba, T.; Bovi, A.; Dalmay, T.; Burgyán, J. The p122 Subunit of Tobacco Mosaic Virus Replicase Is a Potent Silencing Suppressor and Compromises Both Small Interfering RNA- and MicroRNA-Mediated Pathways. J. Virol. 2007, 81, 11768–11780. [Google Scholar] [CrossRef]
- Jimenez-Diaz, R.M.; Cirulli, M.; Bubici, G.; del Mar Jimenez-Gasco, M.; Antoniou, P.P.; Tjamos, E.C. Verticillium wilt, a major threat to olive production: Current status and future prospects for its management. Plant Dis. 2012, 96, 304–329. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, A.R. The history of Botrytis taxonomy, the rise of phylogenetics, and implications for species recognition. Phytopathology 2021, 111, 437–454. [Google Scholar] [CrossRef]
- Andika, I.B.; Wei, S.; Cao, C.; Salaipeth, L.; Kondo, H.; Sun, L. Phytopathogenic fungus hosts a plant virus: A naturally occurring cross-kingdom viral infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12267–12272. [Google Scholar] [CrossRef] [PubMed]
- Sande, W.W.J.; Lo-Ten-Foe, J.R.; Belkum, A.; Netea, M.G.; Kullberg, B.J.; Vonk, A.G. Mycoviruses: Future therapeutic agents of invasive fungal infections in humans? Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 755–763. [Google Scholar] [CrossRef]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal viruses unveiled: A comprehensive review of mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- De Wit, P.J.; Mehrabi, R.; Van den Burg, H.A.; Stergiopoulos, I. Fungal effector proteins: Past, present and future. Mol. Plant Pathol. 2009, 10, 735–747. [Google Scholar] [CrossRef]
- Mascia, T.; Gallitelli, D.; Palukaitis, P. Something new to explore: Plant viruses infecting and inducing gene silencing in filamentous fungi. Mob. Genet. Elem. 2014, 4, e29782. [Google Scholar] [CrossRef] [PubMed]
- Segers, G.C.; Zhang, X.; Deng, F.; Sun, Q.; Nuss, D.L. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc. Natl. Acad. Sci. USA 2007, 104, 12902–12906. [Google Scholar] [CrossRef]
- Ding, S.-W.; Voinnet, O. Antiviral immunity directed by small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef]
- Kondo, H.; Chiba, S.; Toyoda, K.; Suzuki, N. Evidence for negative-strand RNA virus infection in fungi. Virology 2013, 435, 201–209. [Google Scholar] [CrossRef]
- Segers, G.C.; van Wezel, R.; Zhang, X.; Hong, Y.; Nuss, D.L. Hypovirus Papain-Like Protease p29 Suppresses RNA Silencing in the Natural Fungal Host and in a Heterologous Plant System. Eukaryot Cell 2006. [Google Scholar] [CrossRef]
- Andika, I.B.; Kondo, H.; Suzuki, N. Dicer functions transcriptionally and posttranscriptionally in a multilayer antiviral defense. Proc. Natl. Acad. Sci. USA 2019, 116, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lee, K.M.; Cho, W.K.; Park, J.Y.; Kim, K.H. Differential contribution of RNA interference components in response to distinct Fusarium graminearum virus infections. J. Virol. 2018, 92, e01756-17. [Google Scholar] [CrossRef] [PubMed]
- Hogenhout, S.A.; Ammar, E.-D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Campo, S.; Gilbert, K.B.; Carrington, J.C. Small RNA-based antiviral defense in the phytopathogenic fungus Colletotrichum higginsianum. PLoS Pathog. 2016, 12, e1005640. [Google Scholar] [CrossRef]
- Pang, T.; Peng, J.; Bian, R.; Liu, Y.; Zhang, D.; Andika, I.B.; Sun, L. Similar characteristics of siRNAs of plant viruses which replicate in plant and fungal hosts. Biology 2022, 11, 1672. [Google Scholar] [CrossRef]
- Csorba, T.; Kontra, L.; Burgyán, J. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 2015, 479, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.S.; Liu, J.; Cheng, N.-H.; Folimonov, A.; Hou, Y.-M.; Bao, Y.; Katagi, C.; Carter, S.A.; Nelson, R.S. The Tobacco Mosaic Virus 126-kDa Protein Associated with Virus Replication and Movement Suppresses RNA Silencing. Mol. Plant-Microbe Interact. 2004, 17, 583–592. [Google Scholar] [CrossRef]
- Atabekova, A.K.; Solovieva, A.D.; Chergintsev, D.A.; Solovyev, A.G.; Morozov, S.Y. Role of Plant Virus Movement Proteins in Suppression of Host RNAi Defense. Int. J. Mol. Sci. 2023, 24, 9049. [Google Scholar] [CrossRef]
- Yu, J.; Park, J.Y.; Heo, J.I.; Kim, K.H. The ORF2 protein of Fusarium graminearum virus 1 suppresses the transcription of FgDICER2 and FgAGO1 to limit host antiviral defences. Mol. Plant Pathol. 2020, 21, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Tauati, S.J.; Pearson, M.N.; Choquer, M.; Foster, G.D.; Bailey, A.M. Investigating the role of dicer 2 (dcr2) in gene silencing and the regulation of mycoviruses in Botrytis cinerea. Microbiology 2014, 83, 140–148. [Google Scholar] [CrossRef]







| Sample | Lesion Size on Cucumber Leaves, cm | Lesion Size on Strawberry Fruits, cm | N° Total Cucumber Leaves | N° Total Strawberry Fruits |
|---|---|---|---|---|
| Bc− | 2.11 ± 0.28 a,* | 2.61 ± 0.11 a,* | 15 | 30 |
| Bc+TMVGFP | 2.43 ± 0.27 a,* | 2.49 ± 0.13 a,* | 15 | 30 |
| Negative Control | 0.00 ± 0.00 * | 0.00 ± 0.00 * | 5 | 10 |
| Sample at 36 dpi | Disease Severity (Scale 0–5) | Disease Severity Index (DSI) | N° Total Plants |
|---|---|---|---|
| Vd− | 2.67 ± 0.14 a,* | 64.28% | 21 |
| Vd+TMVGFP | 2.76 ± 0.11 a,* | 69.05% | 21 |
| Negative control | 0.0 ± 0.0 * | 0.00% | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnaba, N.F.; Vaccaro, L.; De Miccolis Angelini, R.M.; Spanò, R.; Nigro, F.; Mascia, T. Tracking of Tobacco Mosaic Virus in Taxonomically Different Plant Fungi. J. Fungi 2025, 11, 619. https://doi.org/10.3390/jof11090619
Barnaba NF, Vaccaro L, De Miccolis Angelini RM, Spanò R, Nigro F, Mascia T. Tracking of Tobacco Mosaic Virus in Taxonomically Different Plant Fungi. Journal of Fungi. 2025; 11(9):619. https://doi.org/10.3390/jof11090619
Chicago/Turabian StyleBarnaba, Natascia Filomena, Lorenza Vaccaro, Rita Milvia De Miccolis Angelini, Roberta Spanò, Franco Nigro, and Tiziana Mascia. 2025. "Tracking of Tobacco Mosaic Virus in Taxonomically Different Plant Fungi" Journal of Fungi 11, no. 9: 619. https://doi.org/10.3390/jof11090619
APA StyleBarnaba, N. F., Vaccaro, L., De Miccolis Angelini, R. M., Spanò, R., Nigro, F., & Mascia, T. (2025). Tracking of Tobacco Mosaic Virus in Taxonomically Different Plant Fungi. Journal of Fungi, 11(9), 619. https://doi.org/10.3390/jof11090619








