A Distinct Clinical Entity of Invasive Cardiac Aspergillosis: Not the Heart Valves This Time
Abstract
1. Introduction
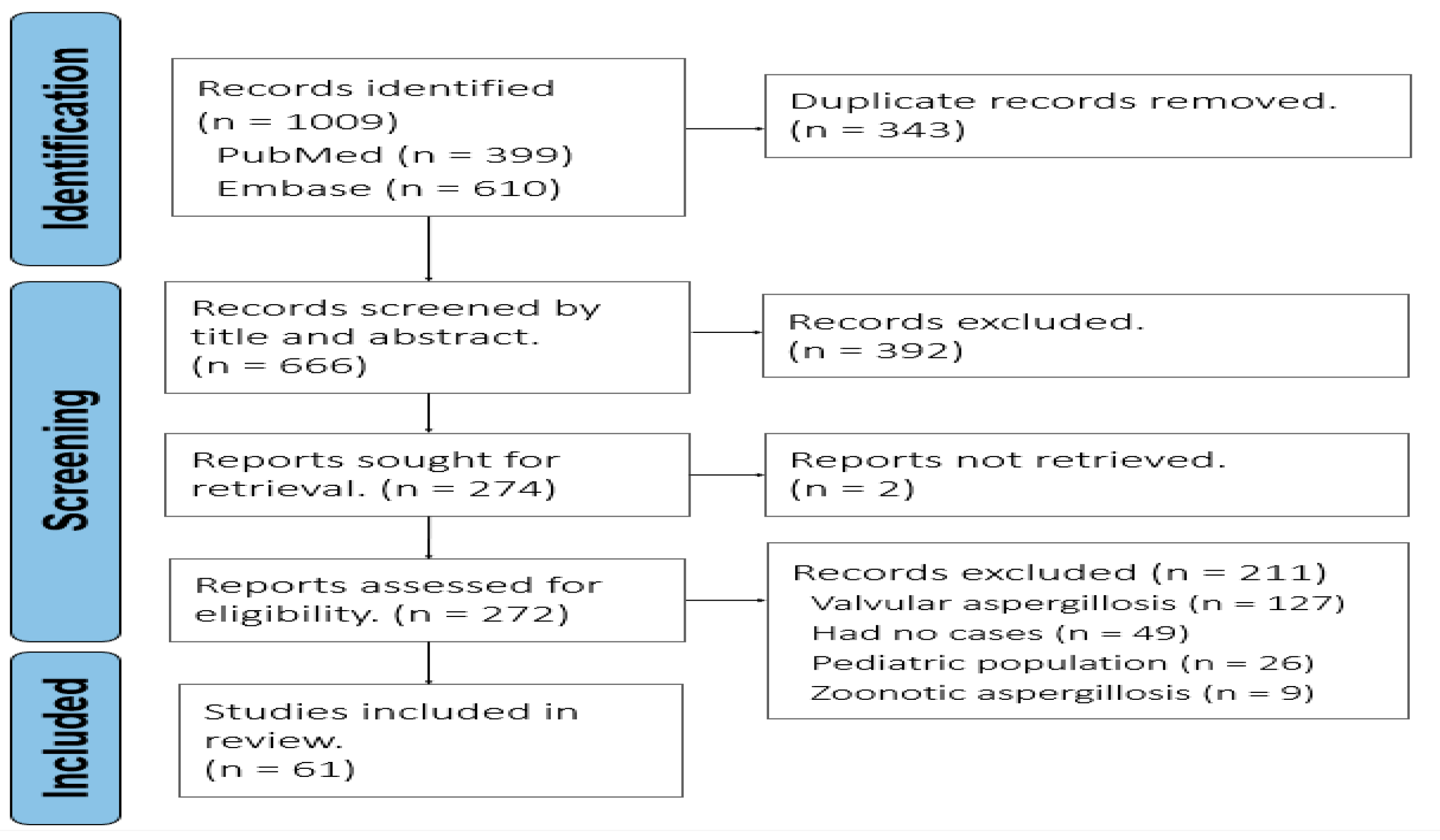
2. Materials and Methods
3. Clinical Vignette
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pasqualotto, A.C. Aspergillosis: From diagnosis to prevention. In Aspergillosis: From Diagnosis to Prevention; Springer: London, UK, 2010. [Google Scholar] [CrossRef]
- Hori, A.; Kami, M.; Kishi, Y.; Machida, U.; Matsumura, T.; Kashima, T. Clinical significance of extra-pulmonary involvement of invasive aspergillosis: A retrospective autopsy-based study of 107 patients. J. Hosp. Infect. 2002, 50, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Bulkley, B.H. Aspergillus pericarditis: Clinical and pathologic features in the immunocompromised patient. Cancer 1982, 49, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Fieguth, H.G.; Aybek, T.; Ujvari, Z.; Moritz, A.; Wimmer-Greinecker, G. Disseminated Aspergillus fumigatus infection with consecutive mitral valve endocarditis in a lung transplant recipient. J. Heart Lung Transplant. 2005, 24, 2297–2300. [Google Scholar] [CrossRef]
- Navaratnam, A.M.D.; Al-Freah, M.; Cavazza, A.; Auzinger, G. A case series of non-valvular cardiac aspergillosis in critically ill solid organ transplant and non-transplant patients and systematic review. J. Intensive Care Soc. 2021, 22, 241–247. [Google Scholar] [CrossRef]
- le Moing, V.; Lortholary, O.; Timsit, J.F.; Couvelard, A.; Bouges-Michel, C.; Wolff, M.; Guillevin, L.; Casassus, P. Aspergillus pericarditis with tamponade: Report of a successfully treated case and review. Clin. Infect. Dis. 1998, 26, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Biso, S.; Lekkham, R.; Climaco, A. Aspergillus Pericarditis with Tamponade in a Renal Transplant Patient. Case Rep. Cardiol. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Alkuwaiti, F.; Elghoneimy, Y.; Alabdrabalrasol, E.; Alshreadah, S. Unusual presentation of aspergillus pericarditis: A case report. Saudi J. Med. Med. Sci. 2019, 7, 175–178. [Google Scholar] [CrossRef]
- Guimaron, S.; Gervais, P.; Voisine, P. A Case of Aspergillus Pericarditis in the Setting of Invasive Aspergillosis: The Role of Surgery in a Multi-modal Treatment. CJC Open 2022, 4, 663–666. [Google Scholar] [CrossRef]
- Hayashi, A.; Kobayashi, S.; Hisauchi, I.; Komatsu, T.; Nakahara, S.; Sakai, Y.; Haruki, K.; Taguchi, I. Long-term favorable course of Aspergillus endo-, myo-, and pericarditis. Int. Heart J. 2017, 58, 1020–1023. [Google Scholar] [CrossRef]
- Kupsky, D.F.; Alaswad, K.; Rabbani, B.T. A Rare Case of Aspergillus Pericarditis with Associated Myocardial Abscess and Echocardiographic Response to Therapy. Echocardiography 2016, 33, 1085–1088. [Google Scholar] [CrossRef]
- Dalhoff, K.; Braun, J.; Gatermann, S.; Djonlagic, H. Fatal Aspergillus pericarditis in acquired immunodeficiency syndrome. Intensive Care Med. 1996, 22, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Aspergillus pancarditis following bone marrow transplantation for chronic myelogenous leukemia. Chest 1989, 95, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Higgins, R.; Alam, Z.; Janakiraman, N.; Gorman, M. Aspergillus fungal mass detected by transesophageal echocardiography. J. Am. Soc. Echocardiogr. 1998, 11, 83–85. [Google Scholar] [CrossRef]
- Itoh, M.; Takahashi, M.; Mori, M.; Tamekiyo, H.; Yoshida, H.; Yago, K.; Shimada, H.; Arai, K. Myocardial infarction caused by Aspergillus embolization in a patient with aplastic anemia. Intern. Med. 2006, 45, 547–550. [Google Scholar] [CrossRef]
- Luce, J.M.; Ostenson, R.C.; Springmeyer, S.C.; Hudson, L.D. Invasive aspergillosis presenting as pericarditis and cardiac tamponade. Chest 1979, 76, 703–705. [Google Scholar] [CrossRef]
- Mortensen, K.L.; Knudsen, J.B.; Jensen-Fangel, S.; Stausbøl-Grøn, B.; Arendrup, M.C.; Petersen, E. Successful management of invasive aspergillosis presenting as pericarditis in an adult patient with chronic granulomatous disease. Mycoses 2011, 54, e233–e236. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A.D.; Weinbaum, D.L.; Aldrich, T.K.; Mandell, G.L. Invasive aspergillosis of the lung and pericardium in a nonimmunocompromised 33 year old man. Am. J. Med. 1981, 71, 903–907. [Google Scholar] [CrossRef]
- Carrel, T.P.; Schaffner, A.; Schmid, E.R.; Schneider, J.; Bauer, E.P.; Laske, A.; von Segesser, L.K.; Turina, M.I. Fatal fungal pericarditis after cardiac surgery and immunosuppression. J. Thorac. Cardiovasc. Surg. 1991, 101, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, L.; Cobos-Alonso, J.; Ventura-Díaz, S.; Mirambeaux-Villalona, R.M.; Cabañero-Sánchez, A.; Muñoz-Molina, G.M.; Chinea-Rodríguez, A. Aspergillus pericardial empyema successfully treated with percutaneous drainage in a patient with invasive pulmonary aspergillosis. Ann. Hematol. 2022, 101, 2793–2794. [Google Scholar] [CrossRef]
- Caballero, M.J.; Mongardon, N.; Haouache, H.; Vodovar, D.; ben Ayed, I.; Auvergne, L.; Hillion, M.L.; Botterel, F.; Dhonneur, G. Aspergillus mediastinitis after cardiac surgery. Int. J. Infect. Dis. 2016, 44, 16–19. [Google Scholar] [CrossRef]
- Müller, N.L.; Miller, R.R.; Ostrow, D.N.; Nelems, B.; Vickars, L.M. Tension pneumopericardium: An unusual manifestation of invasive pulmonary aspergillosis. Am. J. Roentgenol. 1987, 148, 678–680. [Google Scholar] [CrossRef]
- Andersson, B.S.; Luna, M.A.; McCredie, K.B. Systemic aspergillosis as cause of myocardial infarction. Cancer 1986, 58, 2146–2150. [Google Scholar] [CrossRef]
- Kemdem, A.; Ahmad, I.; Ysebrand, L.; Nouar, E.; Silance, P.G.; Aoun, M.; Bron, D.; Vandenbossche, J.L. An Aspergillus Myocardial Abscess Diagnosed by Echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 1177.e3–1177.e5. [Google Scholar] [CrossRef]
- Yu, G.; Zhong, F.; Ye, B. Destroyed lung with constrictive pericarditis. Med. Clin. 2020, 155, 471. [Google Scholar] [CrossRef]
- Hori, M.K.; Knight, L.L.; Carvalho, P.G.; Stevens, D.L. Aspergillar myocarditis and acute coronary artery occlusion in an immunocompromised patient. West J. Med. 1991, 155, 525–527. [Google Scholar] [PubMed] [PubMed Central]
- Ohya, I.; Bunai, Y.; Tsujinaka, M.; Akaza, K.; Nakamura, I. Fatal Aspergillus pancarditis after incompatible blood transfusion intended to be an autologous blood transfusion. Leg. Med. 2001, 3, 246–251. [Google Scholar] [CrossRef]
- Romagnuolo, J.; Jewell, L.D.; Kneteman, N.M.; Bain, V.G. Graft-versus-host disease after liver transplantation complicated by systemic aspergillosis with pancarditis. Can. J. Gastroenterol. 2000, 14, 637–640. [Google Scholar] [CrossRef][Green Version]
- Rouby, Y.; Combourieu, E.; Perrier-Gros-Claude, J.D.; Saccharin, C.; Huerre, M. A case of aspergillus myocarditis associated with septic shock. J. Infect. 1998, 37, 295–297. [Google Scholar] [CrossRef]
- Sulik-Tyszka, B.; Kacprzyk, P.; Mądry, K.; Ziarkiewicz-Wróblewska, B.; Jędrzejczak, W.; Wróblewska, M. Aspergillosis of the Heart and Lung and Review of Published Reports on Fungal Endocarditis. Mycopathologia 2016, 181, 583–588. [Google Scholar] [CrossRef][Green Version]
- Peterson, S.P.; Schiller, N.; Stricker, R.B. Failure of two-dimensional echocardiography to detect Aspergillus endocarditis. Chest 1984, 85, 291–294. [Google Scholar] [CrossRef]
- Bhat, M.; Ghali, P.; Wong, P.; Marcus, V.; Michel, R.; Cantarovich, M.; Metrakos, P.; Deschenes, M. Immunosuppression with budesonide for liver transplant recipients with severe infections. Liver Transplant. 2012, 18, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Rueter, F.; Hirsch, H.H.; Kunz, F.; Buser, P.; Habicht, J.M.; Moch, H.; Fluckiger, U.; Zerkowski, H.R. Late Aspergillus fumigatus endomyocarditis with brain abscess as a lethal complication after heart transplantation. J. Heart Lung Transplant. 2002, 21, 1242–1245. [Google Scholar] [CrossRef]
- Jones, R.E.; Groom, K.M.; Singh, A.; Lai, H.; Dungu, J.N.; Medeiros, F.; Lal, A.; Barbagallo, R.M. Non-Valvular Cardiac Aspergilloma: A Rare Presentation of a Rare Condition. Heart Lung Circ. 2019, 28, e115–e116. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.L.; Oliver, D.H.; Barasch, E. Aspergillus mural vegetation identified by transesophageal echocardiography. Echocardiography 1997, 14, 283–285. [Google Scholar] [CrossRef]
- Pavlina, A.A.; Peacock, J.W.; Ranginwala, S.A.; Pavlina, P.M.; Ahier, J.; Hanak, C.R. Aspergillus mural endocarditis presenting with multiple cerebral abscesses. J. Cardiothorac. Surg. 2018, 13, 107. [Google Scholar] [CrossRef]
- Poupelin, J.C.; Philit, F.; Richard, J.C.; Badet, M.; Lemasson, S.; Bayle, F.; Guérin, C. Pericardial and pleural diffusion of voriconazole during disseminated invasive aspergillosis: Report of a case with successful outcome. Intensive Care Med. 2006, 32, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, G.; Vanstraelen, G.; Hustinx, R.; Delvenne, P.; Hayette, M.P.; Massion, P.; Canivet, J.L. Péricardite aspergillaire avec tamponnade cardiaque et syndrome hémophagocytaire: Un cas non classique d’immunodéficience [Aspergillus pericarditis with cardiac tamponade and haemophagocytic syndrome: A non-classical case of immunodeficiency]. Rev. Med. Liege 2006, 61, 713–718. (In French) [Google Scholar] [PubMed]
- Welsh, R.A.; Buchness, J.M. Aspergillus endocarditis, myocarditis and lung abscesses; report of a case. Am. J. Clin. Pathol. 1955, 25, 782–786. [Google Scholar] [CrossRef]
- Rogers, J.G.; Windle, J.R.; McManus, B.M.; Easley, A.R. Aspergillus myocarditis presenting as myocardial infarction with complete heart block. Am. Heart J. 1990, 120, 430–432. [Google Scholar] [CrossRef]
- Sergi, C.; Hofmann, W.J.; Sinn, P.; Schnabel, P.A.; Otto, H.F.; Weitz, J.; Otto, G.; Eckart, A. Aspergillus endocarditis, myocarditis and pericarditis complicating necrotizing fasciitis. Virchows Archiv. 1996, 429, 177–180. [Google Scholar] [CrossRef]
- Kaplan, R.; Duncalf, D.; Cizmar, S. Aspergillus pancarditis and cardiac arrest during anesthesia. Anesth. Analg. 1981, 60, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Salanitri, G.C.; Huo, E.; Miller, F.H.; Gupta, A.; Pereles, F.S. MRI of mycotic sinus of valsalva pseudoaneurysm secondary to Aspergillus pericarditis. AJR. Am. J. Roentgenol. 2005, 184, S25–S27. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.M.; Leisen, J.C.; Elliott, J.P.; Lewis, J.W.; Wendt, D.J.; Quinn, E.L. Echocardiographically silent Aspergillus mural endocarditis. West J. Med. 1988, 149, 334–338. [Google Scholar] [PubMed] [PubMed Central]
- Xie, L.; Gebre, W.; Szabo, K.; Lin, J.H. Cardiac aspergillosis in patients with acquired immunodeficiency syndrome: A case report and review of the literature. Arch. Pathol. Lab. Med. 2005, 129, 511–515. [Google Scholar] [CrossRef]
- Kombade, S.P.; Abhishek, K.S.; Nag, V.L. Fungal pericarditis due to Aspergillus nidulans: A rare case report. J. Clin. Diagn. Res. 2018, 12, DD5–DD6. [Google Scholar] [CrossRef]
- Cishek, M.B.; Yost, B.; Schaefer, S. Cardiac aspergillosis presenting as myocardial infarction. Clin. Cardiol. 1996, 19, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Vaideeswar, P. Aspergillus pancarditis manifesting as hospital-acquired infection: Report of two cases and review of literature. Cardiovasc. Pathol. 2010, 19, e253–e257. [Google Scholar] [CrossRef]
- Bullis, S.S.; Krywanczyk, A.; Hale, A.J. Aspergillosis myocarditis in the immunocompromised host. IDCases 2019, 17, e00567. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bal, A.; Singhal, M.; Vijayvergiya, R.; Das, A. Fibrosing mediastinitis due to Aspergillus with dominant cardiac involvement: Report of two autopsy cases with review of literature. Cardiovasc. Pathol. 2014, 23, 354–357. [Google Scholar] [CrossRef]
- Miyoshi, I.; Saito, T.; Machida, H.; Uemura, Y.; Kuroda, N.; Taguchi, H. Fungal effusions associated with invasive pulmonary aspergillosis. Intern. Med. 2006, 45, 1019–1020. [Google Scholar] [CrossRef]
- Cabot, R.C.; Scully, R.E.; Galdabini, J.J.; McNeely, B.U.; Wheeler, E.O.; Robboy, S.J. Case 39-1976. N. Engl. J. Med. 1976, 295, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Frantzeskaki, F.; Kosmopoulos, M.; Taccone, F.S.; van den Abeele, A.M.; Bulpa, P.; Forêt, F.; Vogelaers, D.; Blot, S.; Blasco-Navalpotro, M.; et al. Endomyocardial and pericardial aspergillosis in critically ill patients. Mycoses 2017, 60, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.S.; Hough, A.J.; Talley, J.D. IT FITS! Intelligence Transfer: From Images to Solutions Myocardial Aspergillosis. J. Interv. Cardiol. 1998, 11, 247–248. [Google Scholar] [CrossRef]
- Williams, A.H. Aspergillus Myocarditis. Am. J. Clin. Pathol. 1974, 61, 247–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cox, J.N.; di Dió, F.; Pizzolato, G.P.; Lerch, R.; Pochon, N. Aspergillus endocarditis and myocarditis in a patient with the acquired immunodeficiency syndrome (AIDS)—A review of the literature. Virchows Arch. A Pathol. Anat. Histopathol. 1990, 417, 255–259. [Google Scholar] [CrossRef]
- Ross, E.M.; Macher, A.M.; Roberts, W.C. Aspergillus Fumigatus thrombi causing total occlusion of both coronary arterial ostia, all four major epicardial coronary arteries and coronary sinus and associated with purulent pericarditis. Am. J. Cardiol. 1985, 56, 499–500. [Google Scholar] [CrossRef]
- Van Ede, A.; de Pauw, B.E.; Meis, J.F.G.M.; Koot, R.A.C.; Heystraten, F.M.J. Pneumopericardium complicating invasive pulmonary aspergillosis: Case report and review. Infection 1994, 22, 102–105. [Google Scholar] [CrossRef]
- Carrascosa Porras, M.; Herreras Martínez, R.; Corral Mones, J.; Ares, M.; Zabaleta Murguiondo, M.; Rüchel, R. Fatal aspergillus myocarditis following short-term corticosteroid therapy for chronic obstructive pulmonary disease. Scand. J. Infect. Dis. 2002, 34, 224–227. [Google Scholar] [CrossRef]
- Gomyo, H.; Murayama, T.; Obayashi, C.; Mizuno, I.; Koizumi, T.; Imoto, S. [Invasive pulmonary aspergillosis complicated by complete atrioventricular block and aspergillus pericarditis after induction chemotherapy in a patient with acute lymphoblastic leukemia]. Rinsho Ketsueki 2003, 44, 1036–1039. (In Japanese) [Google Scholar] [PubMed]
- Yamamoto, S.; Shichijo, T.; Hino, N. [Pericardial abscess by aspergillus mimicking a cardiac tumor]. Kyobu Geka 2005, 58, 783–786. (In Japanese) [Google Scholar] [PubMed]
- Kalokhe, A.S.; Rouphael, N.; el Chami, M.F.; Workowski, K.A.; Ganesh, G.; Jacob, J.T. Aspergillus endocarditis: A review of the literature. Int. J. Infect. Dis. 2010, 14, e1040–e1047. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Abeele, A.-M.V.D.; Bulpa, P.; Misset, B.; Meersseman, W.; Cardoso, T.; Paiva, J.-A.; Blasco-Navalpotro, M.; De Laere, E.; Dimopoulos, G.; et al. Epidemiology of invasive aspergillosis in critically ill patients: Clinical presentation, underlying conditions, and outcomes. Crit. Care 2015, 19, 1–15. [Google Scholar] [CrossRef]
- Pfeiffer, C.D.; Fine, J.P.; Safdar, N. Diagnosis of invasive aspergillosis using a galactomannan assay: A meta-analysis. Clin. Infect. Dis. 2006, 42, 1417–1727. [Google Scholar] [CrossRef]
- Pickering, J.W.; Sant, H.W.; Bowles, C.A.P.; Roberts, W.L.; Woods, G.L. Evaluation of a (1→3)-β-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2005, 43, 5957–5962. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Urban, A.K.; Freeman, A.F.; Hammoud, D.A. An Unusual Pattern of Premature Cervical Spine Degeneration in STAT3-LOF. J. Clin. Immunol. 2021, 41, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Grimbacher, B.; Holland, S.M.; Gallin, J.I.; Greenberg, F.; Hill, S.C.; Malech, H.L.; Miller, J.A.; O’Connell, A.C.; Puck, J.M. Hyper-IgE Syndrome with Recurrent Infections—An Autosomal Dominant Multisystem Disorder. N. Engl. J. Med. 1999, 340, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Walsh, T.J. Current approaches to diagnosis and treatment of invasive aspergillosis. Am. J. Respir. Crit. Care Med. 2006, 173, 707–717. [Google Scholar] [CrossRef]
- Oh, N.A.; Hennecken, C.; van den Eynde, J.; Doulamis, I.P.; Avgerinos, D.v.; Kampaktsis, P.N. Pericardiectomy and Pericardial Window for the Treatment of Pericardial Disease in the Contemporary Era. Curr. Cardiol. Rep. 2022, 24, 1619–1631. [Google Scholar] [CrossRef]
| Variables | Total (n = 67) |
|---|---|
| Demographics | |
| Age (mean, range) | 44.6 years (18–79) |
| Male sex | 43 (64%) |
| Comorbidities | |
| Hematologic Malignancies (Leukemia, Lymphoma, MDS) | 20 (30%) |
| Other Hematologic disorders | 4 (6%) |
| Transplant history | 16 (24%) |
| HIV/AIDS | 4 (6%) |
| Primary Immunodeficiency | 3 (4.5%) |
| Chronic steroids | 2 (3%) |
| Diabetes mellitus | 5 (7.5%) |
| Hypertension | 4 (6%) |
| Cardiac disorders | 9 (13.5%) |
| Pulmonary disorders | 9 (13.5%) |
| Gastrointestinal disorders | 3 (4.5%) |
| Hepatobiliary disorders | 5 (7.5%) |
| Rheumatologic disorders | 10 (15%) |
| Spine disorders | 2 (3%) |
| Clinical presentation | |
| Fever | 33 (49%) |
| Shortness of breath | 31 (46%) |
| Cough (±hemoptysis) | 14 (21%) (±6 [9%]) |
| Chest pain | 10 (15%) |
| Abdominal pain | 8 (12%) |
| Nausea, vomiting, and/or Diarrhea | 6 (9%) |
| Neurologic/Ocular signs | 10 (15%) |
| Skin manifestations | 3 (4.5%) |
| Shock or hypotension | 15 (22%) |
| Other end-organ damage | 11 (17.5%) |
| Echocardiogram findings | |
| Pericardial effusion/Tamponade | 33 (49%) |
| Cardiac mass | 23 (34%) |
| Valvular vegetation | 8 (12%) |
| Fibrin deposits/bridges | 2 (3%) |
| Pneumopericardium | 1 (2%) |
| Low ejection fraction | 33 (49%) |
| Level of cardiac involvement | |
| Pancardium | 18 (27%) |
| Pericardium alone | 26 (39%) |
| Myocardium alone | 10 (15%) |
| Myocardium and Endocardium | 5 (7%) |
| Pericardium and Endocardium | 2 (3%) |
| Pericardium and Myocardium | 1 (2%) |
| Cardiac mass | 5 (7%) |
| Systems involved in Invasive Aspergillosis | |
| NVCA alone | 14 (21%) |
| NVCA + Lung | 10 (15%) |
| NVCA + Lung + Other distant organs | 35 (52%) |
| Aspergillus species Identified | |
| Not specified | 33 (49%) |
| A. fumigatus | 23 (34%) |
| A. flavus | 8 (12%) |
| A. nidulans | 2 (3%) |
| A. niger | 1 (2%) |
| Concomitant infections | |
| Gram negative bacteremia | 8 (12%) |
| Gram positive bacteremia | 16 (24%) |
| Viral (unspecified) | 9 (13%) |
| Respiratory coinfections | 15 (22%) |
| Cardiac coinfections | 4 (6%) |
| Gastrointestinal infections | 9 (13%) |
| Wound infections | 2 (3%) |
| Leptospirosis | 4 (6%) |
| Total Patients 1 (n, %) | Survived (n, %) | Died (n, %) | |
|---|---|---|---|
| Untreated 2 | 19 (28%) | 0 (0%) | 17 (89.5%) |
| Amphotericin B (AMB) | 30 (45%) | 6 (20%) | 24 (80%) |
| IV L-AmB +/− IP 3 | 5 (7.5%) | 2 (40%) | 3 (60%) |
| IV ABD | 11 (16.5%) | 2 (18%) | 9 (82%) |
| IV ABD switched to IV LAmB | 1 (1.5%) | 0 (0%) | 1 (100%) |
| IV AMB Not specified ± IP 4 | 13 (19.5%) | 2 (15%) | 11 (85%) |
| Azole 5 | 21 (31%) | 12 (57%) | 9 (43%) |
| Voriconazole | 15 (22%) | 9 (60%) | 6 (40%) |
| Fluconazole | 4 (6%) | 0 (0%) | 4 (100%) |
| Itraconazole | 3 (4.5%) | 1 (33%) | 2 (67%) |
| Isavuconazole | 1 (1.5%) | 1 (100%) | 0 (0%) |
| Ketoconazole | 1 (1.5%) | 1 (100%) | 0 (0%) |
| Echinocandin | 9 (13.5%) | 4 (44%) | 5 (56%) |
| Caspofungin | 7 (10.5%) | 3 (43%) | 4 (57%) |
| Micafungin | 1 (1.5%) | 0 (0%) | 1 (100%) |
| Anidulafugin | 1 (1.5%) | 1 (100%) | 0 (0%) |
| Flucytosine | 3 (4.5%) | 0 (0%) | 3 (100%) |
| Antifungal—Not specified | 4 (6%) | 2 (50%) | 2 (50%) |
| Procedure 6 | 27 (40%) | 11 (40.7%) | 15 (55.6%) |
| Pericardiocentesis | 9 (13.5%) | 4 (44.5%) | 5 (55.5%) |
| Pericardial drain or window | 10 (15%) | 3 (30%) | 7 (70%) |
| Mass excision 7 | 5 (7.5%) | 2 (40%) | 2 (40%) |
| Valve replacement | 3 (4.5%) | 1 (33%) | 2 (67%) |
| Pericardial purulent tissue removal | 1 (1.5%) | 1 (100%) | 0 (0%) |
| PTCA 8 attempted | 1 (1.5%) | 0 (0%) | 1 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khouri, Z.; Smeltzer, H.; Al Qura’an, A.; Khan, M.H.; Malek, A.E. A Distinct Clinical Entity of Invasive Cardiac Aspergillosis: Not the Heart Valves This Time. J. Fungi 2025, 11, 486. https://doi.org/10.3390/jof11070486
Al Khouri Z, Smeltzer H, Al Qura’an A, Khan MH, Malek AE. A Distinct Clinical Entity of Invasive Cardiac Aspergillosis: Not the Heart Valves This Time. Journal of Fungi. 2025; 11(7):486. https://doi.org/10.3390/jof11070486
Chicago/Turabian StyleAl Khouri, Zaid, Hunter Smeltzer, Anood Al Qura’an, Mohammad H. Khan, and Alexandre E. Malek. 2025. "A Distinct Clinical Entity of Invasive Cardiac Aspergillosis: Not the Heart Valves This Time" Journal of Fungi 11, no. 7: 486. https://doi.org/10.3390/jof11070486
APA StyleAl Khouri, Z., Smeltzer, H., Al Qura’an, A., Khan, M. H., & Malek, A. E. (2025). A Distinct Clinical Entity of Invasive Cardiac Aspergillosis: Not the Heart Valves This Time. Journal of Fungi, 11(7), 486. https://doi.org/10.3390/jof11070486






