Integrated Management Strategies for Blackleg Disease of Canola Amidst Climate Change Challenges
Abstract
1. Introduction
2. Survival Structures and Infection Biology of Blackleg Pathogen in Canola
3. Current IDM Strategies for Blackleg of Canola
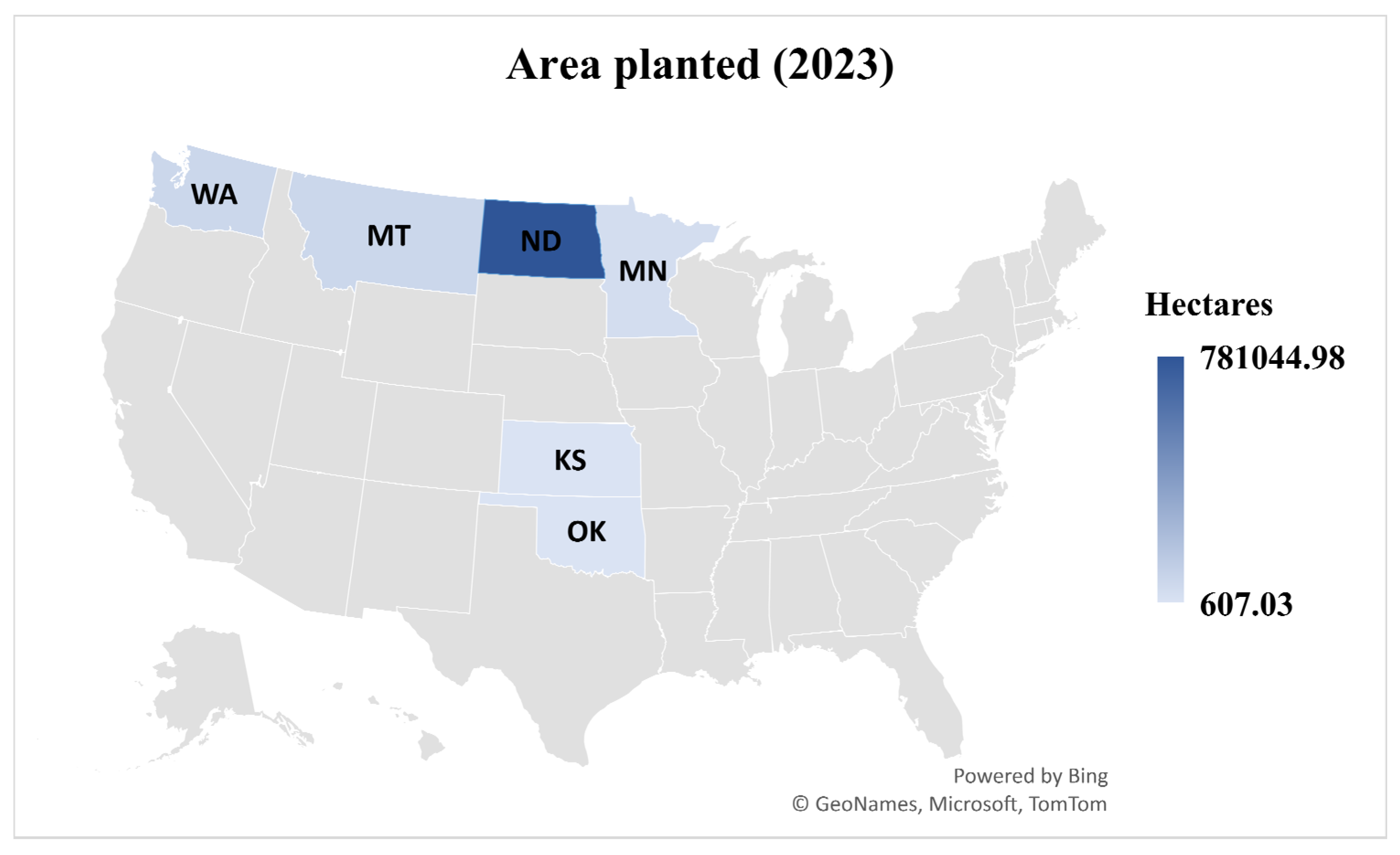
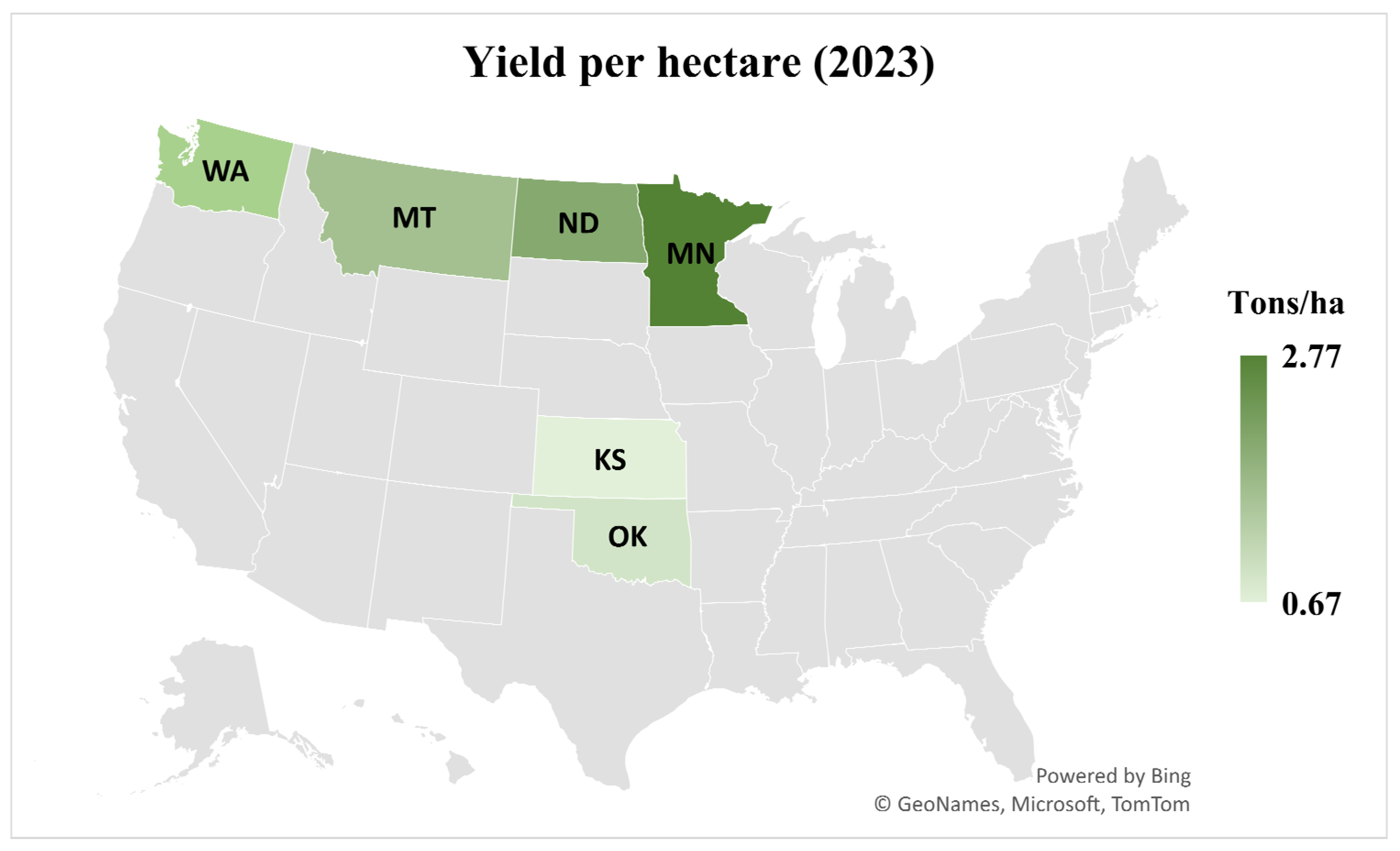
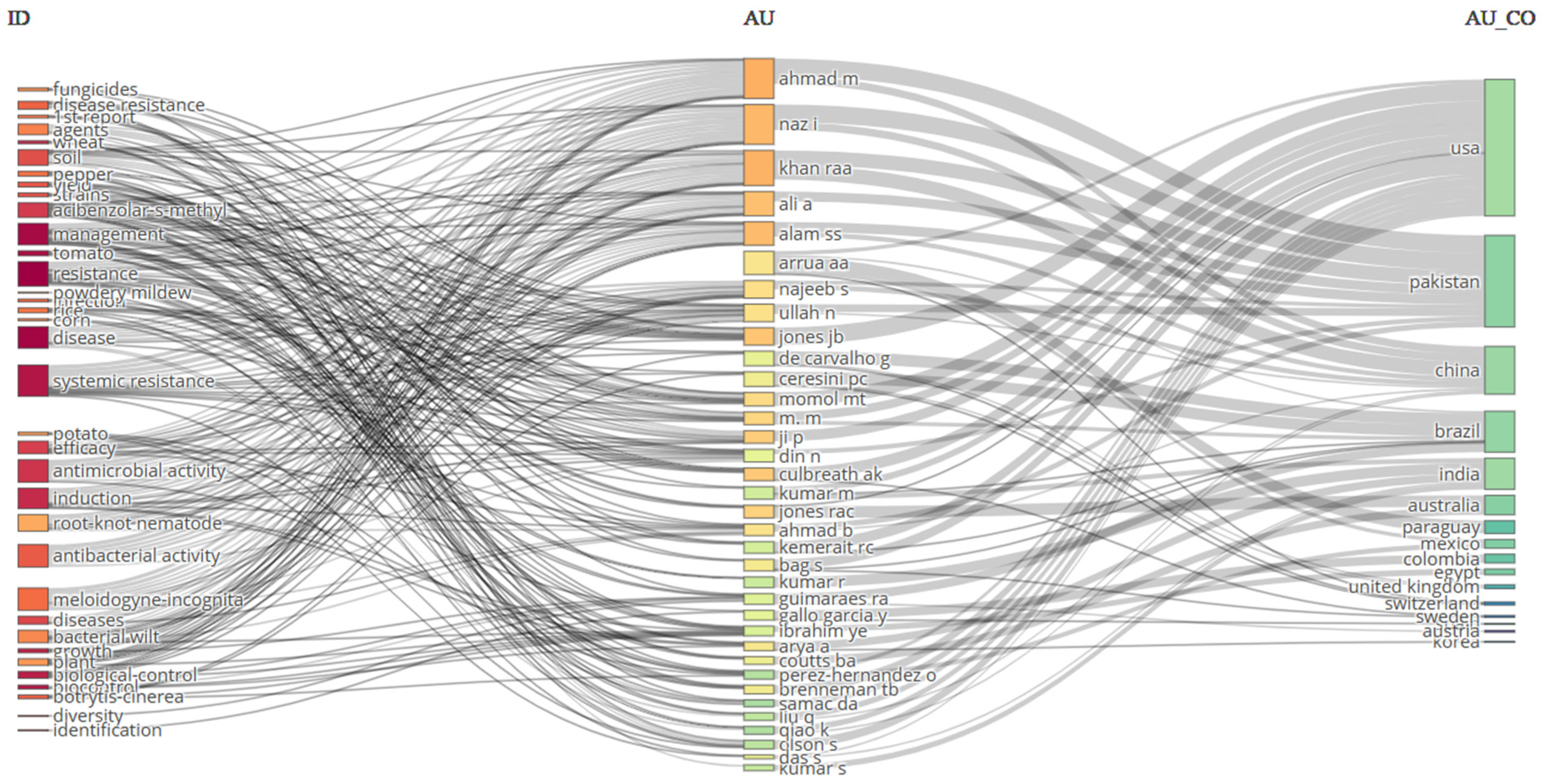
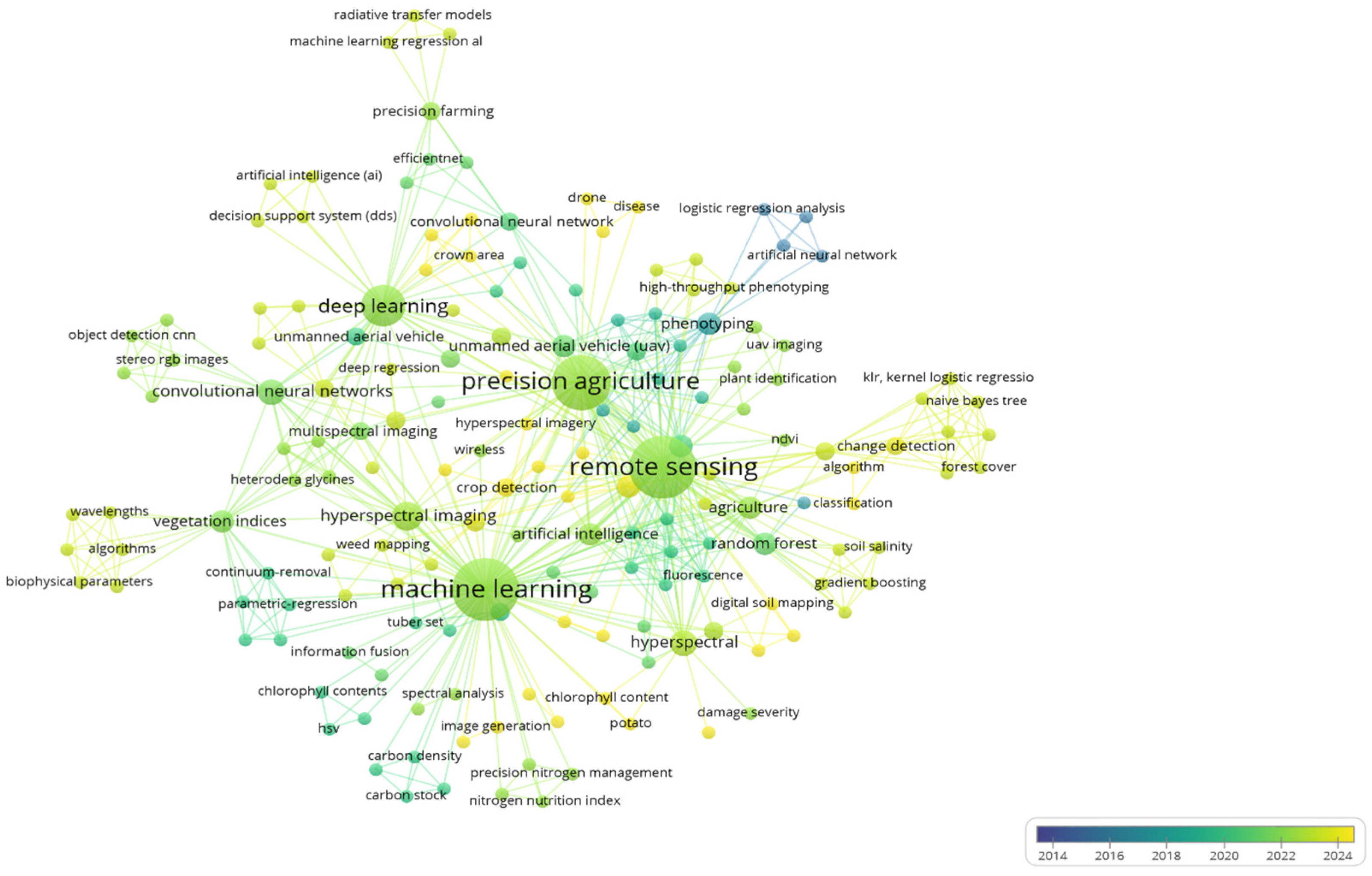
4. Methodology of Bibliometric Analysis
5. Host Resistance Breeding and Its Effectiveness Against Changing Climatic Conditions
6. Cultural Practices (Crop Rotation and Residue Management) for Disease Management
7. Chemical Control Methods
8. Biological Control Agents Used in Blackleg Management
9. Challenges and Limitations of Current IDM Strategies
10. Innovative IDM Approaches for Mitigating Climatic Changes
11. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA National Agricultural Statistics Service. Crop Production 2023 Summary; USDA: Washington, DC, USA, 2024. Available online: https://ageconsearch.umn.edu/record/350164/?v=pdf (accessed on 30 June 2025).
- Mendoza, L.E.d.R.; Nepal, A.; Markell, S. Outbreak of blackleg in canola in North Dakota is caused by new pathogenicity groups. Plant Health Prog. 2012, 13, 19. [Google Scholar]
- Wang, Y.; Strelkov, S.E.; Hwang, S.-F. Yield losses in canola in response to blackleg disease. Can. J. Plant Sci. 2020, 100, 488–494. [Google Scholar]
- Wang, Y.; Strelkov, S.E.; Hwang, S.-F. Blackleg yield losses and interactions with verticillium stripe in canola (Brassica napus) in Canada. Plants 2023, 12, 434. [Google Scholar] [CrossRef]
- Hammond, K.I.M.E.; Lewis, B.G.; Musa, T.M. A systemic pathway in the infection of oilseed rape plants by Leptosphaeria maculans. Plant Pathol. 1985, 34, 557–565. [Google Scholar]
- Hammond, K.E.; Lewis, B.G. Variation in Stem Infections Caused by Aggressive and Non-aggressive Isolates of Leptosphaeria maculans on Brassica napus var. oleifera. Plant Pathol. 1987, 36, 53–65. [Google Scholar]
- Haddadi, P.; Ma, L.; Wang, H.; Borhan, M.H. Genome-Wide Transcriptomic Analyses Provide Insights into the Lifestyle Transition and Effector Repertoire of Leptosphaeria maculans during the Colonization of Brassica napus Seedlings. Mol. Plant Pathol. 2016, 17, 1196–1210. [Google Scholar]
- Travadon, R.; Bousset, L.; Saint-Jean, S.; Brun, H.; Sache, I. Splash Dispersal of Leptosphaeria maculans Pycnidiospores and the Spread of Blackleg on Oilseed Rape. Plant Pathol. 2007, 56, 595–603. [Google Scholar]
- Bousset, L.; Sprague, S.J.; Thrall, P.H.; Barrett, L.G. Spatio-Temporal Connectivity and Host Resistance Influence Evolutionary and Epidemiological Dynamics of the Canola Pathogen Leptosphaeria maculans. Evol. Appl. 2018, 11, 1354–1370. [Google Scholar]
- Bailey, K.L.; Johnston, A.M.; Kutcher, H.R.; Gossen, B.D.; Morrall, R.A.A. Managing Crop Losses from Foliar Diseases with Fungicides, Rotation, and Tillage in the Saskatchewan Parkland. Can. J. Plant Sci. 2000, 80, 169–175. [Google Scholar]
- Van de Wouw, A.P.; Elliott, V.L.; Ware, A.; Lindbeck, K.; Howlett, B.J.; Marcroft, S.J. Infection of Canola Pods by Leptosphaeria maculans and Subsequent Seed Contamination. Eur. J. Plant Pathol. 2016, 145, 687–695. [Google Scholar]
- Kogan, F. Global Warming Impacts on Earth Systems. In Remote Sensing Land Surface Changes: The 1981–2020 Intensive Global Warming; Springer: Cham, Switzerland, 2023; pp. 21–66. [Google Scholar]
- Sosnowski, M.R.; Scott, E.S.; Ramsey, M.D. Temperature, Wetness Period and Inoculum Concentration Influence Infection of Canola (Brassica napus) by Pycnidiospores of Leptosphaeria maculans. Australia’s. Plant Pathol. 2005, 34, 339–344. [Google Scholar]
- Lindsey, R.; Dahlman, L. Climate Change: Global Temperature. Clim. Gov. 2020, 16, 1–5. [Google Scholar]
- Kelder, T.; Müller, M.; Slater, L.J.; Marjoribanks, T.I.; Wilby, R.L.; Prudhomme, C.; Bohlinger, P.; Ferranti, L.; Nipen, T. Using UNSEEN Trends to Detect Decadal Changes in 100-Year Precipitation Extremes. npj Com. Atmos. Sci. 2020, 3, 47. [Google Scholar]
- Amjadi, Z.; Hamzehzarghani, H.; Rodriguez, V.M. Studying Temperature’s Impact on Brassica napus Resistance in Order to Identify Key Regulatory Mechanisms Using Comparative Metabolomics. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Bondad, J.J.; Whish, J.P.M.; Sprague, S.J.; Maher, R.; Barry, K.M.; Harrison, M.T. Environmental and Management Determinants of Blackleg Crown Canker Disease (Leptosphaeria maculans) of Canola (Brassica napus). Australas. Plant Pathol. 2025, 54, 13–24. [Google Scholar] [CrossRef]
- Lamichhane, J.R. Rising Risks of Late-Spring Frosts in a Changing Climate. Nat. Clim. Change 2021, 11, 554–555. [Google Scholar]
- Dawidziuk, A.; Kaczmarek, J.; Jedryczka, M. The Effect of Winter Weather Conditions on the Ability of Pseudothecia of Leptosphaeria maculans and L. biglobosa to Release Ascospores. Eur. J. Plant Pathol. 2012, 134, 329–343. [Google Scholar]
- Hubbard, M.; Peng, G. Quantitative Resistance against an Isolate of Leptosphaeria maculans (Blackleg) in Selected Canadian Canola Cultivars Remains Effective under Increased Temperatures. Plant Pathol. 2018, 67, 1329–1338. [Google Scholar]
- Berghuis, B. Managing Economically Important Diseases of Sunflower and Oilseed Rape in North Dakota, California, and Schleswig-Holstein. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 2021. [Google Scholar]
- Fernandez, J. Phenotypic Characterization of the Pathogenicity Groups of Leptosphaeria maculans on Brassica napus. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2015. [Google Scholar]
- Van de Wouw, A.P.; Marcroft, S.J.; Sprague, S.J.; Scanlan, J.L.; Vesk, P.A.; Idnurm, A. Epidemiology and Management of Blackleg of Canola in Response to Changing Farming Practices in Australia. Australas. Plant Pathol. 2021, 50, 137–149. [Google Scholar] [CrossRef]
- Khangura, R.; MacLeod, W.J. Yield Losses from Blackleg (Leptosphaeria maculans) in Canola Varieties under Moderate Disease Pressure. In Proceedings of the 17th Australian Research Assembly on Brassicas (ARAB), Wagga, Australia, 15–17 August 2011; p. 89. [Google Scholar]
- Dolatabadian, A.; Cornelsen, J.; Huang, S.; Zou, Z.; Fernando, W.G.D. Sustainability on the Farm: Breeding for Resistance and Management of Major Canola Diseases in Canada Contributing towards an IPM Approach. Can. J. Plant Pathol. 2022, 44, 157–190. [Google Scholar] [CrossRef]
- Zhao, L.; Harding, M.W.; Peng, G.; Lange, R.; Walkowiak, S.; Fernando, W.G.D. Artificial Intelligence Analysis of Contributive Factors in Determining Blackleg Disease Severity in Canola Farmlands. Can. J. Plant Pathol. 2024, 46, 114–127. [Google Scholar] [CrossRef]
- Peng, G.; Liu, C.; Fernando, W.G.D.; Lang, R.; McLaren, D.L.; Johnson, E.N.; Kutcher, H.R.; Singh, G.; Turkington, T.K.; Yu, F. Early Fungicide Treatment Reduces Blackleg on Canola, but Yield Benefit Is Realized Only on Susceptible Cultivars under High Disease Pressure. Can. J. Plant Pathol. 2021, 43, 384–393. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sain, S.K.; Singh, P.S. A Perspective on Integrated Disease Management in Agriculture. Biol. Bull. 2016, 2, 79–85. [Google Scholar]
- Hossard, L.; Souchère, V.; Jeuffroy, M.H. Effectiveness of Field Isolation Distance, Tillage Practice, Cultivar Type and Crop Rotations in Controlling Phoma Stem Canker on Oilseed Rape. Agric. Ecosyst. Environ. 2018, 268, 48–57. [Google Scholar]
- Reddy, N.K.; Rakhonde, G.; Purushotham, P.; Patel, P.S.; Ahale, S. Impact of Climate Change on Forage Crop Production with Special Emphasis on Diseases and Mitigation Strategies through Breeding and Molecular Approaches. In Molecular Interventions for Developing Climate-Smart Crops: A Forage Perspective; Springer: Singapore, 2023; pp. 75–97. [Google Scholar] [CrossRef]
- Brun, H.; Chèvre, A.M.; Fitt, B.D.L.; Powers, S.; Besnard, A.L.; Ermel, M.; Huteau, V.; Marquer, B.; Eber, F.; Renard, M.; et al. Quantitative Resistance Increases the Durability of Qualitative Resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2010, 185, 285–299. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Scanlan, J.L.; Marcroft, S.J.; Smith, A.J.; Sheedy, E.M.; Perndt, N.W.; Harrison, C.E. Fungicide Sensitivity and Resistance in the Blackleg Fungus, Leptosphaeria maculans, across Canola Growing Regions in Australia. Crop Pasture Sci. 2021, 72, 735–744. [Google Scholar]
- Guo, X. Studies on Effects of Crop Rotation and Tillage on Blackleg Disease (Leptosphaeria maculans) in Canola (Brassica napus), Dispersal Patterns of L. maculans Spores. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2004. [Google Scholar]
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and Management of Leptosphaeria maculans (Phoma Stem Canker) on Oilseed Rape in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Toscano-Underwood, C.; Huang, Y.J.; Fitt, B.D.L.; Hall, A.M. Effects of Temperature on Maturation of Pseudothecia of Leptosphaeria maculans and L. biglobosa on Oilseed Rape Stem Debris. Plant Pathol. 2003, 52, 726–736. [Google Scholar] [CrossRef]
- Guo, X.W.; Fernando, W.G.D.; Entz, M. Effects of Crop Rotation and Tillage on Blackleg Disease of Canola. Can. J. Plant Pathol. 2005, 27, 53–57. [Google Scholar] [CrossRef]
- Khangura, R.; Speijers, J.; Barbetti, M.J.; Salam, M.U.; Diggle, A.J. Epidemiology of Blackleg (Leptosphaeria maculans) of Canola (Brassica napus) in Relation to Maturation of Pseudothecia and Discharge of Ascospores in Western Australia. Phytopathology 2007, 97, 1011–1021. [Google Scholar] [CrossRef]
- Petrie, G.A. Long-Term Survival and Sporulation of Leptosphaeria maculans (Blackleg) on Naturally Infected Rapeseed/Canola Stubble in Saskatchewan. Can. Plant Dis. Surv. 1995, 75, 57–63. [Google Scholar]
- Petrie, G.A. Effects of Temperature and Moisture on the Number, Size and Septation of Ascospores Produced by Leptosphaeria maculans (Blackleg) on Rapeseed Stubble. Can. Plant Dis. Surv. 1994, 74, 57–61. [Google Scholar]
- Salam, M.U.; Khangura, R.K.; Diggle, A.J.; Barbetti, M.J. Blackleg Sporacle: A Model for Predicting Onset of Pseudothecia Maturity and Seasonal Ascospore Showers in Relation to Blackleg of Canola. Phytopathology 2003, 93, 1073–1081. [Google Scholar] [CrossRef]
- Petrie, G.A. Patterns of Ascospore Discharge by Leptosphaeria maculans (Blackleg) from 9- to 13-Month-Old Naturally Infected Rapeseed/Canola Stubble from 1977 to 1993. Can. Plant Dis. Surv. 1995, 75, 65–69. [Google Scholar]
- Wang, Y. Yield Losses and Pyraclostrobin Sensitivity in Blackleg (Leptosphaeria maculans) of Canola. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2019. [Google Scholar]
- Upadhaya, S. Developing a Blackleg Management Package for North Dakota. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2019. [Google Scholar]
- Pérès, A.; Poisson, B.; Le Sourne, V.; Maisonneuve, C. Leptosphaeria maculans: Effect of Temperature, Rainfall and Humidity on the Formation of Pseudothecia. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999; pp. 26–29. [Google Scholar]
- McGee, D.C.; Petrie, G.A. Seasonal Patterns of Ascospore Discharge by Leptosphaeria maculans in Relation to Blackleg of Oilseed Rape. Phytopathology 1979, 69, 586–589. [Google Scholar]
- Huang, Y.J.; Fitt, B.D.L.; Jedryczka, M.; Dakowska, S.; West, J.S.; Gladders, P.; Steed, J.M.; Li, Z.Q. Patterns of Ascospore Release in Relation to Phoma Stem Canker Epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa). Eur. J. Plant Pathol. 2005, 111, 263–277. [Google Scholar] [CrossRef]
- Marcroft, S.J.; Sprague, S.J.; Pymer, S.J.; Salisbury, P.A.; Howlett, B.J. Crop Isolation, Not Extended Rotation Length, Reduces Blackleg (Leptosphaeria maculans) Severity of Canola (Brassica napus) in South-Eastern Australia. Aust. J. Exp. Agric. 2004, 44, 601–606. [Google Scholar]
- Travadon, R.; Bousset, L.; Saint-Jean, S.; Brun, H.; Sache, I. Rain-Splash Is an Effective Mechanism of Dispersal of Blackleg (Leptosphaeria maculans) Pycnidiospores. In Proceedings of the 12th International Rapeseed Congress, Wuhan, China, 26–30 March 2007. [Google Scholar]
- Kharbanda, P.D.; Tewari, J.P. Integrated Management of Canola Diseases Using Cultural Methods. Can. J. Plant Pathol. 1996, 18, 168–175. [Google Scholar] [CrossRef]
- Zhang, X.; Fernando, W.G.D. Insights into Fighting against Blackleg Disease of Brassica napus in Canada. Crop Pasture Sci. 2017, 69, 40–47. [Google Scholar]
- Lane, A.; Jarvis, A. Changes in Climate Will Modify the Geography of Crop Suitability: Agricultural Biodiversity Can Help with Adaptation; Bioversity International: Rome, Italy, 2007. [Google Scholar]
- He, Y.; Wang, H.; Qian, B.; McConkey, B.; Cutforth, H.; Lemke, R.; DePauw, R.; Brandt, K.; McCaig, T.; Hu, K.; et al. Effects of Climate Change on Killing Frost in the Canadian Prairies. Clim. Change 2012, 54, 221–231. [Google Scholar] [CrossRef]
- Komluski, J.; Habig, M.; Stukenbrock, E.H. Repeat-Induced Point Mutation and Gene Conversion Coinciding with Heterochromatin Shape the Genome of a Plant-Pathogenic Fungus. mBio 2023, 14, e03290-22. [Google Scholar] [CrossRef]
- Proano, C. Analysis of the Leptosphaeria maculans Race Structure and Identification of Major-Gene Resistance to Black Leg in Winter Canola. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2015. [Google Scholar]
- Soomro, W. Characterizing Avr Genes of Leptosphaeria maculans and Resistance Responses among Canadian Canola Cultivars in Western Canada. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2016. [Google Scholar]
- Sprague, S.J.; Marcroft, S.J.; Hayden, H.L.; Howlett, B.J. Major Gene Resistance to Blackleg in Brassica napus Overcome Within Three Years of Commercial Production in Southeastern Australia. Plant Dis. 2006, 90, 190–198. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, G.; Kutcher, H.R.; Balesdent, M.H.; Delourme, R.; Fernando, W.G.D. Breakdown of Rlm3 Resistance in the Brassica napus–Leptosphaeria maculans Pathosystem in Western Canada. Eur. J. Plant Pathol. 2016, 145, 659–674. [Google Scholar] [CrossRef]
- Rimmer, S.R. Resistance Genes to Leptosphaeria maculans in Brassica napus. Can. J. Plant Pathol. 2006, 28, S288–S297. [Google Scholar]
- Cantila, A.Y.; Saad, N.S.M.; Amas, J.C.; Edwards, D.; Batley, J. Recent Findings Unravel Genes and Genetic Factors Underlying Leptosphaeria maculans Resistance in Brassica napus and Its Relatives. Int. J. Mol. Sci. 2020, 21, 5247. [Google Scholar]
- Delourme, R.; Chèvre, A.M.; Brun, H.; Rouxel, T.; Balesdent, M.H.; Dias, J.S.; Salisbury, P.; Renard, M.; Rimmer, S.R. Major Gene and Polygenic Resistance to Leptosphaeria maculans in Oilseed Rape (Brassica napus). In Sustainable Strategies for Managing Brassica napus Resistance; Springer: Dordrecht, The Netherlands, 2006; pp. 41–52. [Google Scholar] [CrossRef]
- Zhu, B.; Rimmer, S.R. Inheritance of Resistance to Leptosphaeria maculans in Two Accessions of Brassica napus. Can. J. Plant Pathol. 2003, 25, 98–103. [Google Scholar] [CrossRef]
- Ansan-Melayah, D.; Balesdent, M.H.; Delourme, R.; Pilet, M.L.; Tanguy, X.; Renard, M.; Rouxel, T. Genes for Race-Specific Resistance Against Blackleg Disease in Brassica napus L. Plant Breed. 1998, 117, 373–378. [Google Scholar] [CrossRef]
- Mayerhofer, R.; Bansal, V.K.; Thiagarajah, M.R.; Stringam, G.R.; Good, A.G. Molecular Mapping of Resistance to Leptosphaeria maculans in Australian Cultivars of Brassica napus. Genome 1997, 40, 294–301. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Rimmer, S.R.; Williams, P.H.; Osborn, T.C. Mapping Loci Controlling Brassica napus Resistance to Leptosphaeria maculans Under Different Screening Conditions. Phytopathology 1995, 85, 213–217. [Google Scholar]
- Leflon, M. The Dispensable Chromosome of Leptosphaeria maculans Shelters an Effector Gene Conferring Avirulence Towards Brassica rapa. Master’s Thesis, INRA, Versailles, France, 2013. [Google Scholar]
- Balesdent, M.H.; Attard, A.; Kühn, M.L.; Rouxel, T. New Avirulence Genes in the Phytopathogenic Fungus Leptosphaeria maculans. Phytopathology 2002, 92, 1122–1133. [Google Scholar] [CrossRef]
- Chèvre, A.M.; Barret, P.; Eber, F.; Dupuy, P.; Brun, H.; Tanguy, X.; Renard, M. Selection of Stable Brassica napus–B. juncea Recombinant Lines Resistant to Blackleg (Leptosphaeria maculans). 1. Identification of Molecular Markers, Chromosomal. Theor. Appl. Genet. 1997, 95, 1104–1111. [Google Scholar] [CrossRef]
- Eber, F.; Lourgant, K.; Brun, H.; Lode, M.; Huteau, V.; Coriton, O.; Alix, K.; Balesdent, M.; Chèvre, A.M. Analysis of Brassica nigra Chromosomes Allows Identification of a New Effective Leptosphaeria maculans Resistance Gene Introgressed in Brassica napus. In Proceedings of the 13th International Rapeseed Congress, Prague, Czech Republic, 5–9 June 2011; pp. 5–9. [Google Scholar]
- Yu, F.; Lydiate, D.J.; Rimmer, S.R. Identification of Two Novel Genes for Blackleg Resistance in Brassica napus. Theor. Appl. Genet. 2005, 110, 969–979. [Google Scholar] [CrossRef]
- Yu, F.; Gugel, R.K.; Kutcher, H.R.; Peng, G.; Rimmer, S.R. Identification and Mapping of a Novel Blackleg Resistance Locus LepR4 in the Progenies from Brassica napus × B. rapa subsp. sylvestris. Theor. Appl. Genet. 2013, 126, 307–315. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Marcroft, S.J.; Barbetti, M.J.; Hua, L.; Salisbury, P.A.; Gout, L.; Rouxel, T.; Howlett, B.J.; Balesdent, M.H. Dual Control of Avirulence in Leptosphaeria maculans Towards a Brassica napus Cultivar with “sylvestris-derived” Resistance Suggests Involvement of Two Resistance Genes. Plant Pathol. 2009, 58, 305–313. [Google Scholar] [CrossRef]
- Yu, F.; Lydiate, D.J.; Rimmer, S.R. Identification and Mapping of a Third Blackleg Resistance Locus in Brassica napus Derived from B. rapa subsp. sylvestris. Genome 2008, 51, 64–72. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Z.; Sun, Z.; Fernando, D.W.G.; McVetty, P.B.E.; Li, G. Identification of Two Blackleg Resistance Genes and Fine Mapping of One of These Two Genes in a Brassica napus Canola Cultivar “Surpass 400”. Theor. Appl. Genet. 2011, 122, 1223–1231. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Lowe, R.G.T.; Elliott, C.E.; Dubois, D.J.; Howlett, B.J. An Avirulence Gene, AvrLmJ1, from the Blackleg Fungus, Leptosphaeria maculans, Confers Avirulence to Brassica juncea Cultivars. Mol. Plant Pathol. 2014, 15, 523–530. [Google Scholar] [CrossRef]
- Parlange, F.; Daverdin, G.; Fudal, I.; Kuhn, M.L.; Balesdent, M.H.; Blaise, F.; Grezes-Besset, B.; Rouxel, T. Leptosphaeria maculans Avirulence Gene AvrLm4-7 Confers a Dual Recognition Specificity by the Rlm4 and Rlm7 Resistance Genes of Oilseed Rape, and Circumvents Their Combined Resistance Specificities. Mol. Microbiol. 2009, 71, 851–863. [Google Scholar] [CrossRef]
- Fudal, I.; Ross, S.; Gout, L.; Blaise, F.; Kuhn, M.L.; Eckert, M.R.; Cattolico, L.; Bernard-Samain, S.; Balesdent, M.H.; Rouxel, T. Heterochromatin-Like Regions as Ecological Niches for Avirulence Genes in the Leptosphaeria maculans Genome: Map-Based Cloning of AvrLm6. Mol. Plant Microbe Interact. 2007, 20, 459–470. [Google Scholar] [CrossRef]
- Balesdent, M.H.; Fudal, I.; Ollivier, B.; Bally, P.; Grandaubert, J.; Eber, F.; Chèvre, A.M.; Leflon, M.; Rouxel, T. The Dispensable Chromosome of Leptosphaeria maculans Shelters an Effector Gene Conferring Avirulence Towards Brassica rapa. New Phytol. 2013, 198, 887–898. [Google Scholar] [CrossRef]
- Plissonneau, C.; Daverdin, G.; Ollivier, B.; Blaise, F.; Degrave, A.; Fudal, I.; Rouxel, T.; Balesdent, M.H. A Game of Hide and Seek between Avirulence Genes AvrLm4-7 and AvrLm3 in Leptosphaeria maculans. New Phytol. 2016, 209, 1613–1624. [Google Scholar] [CrossRef]
- Ghanbarnia, K.; Fudal, I.; Larkan, N.J.; Links, M.G.; Balesdent, M.H.; Profotova, B.; Fernando, W.G.D.; Rouxel, T.; Borhan, M.H. Rapid Identification of the Leptosphaeria maculans Avirulence Gene AvrLm2 Using an Intraspecific Comparative Genomics Approach. Mol. Plant Pathol. 2015, 16, 699–709. [Google Scholar] [CrossRef]
- Gout, L.; Fudal, I.; Kuhn, M.L.; Blaise, F.; Eckert, M.; Cattolico, L.; Balesdent, M.H.; Rouxel, T. Lost in the Middle of Nowhere: The AvrLm1 Avirulence Gene of the Dothideomycete Leptosphaeria maculans. Mol. Microbiol. 2006, 60, 67–80. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Zhang, X.; Selin, C.; Zou, Z.; Liban, S.H.; McLaren, D.L.; Kubinec, A.; Parks, P.S.; Rashid, M.H.; Padmathilake, K.R.E.; et al. A Six-Year Investigation of the Dynamics of Avirulence Allele Profiles, Blackleg Incidence, and Mating Type Alleles of Leptosphaeria maculans Populations. Plant Dis. 2018, 102, 790–798. [Google Scholar] [CrossRef]
- Noel, K.; Wolf, I.R.; Hughes, D.; Valente, G.T.; Qi, A.; Huang, Y.-J.; Fitt, B.D.L.; Stotz, H.U. Transcriptomics of Temperature-Sensitive R Gene-Mediated Resistance Identifies a WAKL10 Protein Interaction Network. Sci. Rep. 2024, 14, 5023. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, X.; Feng, B.; Sheen, J.; Shan, L.; He, P. Plant Immune Response to Pathogens Differs with Changing Temperatures. Nat. Commun. 2013, 4, 2530. [Google Scholar] [CrossRef]
- Erickson, F.L.; Holzberg, S.; Calderon-Urrea, A.; Handley, V.; Axtell, M.; Corr, C.; Baker, B. The Helicase Domain of the TMV Replicase Proteins Induces the N-Mediated Defence Response in Tobacco. Plant J. 1999, 18, 67–75. [Google Scholar] [CrossRef]
- Yang, C. The Regulation of Intrinsic Signaling in Brassica napus Defending Against Leptosphaeria maculans. Ph.D. Thesis, The University of Manitoba, Winnipeg, MB, Canada, 2021. [Google Scholar]
- Chen, S.; Zhang, W.; Bolus, S.; Rouse, M.N.; Dubcovsky, J. Identification and Characterization of Wheat Stem Rust Resistance Gene Sr21 Effective against the Ug99 Race Group at High Temperature. PLoS Genet. 2018, 14, e1007287. [Google Scholar] [CrossRef]
- Huang, Y.J.; Pirie, E.J.; Evans, N.; Delourme, R.; King, G.J.; Fitt, B.D.L. Quantitative Resistance to Symptomless Growth of Leptosphaeria maculans (Phoma Stem Canker) in Brassica napus (Oilseed Rape). Plant Pathol. 2009, 58, 314–323. [Google Scholar] [CrossRef]
- Vasquez-Teuber, P.; Rouxel, T.; Mason, A.S.; Soyer, J.L. Breeding and Management of Major Resistance Genes to Stem Canker/Blackleg in Brassica Crops. Theor. Appl. Genet. 2024, 137, 192. [Google Scholar] [CrossRef]
- Kutcher, H.R.; Brandt, S.A.; Smith, E.G.; Ulrich, D.; Malhi, S.S.; Johnston, A.M. Blackleg Disease of Canola Mitigated by Resistant Cultivars and Four-Year Crop Rotations in Western Canada. Can. J. Plant Pathol. 2013, 35, 209–221. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Yu, Z.; Shi, X.; Zhou, X.; Wang, P.; Song, Y. Pyramiding of Multiple Genes Generates Rapeseed Introgression Lines with Clubroot and Herbicide Resistance, High Oleic Acid Content, and Early Maturity. Crop J. 2023, 11, 895–903. [Google Scholar]
- Lin, L.; Zhang, X.; Fan, J.; Li, J.; Ren, S.; Gu, X.; Wu, J. Natural Variation in BnaA07.MKK9 Confers Resistance to Sclerotinia Stem Rot in Oilseed Rape. Nat. Commun. 2024, 15, 5059. [Google Scholar]
- Balesdent, M.H.; Gautier, A.; Plissonneau, C.; Le Meur, L.; Loiseau, A.; Leflon, M.; Rouxel, T. Twenty Years of Leptosphaeria maculans Population Survey in France Suggests Pyramiding Rlm3 and Rlm7 in Rapeseed Is a Risky Resistance Management Strategy. Phytopathology 2022, 112, 2126–2137. [Google Scholar]
- Van de Wouw, A.P.; Howlett, B.J.; Idnurm, A. Changes in Allele Frequencies of Avirulence Genes in the Blackleg Fungus, Leptosphaeria maculans, over Two Decades in Australia. Crop Pasture Sci. 2017, 69, 20–29. [Google Scholar]
- Sosnowski, M.R.; Scott, E.S.; Ramsey, M.D. Survival of Leptosphaeria maculans in Soil on Residues of Brassica napus in South Australia. Plant Pathol. 2006, 55, 200–206. [Google Scholar] [CrossRef]
- Hegewald, H.; Wensch-Dorendorf, M.; Stachow, U.; Scholten, T.; Schmidt, K. Impacts of Break Crops and Crop Rotations on Oilseed Rape Productivity: A Review. Eur. J. Agron. 2018, 94, 31–42. [Google Scholar]
- Van de Wouw, A.P.; Marcroft, S.J.; Ware, A.; Lindbeck, K.; Khangura, R.; Howlett, B.J. Breakdown of Resistance to the Fungal Disease Blackleg Is Averted in Commercial Canola (Brassica napus) Crops in Australia. Field Crop Res. 2014, 166, 144–151. [Google Scholar]
- Cornelsen, J.; Zou, Z.; Huang, S.; Parks, P.; Lange, R.; Peng, G.; Fernando, W.G.D. Validating the Strategic Deployment of Blackleg Resistance Gene Groups in Commercial Canola Fields on the Canadian Prairies. Front. Plant Sci. 2021, 12, 669997. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Cozijnsen, A.J.; Hane, J.K.; Brunner, P.C.; McDonald, B.A.; Oliver, R.P.; Howlett, B.J. Evolution of Linked Avirulence Effectors in Leptosphaeria maculans Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants. PLoS Pathog. 2010, 6, e1001180. [Google Scholar] [CrossRef]
- Marcroft, S.J.; Van de Wouw, A.P.; Salisbury, P.A.; Potter, T.D.; Howlett, B.J. Effect of Rotation of Canola (Brassica napus) Cultivars with Different Complements of Blackleg Resistance Genes on Disease Severity. Plant Pathol. 2012, 61, 934–944. [Google Scholar] [CrossRef]
- Sprague, S.J.; Balesdent, M.H.; Brun, H.; Hayden, H.L.; Marcroft, S.J.; Pinochet, X.; Rouxel, T.; Howlett, B.J. Major Gene Resistance in Brassica napus (Oilseed Rape) Is Overcome by Changes in Virulence of Populations of Leptosphaeria maculans in France and Australia. In Sustainable Strategies for Managing Brassica napus Resistance; Springer: Dordrecht, The Netherlands, 2006; pp. 33–40. [Google Scholar] [CrossRef]
- Crété, R.; Pires, R.N.; Barbetti, M.J.; Renton, M. Rotating and Stacking Genes Can Improve Crop Resistance Durability While Potentially Selecting Highly Virulent Pathogen Strains. Sci. Rep. 2020, 10, 19855. [Google Scholar] [CrossRef]
- Janowski, K. Understanding the Epidemiology and Genetic Diversity of Leptosphaeria maculans, and Exploring Chemical Control Strategies to Manage Blackleg of Winter Canola. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2021. [Google Scholar]
- Gugel, R.K.; Petrie, G.A. History, Occurrence, Impact, and Control of Blackleg of Rapeseed. Can. J. Plant Pathol. 1992, 14, 36–45. [Google Scholar]
- Rempel, C.B.; Hall, R. Effects of Time and Rate of Application of Triazole Fungicides on Incidence and Severity of Blackleg and Growth and Yield of Canola. Can. J. Plant Sci. 1995, 75, 737–743. [Google Scholar] [CrossRef]
- Marcroft, S.J.; Sosnowski, M.R.; Scott, E.S.; Ramsey, M.D.; Salisbury, P.A.; Howlett, B.J. Brassica napus Plants Infected by Leptosphaeria maculans after the Third to Fifth Leaf Growth Stage in South-Eastern Australia Do Not Develop Blackleg Stem Canker. Eur. J. Plant Pathol. 2005, 112, 289–292. [Google Scholar] [CrossRef]
- Peng, G.; Liu, X.; McLaren, D.L.; McGregor, L.; Yu, F. Seed Treatment with the Fungicide Fluopyram Limits Cotyledon Infection by Leptosphaeria maculans and Reduces Blackleg of Canola. Can. J. Plant Pathol. 2020, 42, 480–492. [Google Scholar] [CrossRef]
- Hall, B. South Australia Research & Development Institute (SARDI). Government of South Australia: Adelaide, Australia, 2012. [Google Scholar]
- Khangura, R.K.; Barbetti, M.J. Time of Sowing and Fungicides Affect Blackleg (Leptosphaeria maculans) Severity and Yield in Canola. Aust. J. Exp. Agric. 2005, 45, 1291–1297. [Google Scholar]
- Xi, K.; Kutcher, H.R.; Westcott, N.D.; Morrall, R.A.A.; Rimmer, S.R. Effect of Seed Treatment and Fertilizer Coated with Flutriafol on Blackleg of Canola (Oilseed Rape) in Western Canada. Can. J. Plant Pathol. 1991, 13, 336–346. [Google Scholar]
- Upadhaya, S.G.; Chapara, V.; Rahman, M.; del Río Mendoza, L.E. Efficacy of Fungicide Seed Treatments in Controlling Blackleg of Canola. Plant Health Prog. 2019, 20, 160–164. [Google Scholar] [CrossRef]
- Rasanie, K.; Padmathilake, E. Investigation of Mechanisms Underlying Blackleg Mitigation in Canola Through Three Novel Integrated Pest Management Approaches. Master’s Thesis, University of North Dakota, Grand Forks, ND, USA, 2022. [Google Scholar]
- Eckert, M.R.; Rossall, S.; Selley, A.; Fitt, B.D.L. Effects of Fungicides on in vitro Spore Germination and Mycelial Growth of the Phytopathogens Leptosphaeria maculans and L. biglobosa (Phoma Stem Canker). Pest Manag. Sci. 2010, 66, 396–405. [Google Scholar] [CrossRef]
- Liu, C. Evaluation of Fungicides for Management of Blackleg Disease on Canola and QoI-Fungicide Resistance in Leptosphaeria maculans in Western Canada. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2014. [Google Scholar]
- Del Río Mendoza, L.E.; Ruud, S.; Mansouripour, S. Timing of Fungicide Applications Is Critical for Blackleg Control in Canola. In Proceedings of the APS Annual Meeting, Tampa, FL, USA, 30 July–3 August 2016; Amer Phytopathological Soc.: St. Paul, MN, USA, 2016; Volume 106, p. 6. [Google Scholar]
- Hanif, S.E. Efficacy and Mechanisms of Bacterial Biocontrol Agents against Leptosphaeria maculans (Desm.) Causing Blackleg Disease of Canola (Brassica napus L.). Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2021. [Google Scholar]
- Yang, Y.; Marcroft, S.J.; Forsyth, L.M.; Zhao, J.; Li, Z.; Van de Wouw, A.P.; Idnurm, A. Sterol Demethylation Inhibitor Fungicide Resistance in Leptosphaeria maculans Is Caused by Modifications in the Regulatory Region of ERG11. Plant Dis. 2020, 104, 1280–1290. [Google Scholar] [CrossRef]
- Ghazanfar, M.U.; Raza, M.; Raza, W.; Qamar, M.I. Trichoderma as Potential Biocontrol Agent, Its Exploitation in Agriculture: A Review. Plant Prot. 2018, 2, 1–7. [Google Scholar]
- Ramarathnam, R.; Fernando, W.G.D.; Rajesh, R.; Fernando, W.G.D. Preliminary Phenotypic and Molecular Screening for Potential Bacterial Biocontrol Agents of Leptosphaeria maculans, the Blackleg Pathogen of Canola. Biocontrol Sci. Technol. 2006, 16, 567–582. [Google Scholar] [CrossRef]
- Jamalzadeh, A.; Darvishnia, M.; Khodakaramian, G.; Zafari, D.; Bazgir, E. Effect of Antagonistic Bacteria Associated with Canola on Disease Suppression. Eur. J. Plant Pathol. 2023, 165, 649–663. [Google Scholar] [CrossRef]
- Shah, U.A.; Kotta-Loizou, I.; Fitt, B.D.L.; Coutts, R.H.A. Mycovirus-Induced Hypervirulence of Leptosphaeria biglobosa Enhances Systemic Acquired Resistance to Leptosphaeria maculans in Brassica napus. Mol. Plant-Microbe Interact. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Chen, Y.; Fernando, W.G.D. Prevalence of Pathogenicity Groups of Leptosphaeria maculans in Western Canada and North Dakota, USA. Can. J. Plant Pathol. 2006, 28, 533–539. [Google Scholar] [CrossRef]
- Potlakayala, S.D.; Reed, D.W.; Covello, P.S.; Fobert, P.R. Systemic Acquired Resistance in Canola Is Linked with Pathogenesis-Related Gene Expression and Requires Salicylic Acid. Phytopathology 2007, 97, 794–802. [Google Scholar] [CrossRef]
- Kakraliya, S.; Abrol, S.; Choskit, D.; Pandit, D. Integrated Disease Management in Agriculture. Just Agric. 2020, 1, 1–5. [Google Scholar]
- Ishii, H. Impact of Fungicide Resistance in Plant Pathogens on Crop Disease Control and Agricultural Environment. Jpn. Agric. Res. Q. 2006, 40, 205–211. [Google Scholar]
- Amaradasa, B.S.; Everhart, S.E. Effects of Sublethal Fungicides on Mutation Rates and Genomic Variation in Fungal Plant Pathogen, Sclerotinia sclerotiorum. PLoS ONE 2016, 11, e0168079. [Google Scholar] [CrossRef]
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide Resistance: Progress in Understanding Mechanism, Monitoring, and Management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Gisi, U.; Sierotzki, H. Molecular and Genetic Aspects of Fungicide Resistance in Plant Pathogens. 2008. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20153030376 (accessed on 3 July 2025).
- Damicone, J.; Smith, D. Fungicide Resistance Management. In Oklahoma Cooperative Extension Fact Sheets; Oklahoma State University: Stillwater, OK, USA, 2009. [Google Scholar]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and Transgenerational Effects of Endocrine Disrupting Chemicals: A Role for Altered Epigenetic Regulation? Semin. Cell Dev. Biol. 2015, 43, 66–75. [Google Scholar] [PubMed]
- Gerage, J.M.; Meira, A.P.G.; da Silva, M.V. Food and Nutrition Security: Pesticide Residues in Food. Nutrire 2017, 42, 1. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.A.; Iglesias, V.P.; Muñoz, M.P.; Cornejo, C.A.; Achu, E.; Baumert, B.; Hanchey, A.; Concha, C.; Brito, A.M.; et al. Chronic Exposure to Organophosphate (OP) Pesticides and Neuropsychological Functioning in Farm Workers: A Review. Int. J. Occup. Environ. Health 2016, 22, 68–79. [Google Scholar] [CrossRef]
- Rhodes, L.A.; McCarl, B.A. An Analysis of Climate Impacts on Herbicide, Insecticide, and Fungicide Expenditures. Agronomy 2020, 10, 745. [Google Scholar] [CrossRef]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop Pests and Pathogens Move Polewards in a Warming World. Nat. Clim. Change 2013, 3, 985–988. [Google Scholar]
- Ladányi, M.; Horváth, L. A Review of the Potential Climate Change Impact on Insect Populations-General and Agricultural Aspects. Appl. Ecol. Environ. Res. 2010, 8, 143–152. [Google Scholar]
- Ziska, L.H. Increasing Minimum Daily Temperatures Are Associated with Enhanced Pesticide Use in Cultivated Soybean along a Latitudinal Gradient in the Mid-Western United States. PLoS ONE 2014, 9, e98516. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar]
- Bustos, N.; Cruz-Alcalde, A.; Iriel, A.; Cirelli, A.F.; Sans, C. Sunlight and UVC-254 Irradiation Induced Photodegradation of Organophosphorus Pesticide Dichlorvos in Aqueous Matrices. Sci. Total Environ. 2019, 649, 592–600. [Google Scholar]
- Savary, S. Plant Health and Food Security. J. Plant Pathol. 2020, 102, 605–607. [Google Scholar]
- Donatelli, M.; Magarey, R.; Bregaglio, S.; Willems, L. Modelling the Impacts of Pests and Diseases on Agricultural Systems. Agric. Syst. 2017, 153, 109–125. [Google Scholar]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier: Burlington, MA, USA, 2005; ISBN 0080473784. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar]
- Rossi, V.; Caffi, T.; Salinari, F. Helping Farmers Face the Increasing Complexity of Decision-Making for Crop Protection. Phytopathol. Mediterr. 2012, 51, 457–479. [Google Scholar]
- Singh, K.P.; Aravind, T.; Srivastava, A.K.; Karibasappa, C.S. Decision-Making Tools for Integrated Disease Management. In Emerging Trends in Plant Pathology; Gaur, R.K., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 703–724. [Google Scholar] [CrossRef]
- Gent, D.H.; Mahaffee, W.F.; McRoberts, N.; Pfender, W.F. The Use and Role of Predictive Systems in Disease Management. Annu. Rev. Phytopathol. 2013, 51, 267–289. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar]
- Sharma, A.; Jain, A.; Gupta, P.; Chowdary, V.M. Machine Learning Applications for Precision Agriculture: A Comprehensive Review. IEEE Access 2020, 8, 69714–69737. [Google Scholar]
- Kukar, M.; Vračar, P.; Košir, D.; Pevec, D.; Bosnić, Z. AgroDSS: A Decision Support System for Agriculture and Farming. Comput. Electron. Agric. 2019, 157, 105–123. [Google Scholar]
- Mahlein, A.K.; Oerke, E.C.; Steiner, U.; Dehne, H.W. Recent Advances in Sensing Plant Diseases for Precision Crop Protection. Eur. J. Plant Pathol. 2012, 133, 197–209. [Google Scholar] [CrossRef]
- Baranowski, P.; Jedryczka, M.; Mazurek, W.; Babula-Skowronska, D.; Siedliska, A.; Kaczmarek, J. Hyperspectral and Thermal Imaging of Oilseed Rape (Brassica napus) Response to Fungal Species of the Genus Alternaria. PLoS ONE 2015, 10, e0122913. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring Plant Diseases and Pests through Remote Sensing Technology: A Review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Ali, Z.; Deng, D.; Shaikh, M.K.; Hasan, R.; Khan, M.A. AI-Based UAV Swarms for Monitoring and Disease Identification of Brassica Plants Using Machine Learning: A Review. Comput. Syst. Sci. Eng. 2024, 45, 963–973. [Google Scholar]
- Singh, K.D.; Duddu, H.S.N.; Vail, S.; Parkin, I.; Shirtliffe, S.J. UAV-Based Hyperspectral Imaging Technique to Estimate Canola (Brassica napus L.) Seedpods Maturity. Can. J. Remote Sens. 2021, 47, 33–47. [Google Scholar] [CrossRef]
- Shaikh, T.A.; Rasool, T.; Lone, F.R. Towards Leveraging the Role of Machine Learning and Artificial Intelligence in Precision Agriculture and Smart Farming. Comput. Electron. Agric. 2022, 193, 106715. [Google Scholar]
- Jha, G.K.; Ranjan, P.; Gaur, M. A Machine Learning Approach to Recommend Suitable Crops and Fertilizers for Agriculture. In Recommender System with Machine Learning and Artificial Intelligence; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 89–99. [Google Scholar] [CrossRef]
- Attri, I.; Awasthi, L.K.; Sharma, T.P. Machine Learning in Agriculture: A Review of Crop Management Applications. Multimed. Tools Appl. 2024, 83, 12875–12915. [Google Scholar] [CrossRef]
- Jedryczka, M.; Kaczmarek, J.; Karolewski, Z.; Olszak-Przybyś, H.; Wacławczyk, J.; Przewodowski, W. Monitoring of Leptosphaeria maculans and L. biglobosa Ascospores around East Sudethian Mountains a Joint Initiative of Poland and the Czech Republic. Res. Proj. Rothamsted Repos. 2010, 31, 49–66. [Google Scholar]
- Duarte, P.A.; Menze, L.; Abdelrasoul, G.N.; Yosinski, S.; Kobos, Z.; Stuermer, R.; Reed, M.; Yang, J.; Li, X.S.; Chen, J. Single Ascospore Detection for the Forecasting of Sclerotinia Stem Rot of Canola. Lab Chip 2020, 20, 3644–3652. [Google Scholar] [CrossRef]
- Twengström, E.; Sigvald, R.; Svensson, C.; Yuen, J. Forecasting Sclerotinia Stem Rot in Spring Sown Oilseed Rape. Crop Prot. 1998, 17, 491–498. [Google Scholar] [CrossRef]
- Mbega, E.R.; Kaijage, S.F. Potential of Mobile-Based Apps and Online Platforms in Fast-Tracking Access of Agriculture Information. ICT Agric. Dev. 2021, 8, 76–85. [Google Scholar]





| Author | Title | Key Strategies Studied | Main Findings |
|---|---|---|---|
| Kutcher et al., 2013 [89] | Blackleg disease of canola mitigated by resistant cultivars and four-year crop rotations in western Canada | Crop rotation (2 to 4 years) + resistant cultivars (Rlm3, RlmS) | 4-year rotation + resistant cultivars showed synergistic reduction in blackleg severity |
| Marcroft et al., 2012 [99] | Effect of rotation of canola cultivars with different complements of blackleg resistance genes on disease severity | Rotation of R genes (Rlm1, Rlm4, Rlm6) in different cultivars | Rotating resistance genes reduced selection pressure and delayed pathogen adaptation |
| Sprague et al., 2006 [100] | Major gene resistance to blackleg in Brassica napus is overcome by changes in virulence of populations of Leptosphaeria maculans | Monitoring resistance breakdown due to widespread use of R genes (Rlm1, Rlm7) | Field-level R gene breakdown observed; highlighted the need for integrated strategies |
| Crété et al., 2020 [101] | Rotating and stacking genes can improve crop resistance durability | Modeling of gene rotation, pyramiding, and mixtures | Rotation outperformed pyramiding in maintaining resistance durability against recombining pathogens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaq, K.; Del Río Mendoza, L.E.; Babakhani, B.; Azizi, A.; Razzaq, H.; Rahman, M. Integrated Management Strategies for Blackleg Disease of Canola Amidst Climate Change Challenges. J. Fungi 2025, 11, 514. https://doi.org/10.3390/jof11070514
Razzaq K, Del Río Mendoza LE, Babakhani B, Azizi A, Razzaq H, Rahman M. Integrated Management Strategies for Blackleg Disease of Canola Amidst Climate Change Challenges. Journal of Fungi. 2025; 11(7):514. https://doi.org/10.3390/jof11070514
Chicago/Turabian StyleRazzaq, Khizar, Luis E. Del Río Mendoza, Bita Babakhani, Abdolbaset Azizi, Hasnain Razzaq, and Mahfuz Rahman. 2025. "Integrated Management Strategies for Blackleg Disease of Canola Amidst Climate Change Challenges" Journal of Fungi 11, no. 7: 514. https://doi.org/10.3390/jof11070514
APA StyleRazzaq, K., Del Río Mendoza, L. E., Babakhani, B., Azizi, A., Razzaq, H., & Rahman, M. (2025). Integrated Management Strategies for Blackleg Disease of Canola Amidst Climate Change Challenges. Journal of Fungi, 11(7), 514. https://doi.org/10.3390/jof11070514







