Abstract
Mycogone perniciosa is the causative agent of wet bubble disease, which induces significant losses in the production of Agaricus bisporus, indicating the high importance of the development of novel inhibitory agents. The isolation, identification, and molecular characterization of five isolates of M. perniciosa from diseased fruit bodies of A. bisporus was done. Moreover, the study evaluated the in vitro and in situ potential of Origanum vulgare essential oil (EO) to limit M. perniciosa growth and provided chemical characterization of its volatile components. The obtained strains differed phenotypically and according to their molecular characteristics. O. vulgare EO has shown more promising antifungal activity than the commercial fungicide Prochloraz-Mn in the microatmospheric method. In the treatment of experimentally induced wet bubble disease on A. bisporus in the growing chambers with 2% of O. vulgare EO and simultaneous application of spore suspension of mycopathogen, O. vulgare EO totally inhibited the growth of M. perniciosa. Carvacrol, p-cymene, γ-terpinene, and thymol were dominant constituents of O. vulgare EO examined in this study. O. vulgare EO has shown promising potential to limit growth of M. perniciosa and should be further explored as a novel biofungicide.
1. Introduction
Mushrooms are popular as food worldwide due to their pleasant taste and health-beneficial properties associated with their intake [1]. Agaricus bisporus is among the most sought-after cultivated mushrooms. It is rich in polysaccharides, like bioactive β-glucans, and associated with numerous beneficial properties [2]. Moreover, this species is abundant in other bioactive mycoconstituents such as phenolic acids, saturated fatty acids, and β-tocopherol [3]. These bioactive molecules, along with the appealing flavor which makes them popular in culinary uses, contribute to the mushroom’s widespread popularity and growing demand for its production. Some studies provide supportive evidence for the cultivation of this species as part of urban agriculture that might provide sustainable solutions for some of the global challenges [4].
Numerous infections pose serious risks to production and quality in the regulated conditions used to cultivate A. bisporus. These consist of bacterial, fungal, and, less frequently, viral agents. The most common and harmful of these are fungal pathogens, particularly in the warm, humid environments found in mushroom-growing units. The most prevalent and economically significant pathogens are Cladobotryum species (cobweb disease), Trichoderma aggressivum (green mold), Verticillium fungicola (dry bubble), Mycogone perniciosa (causative agent of wet bubble disease), and bacterial pathogens like Pseudomonas tolaasii (bacterial blotch) [5].
One of the most common diseases of A. bisporus in mushroom farms is wet bubble disease (WBD), caused by the fungus Hypomyces perniciosus (formerly Mycogone perniciosa) [5]. M. perniciosa can easily spread from the initial source of infection by using air currents, water splashes, and phorid and sciarid flies, with its growth being stimulated by metabolites of the growing A. bisporus mushroom mycelium. It is noted that the duration from infestation to symptom expression varies from 2–3 to 12 days in most of the cases [6]. Wet bubble disease is the reason for drastic economic losses in button mushroom production, with China, for example, losing about 15–30% of mushroom yields due to this disease [7]. M. perniciosa infections are not limited to A. bisporus but can affect other species such as Pleurotus ostreatus, P. citrinopileatus, and Volvariella volvacea [8], but also found in the wild Agaricus arvensis [9].
There are different fungicides for the control of this disease. However, more recently, some studies report on the problem of resistance being developed to the available protective chemicals [7]. On the contrary, another study [8] found prochloraz-Mn and carbendazim to be effective in inhibiting the growth of M. perniciosa and highlighted the low risk for the development of resistance. However, even if the resistance is not in question, some concerns are raised in regard to the application of fungicides. For example, carbendazim interferes with the antioxidant defense system and is teratogenic, mutagenic, and aneugenic. The range of abnormalities induced by this fungicide includes hematological abnormalities, mitotic spindle deformity, endocrine disruption, embryo toxicity, infertility, and others [10].
Fungicides based on plant products have diverse beneficial properties, such as having lower resistance and being environmentally friendly [11]. EOs are especially known for their antifungal activity, with their mechanisms often being related to the interference with cell membrane or cell morphology by EO and its constituents [12]. Among the most studied antifungal EOs is Origanum vulgare. O. vulgare, oregano, is one of the most used aromatic species, both as a spice and medicinal plant, mainly due to the high content of its EO, with commercial O. vulgare being both wild collected and cultivated [13]. This oil can inhibit the growth of different fungal pathogens such as Botrytis cinerea [14] and Candida spp. [15]. The antifungal activity of O. vulgare EO could be attributed to diverse phytochemicals with strong antifungal potential that are present in the oil, such as thymol and carvacrol [16].
Despite the abundant potential of O. vulgare EO to reduce growth rates of different fungal pathogens, not much is known regarding its effect on the most common pathogen of button mushrooms, M. perniciosa. Furthermore, research on the bioactive properties of O. vulgare EO with in situ studies is at the moment scarce, highlighting a significant gap in the current knowledge. Our study aimed to determine the antifungal activity of O. vulgare EO towards different strains of M. perniciosa isolated from button mushrooms. Furthermore, we have evaluated the efficacy of the oil in the in vitro and in situ infection models. Additionally, chemical characterization of the EO was conducted.
2. Materials and Methods
2.1. Fungal Strains and Growth Media
Samples of diseased A. bisporus fruiting bodies were collected in mushroom farms from Serbia (isolates MPPS (Padinska Skela) and MPR (Ripanj)) and Bosnia and Herzegovina isolate MPS (Sarajevo). On the surface of the fruiting bodies, after 3 days of incubation in a humid chamber, a white mycelium developed. The mycelia were isolated on a medium MA (Torlak, Belgrade, Serbia) with antibiotics streptomycin (Sigma Aldrich, Saint Louis, MO, USA) in order to eliminate bacteria, and then the pure culture was isolated on PDA (Neogen, Lansig, MI, USA) [17]. The isolates were identified according to the taxonomic criteria described by Brady and Gibson [18]. The two strains that were obtained from Mushroom Experimental Station (Horst, The Netherlands) (MPH1—CBS 815,73; MPH2—CBS 322,52), are from international culture collections Centraalbureau voor Schimmelcultures (CBS, Utrecht, The Netherlands). Pure cultures of the M. perniciosa were deposited at the Fungal Collection Unit of the Institute for Biological Research “Siniša Stanković”—National Institute of the Republic of Serbia; University of Belgrade. The mycopathogens were maintained on potato dextrose agar (PDA). The cultures were stored at 4 °C and subcultured once a month [17].
2.2. DNA Extraction and RAPD-PCR Analysis
2.2.1. DNA Extraction
To produce fungal biomass for DNA extraction, the Mycogone isolates were grown for 7 days at 25 °C in stationary conditions in Erlenmeyer flasks (500 mL) containing 200 mL liquid potato dextrose yeast medium (PDY) (Torlak, Belgrade, Serbia), harvested by filtration, then washed twice with sterile water and dried at room temperature [19].
DNA extraction was carried out using the methods of Mello et al. [20] with slight modifications. Approximately 250–300 mg of tissue was crushed in the presence of liquid nitrogen and homogenized with 700 µL extraction buffer (100 mM Tris–HCl pH 8.0, 1.4 M NaCl, 20 mM EDTA, 2% CTAB, 0.2% β-mercaptoethanol, Thermo Scientific, Waltham, MA, USA). All samples were incubated at 65 °C for 1 h, centrifuged for 5 min at 13,000 rpm, and extracted with 1 volume of chloroform/isoamyl alcohol (24:1) (Acros Organics, Geel, Belgium). Nucleic acid in the supernatant was precipitated with 1.5 volumes of isopropanol (Fisher Scientific, Waltham, MA, USA) and centrifuged for 15 min at 13,000 rpm for each extraction. Samples were incubated at −20 °C for 30 min, centrifuged at 13,000 rpm for 30 min, and the pellet washed with 1 mL of 70% ethanol (Zorka, Šabac, Serbia), well dried, and resuspended in 200 µL of 1× TE buffer (10 mM Tris–HCl pH 8.0, 1 mM EDTA) at 4 °C during the night. DNA is treated with RNase (1 mg/mL) (Acros Organics, Geel, Belgium). To 100 µL of DNA solution, 5 µL of RNase was added and incubated at 37 °C for 30 min. The final DNA concentration was estimated by agarose gel electrophoresis at 1.5% agarose gel in 1× TBE buffer (45 mM Tris-borate, 1 mM EDTA, pH 8.0).
2.2.2. RAPD-PCR Analyses
For RAPD analysis [19], primers 268 (AGGCCGCTTA), 266 (CCACTCACCG), and 280 (CTGGGAGTGG) supplied by Applied Biosystems UK were used. In a total volume of 25 µL (10 ng DNA, reaction buffer 10× (Applied Biosystems UK, Warrington, UK), 2.5 mM dNTP MIX (Applied Biosystems UK), 5 μM decamer primers, 5 U/μL Ampli Taq Gold (Applied Biosystems, Warrington, UK), and 2 mM MgCl2. Amplification was performed in a Genius-Techne thermal cycler (Cambridge, UK) as follows: initial denaturation for 2 min at 94 °C, then 35 cycles of 1 min at 94 °C, 2 min at 37 °C to 20 °C (35 cycles—in each cycle temperature is lower for 0.5 °C), and 2 min at 72 °C, followed by a final extension for 5 min at 72 °C. Amplification products were separated by electrophoresis on 1.5% agarose gel in 1× TBE buffer, stained with ethidium bromide, and visualized under UV light.
2.3. GC-MS Analysis of O. vulgare Volatile Compounds
O. vulgare EO was produced by the distillation company Sanicula Co., Ltd., Paraćin, Serbia. O. vulgare EO was dissolved in absolute ethanol (1:99), and the sample was transferred to a 2 mL vial for GC-MS analysis. Analysis of volatile compounds was performed on an Agilent 8890/5977BMSD/FID GC-MS system using an Agilent (Santa Clara, CA, USA) HP-INNOWax (PEG stationary phase) column with a length of 30 cm, an internal diameter of 250 μm, and a coating thickness of 0.25 μm. From each sample, 1 μL was injected in a splitless flow mode at the injector temperature of 250 °C. Helium 5.0 was used as carrier gas at a flow rate of 0.8 mL/min. The oven temperature program included an initial temperature of 65 °C, at a rate of 3 °C/min, ramping up to 220 °C and holding for 15 min. The mass detector temperature was set at 150 °C, and the transfer line temperature was 250 °C. Ionization was achieved through electron impact (EI) at 70 eV. To establish the retention index, a mixture of C10-C40 normal alkanes (Sigma, St. Louis, MO, USA) was used in conjunction with AMDIS ver. 2.73 (National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA). The FID detector operated at a temperature of 300 °C. Compounds were identified by comparing the Kovach retention index and mass spectrum with those of standard substances and mass spectral data from the National Institute of Standards and Technology (NIST MS Search, Version 2.4) libraries, the Robert Adams library, and pertinent literature data. MSD ChemStation software (F.01.03.2357, Agilent Technologies, Inc., Santa Clara, CA, USA) was used to integrate and determine the peak areas of individual volatile compounds. The amounts of individual components were expressed as a percentage of the total chromatogram area based on data from the FID detector [21].
2.4. In Vitro Antifungal Activity
The modified microatmosphere method [22] was used for the investigation of the antifungal activity of O. vulgare EO. Petri plates measuring 50 mm were filled with 10 mL of PDA medium and then were seeded with a small amount of 7-day-old mycelium culture of the M. perniciosa strains. The Petri dishes were then inverted, and the determined amount (0.001–20 μL/mL) of pure oil impregnated on sterile filter paper discs (6 mm in diameter) was deposited on the inverted lid. Prochloraz-Mn, (((1-N-propyl-N-2(2,4,6-trichlorophenoxy) ethyl) carbomoyl imidazole + Manganese)) (“Mirage45-EC”, Makhteshim Chemical Works, Beer-Sheva, Israel) was used as a positive control (1 mg/mL; 5–>50 μL/disc). Minimal inhibitory quantities (MIQ) and minimal fungicidal quantities (MFQ) of EOs were noted upon 7 days of incubation at 25 °C.
2.5. In Situ Antifungal Activity
The experiments were carried out in the cultivation chamber in bags, 40 × 60 × 25 cm, filled with compost phase III spawned with A15 mushroom strain from a commercial mushroom compost yard (Company EKOFUNGI, Padinska skela, Serbia). The surface of the compost was covered with an 8 cm layer of black peat. The conditions in the mushroom house chamber were fully controlled for routine production; that is, the temperature was 24 °C, the carbon dioxide concentration was 3000 mg/L, and the relative humidity was 95%. After 8 days, when the mycelium reached the surface of the casing, the temperature and carbon dioxide concentrations were lowered to 18 °C and to 800 mg/L, respectively.
To treat experimentally induced WBD in situ, we tested the antifungal activity of O. vulgare EO when applied in casing soil in different ways. Yield performance of A. bisporus fruiting bodies was measured under different treatment conditions: 1—Untreated control; 2—Treated with prochloraz-Mn; 3—Treated with 2% O. vulgare EO; 4—Inoculated with M. perniciosa; 5—2.0% oil added to casing soil, and 4 h later the spore suspension of 1.2 × 106 spors/mL was applied and put on the compost; 6—2% O. vulgare EO added simultaneously with a suspension of 1.2 × 106 spors/mL in casing soil and put on the compost layer immediately. The suspensions of the M. perniciosa spores were prepared on the day of A. bisporus inoculation. Conidia were prepared by washing the surface of agar slants (PDA) 2-week-old pure cultures of M. perniciosa with nonpyrogenic sterile physiological saline with Tween 40 (Fisher Bioreagents, Pittsburgh, PA, USA). Obtained conidia were counted using an improved Neubauer hemocytometer (Neubauer chamber, Paul Marienfeld, England) and adjusted to the desired concentration. The occurrence of fruiting of A. bisporus was monitored for 25 days, the appearance and number of fruiting bodies, and the appearance of disease symptoms.
2.6. Statistical Analysis
Experiments were performed in three replicates, and statistical analysis was calculated by two-way ANOVA (GraphPad Prism 9.0.0).
3. Results and Discussion
3.1. The Isolation of M. perniciosa Isolates
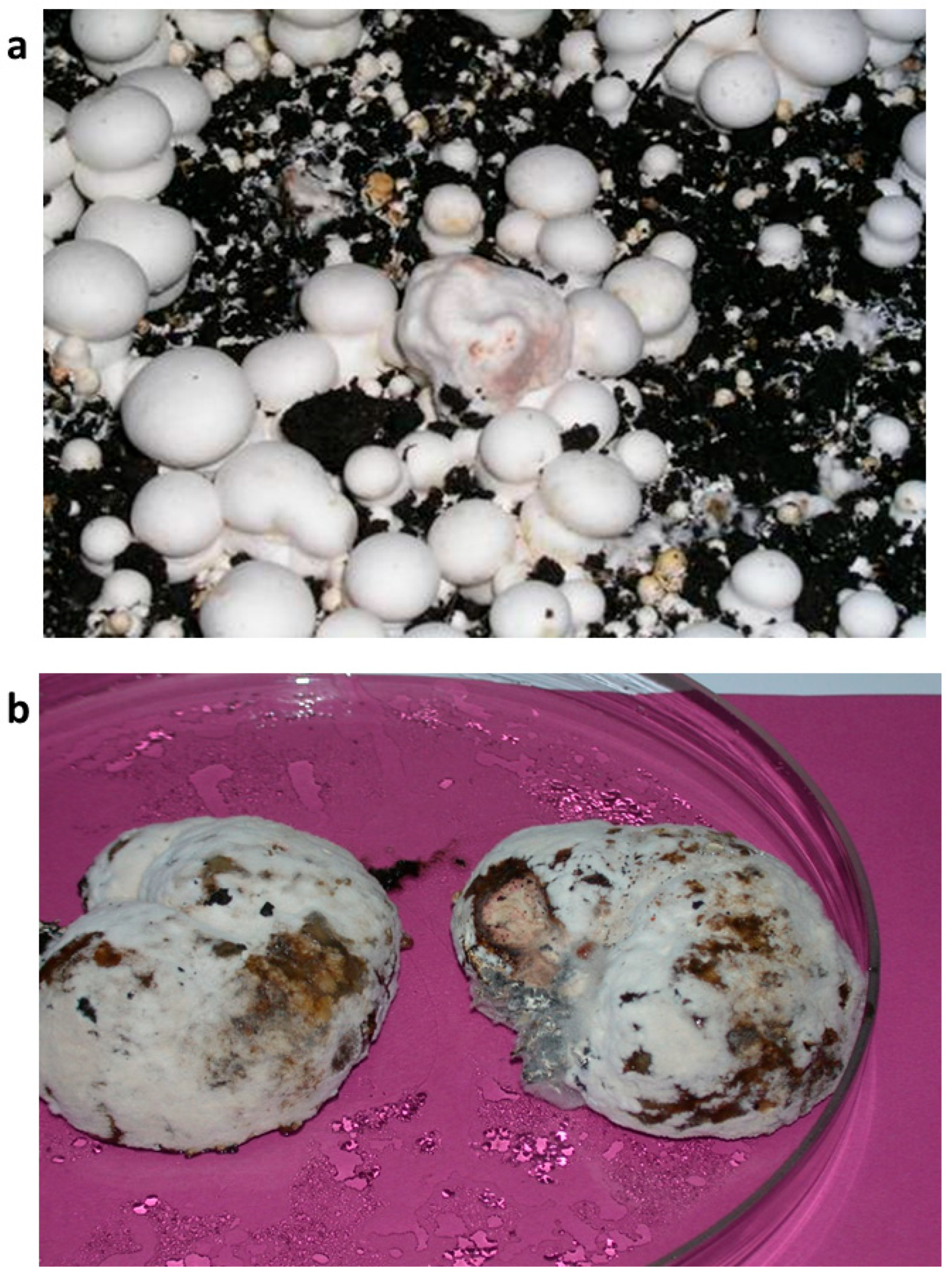
The isolation and identification of isolates of M. perniciosa from diseased fruiting bodies of white button mushrooms from mushroom units in Serbia and Bosnia and Herzegovina were made (Figure 1).
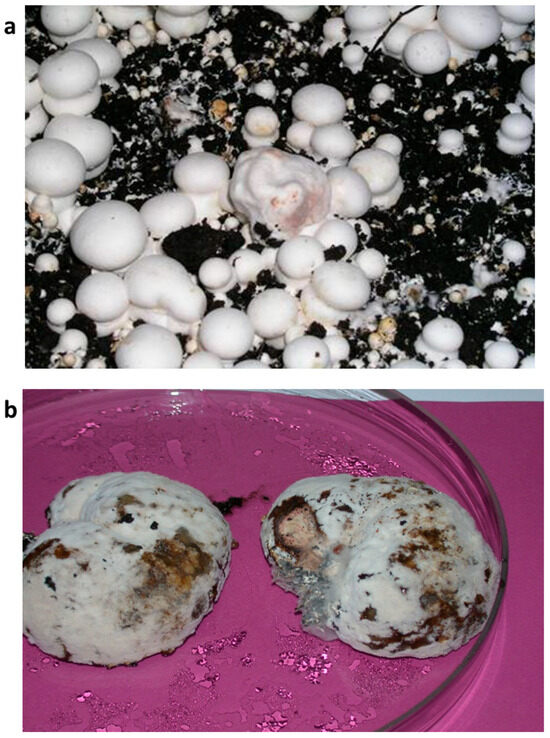
Figure 1.
Fruiting bodies of A. bisporus with symptoms of WBD, fresh (a) and on the third day in a humid chamber (b).
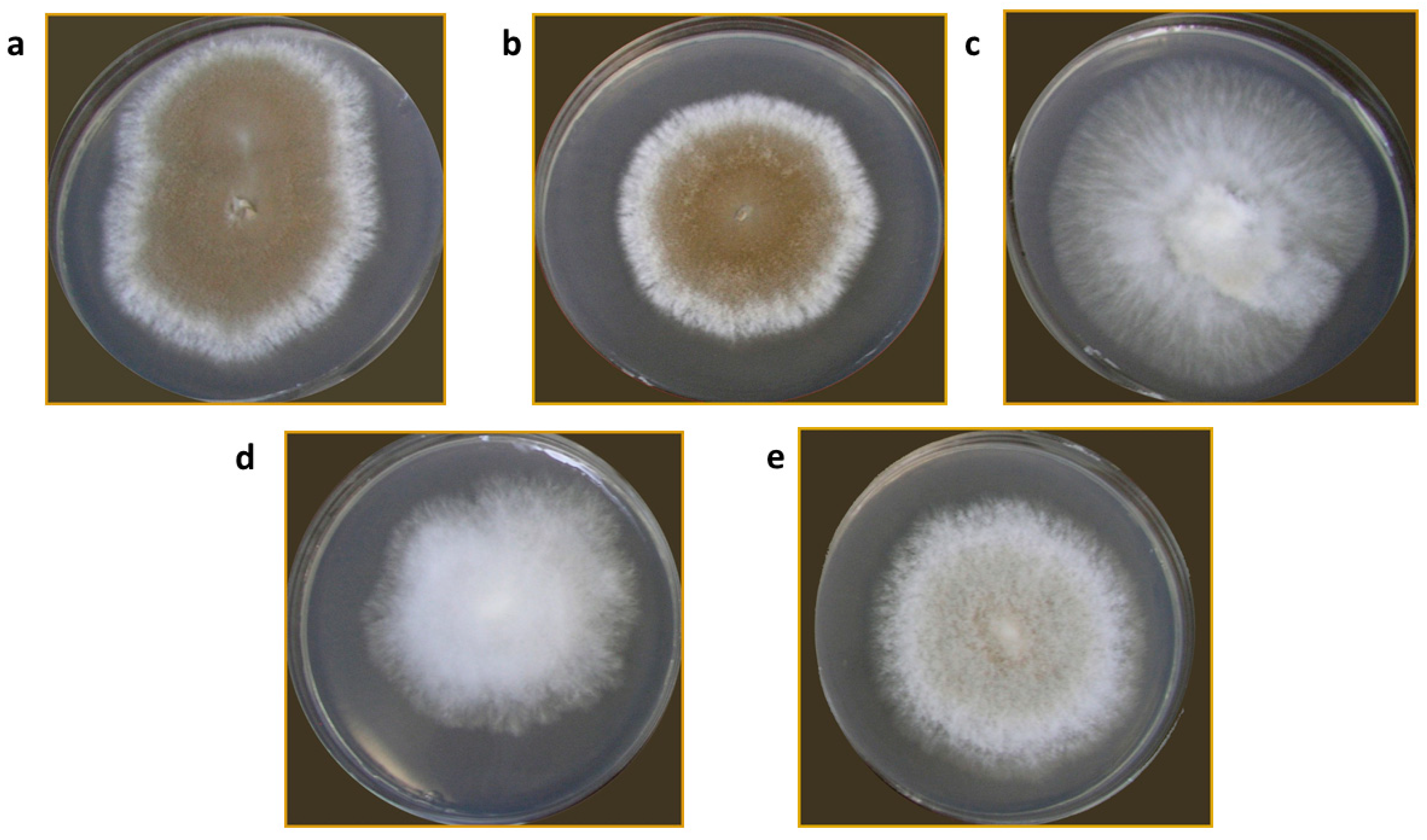
Colony characteristics of all isolates varied. The colonies were very regular in growth with either dense aerial mycelium MPH2, MPPS, and MPR, or sparse MPH1. Only the MPS isolate had a colony, which grew in compact and aerial sectors, irregular in growth. Colony color, which to some extent indicates the production of aleuriospores, varied from white for the MPS isolate to dark brown for the MPPS isolate (Figure 2).
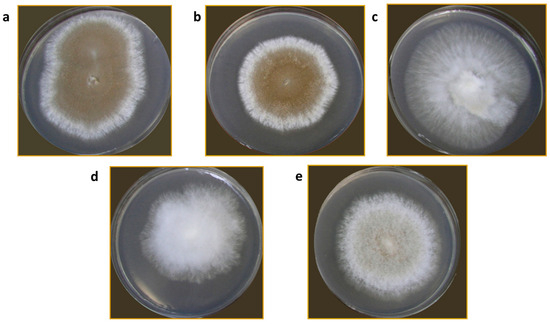
Figure 2.
M. perniciosa isolates on PDA plates: MPPS (a); MPR (b); MPS (c); MPH1 (d); MPH2 (e).
Mycopathogenic and different competing types of microorganisms inhabit the cover of A. bisporus mycelium, including species that cause WBD and M. perniciosa [23]. The presented isolation of different strains of M. perniciosa from A. bisporus collected from mushroom farms in different European countries confirms the widespread presence of the causal agent of WBD across Europe [11,24]. The variability in colony morphology among different isolates indicates potential diversity within M. perniciosa strains from different origins. This phenotypic diversity observed on PDA plates might be the consequence of genetic variability that is further explored in this manuscript. Additionally, two isolates from Serbia, MPPS and MPR, have shown similar growth patterns. Moreover, in the previous study, authors have implied different growth rates and characteristics on PDA of strains from different origins [25]. The study by Li et al. [8] pointed out the possible connection between the color of the colonies and the strains pathogenicity, which is another feature that should be explored in the future.
3.2. RAPD-PCR Analyses of M. perniciosa Isolates
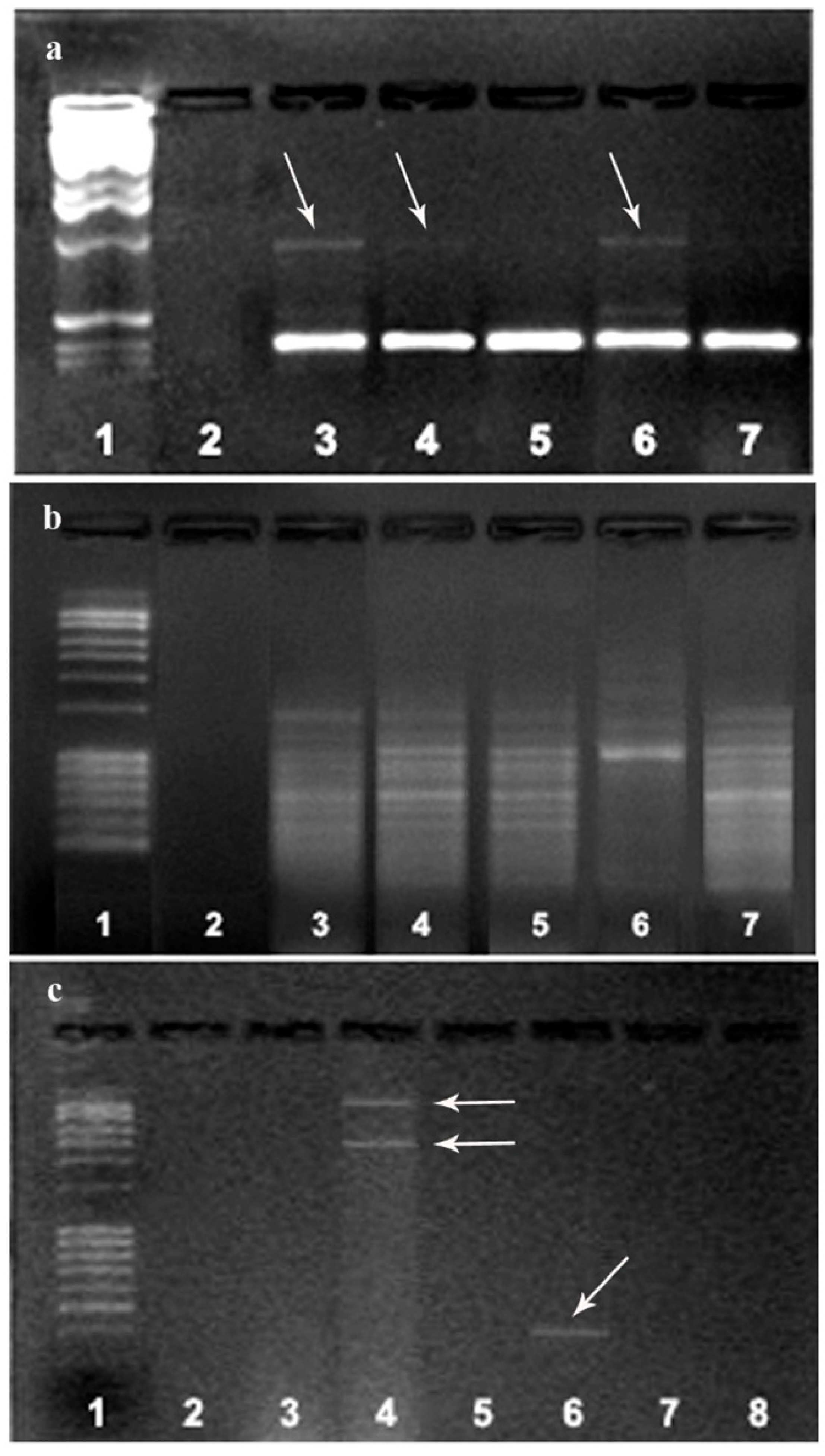
The obtained M. perniciosa isolates were molecularly characterized by using RAPD markers. RAPD profiles of MPH1, MPH2, and MPPS showed relative heterogeneity (Figure 3a). Primer 266 did not reveal any intra-isolate variability among major bands (Figure 3b), whereas primer 280 showed a high degree of polymorphism between isolates from Serbia (MPPS and MPR) and the other two groups (Figure 3c).
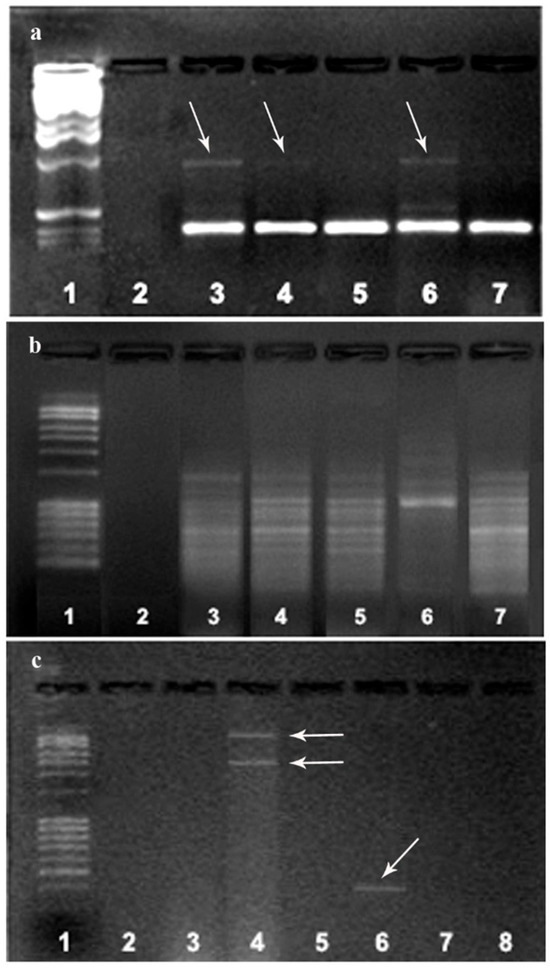
Figure 3.
Comparison of the RAPD patterns of M. perniciosa isolates: RAPD fragments obtained with primer 268 (a) and 266 (b): marker (line 1); negative control (line 2); MPH1 (line 3); MPH2 (line 4); MPS (line 5); MPPS (line 6); MPR (line 7); (c) RAPD fragments obtained with primer 280: marker (line 1); MPH1 (line 2); MPH2 (line 3); MPPS (line 4); MPS (line 5); MPR (line 6); negative controls (lines 7, 8). The arrows represent the detected polymorphism between the tested isolates MPH1, MPH2 and MPPS in relation to the other isolates with primer 268 (a) and two fragments obtained in the MPPS isolate that did not occur in of another isolate. Fragments are different sizes are also present in MPR isolates with primer 266 (c).
According to the morpho-physiological characteristics and RAPD analyses of five M. perniciosa isolates, isolates from Serbia were similar; the isolates from the Netherlands showed mutually similar characteristics, but they were different from isolates from Serbia. The isolate from Bosnia and Herzegovina is different from these two groups. For further tests, the MPS isolate was used.
It seems that the combination of morpho-physiological traits previously observed and RAPD patterns supports the classification of isolates according to their geographical origin. This might have potential implications for disease management strategies in different regions. Previously, the study by Li et al. [8] found no evidence of an association between the genetic diversity and the geographical origin of the isolates from different regions of China, suggesting that further research on more strains and additional locations is needed to provide strong evidence. On the other hand, genetic diversity of 38 M. perniciosa isolates from different areas in China was analyzed by sequence-related amplification polymorphism (SRAP), with the strains from the same provinces being clustered in the same clade [25]. The limits of this evaluation of diversity are limited sample size and geographical coverage, future studies should include additional regions and additional samples from the same region in order to provide further evidence for the link between the origin of the strains and their characteristics. Additionally, whole-genome sequencing of the strains could reveal other differences among them and could provide a more detailed understanding of their evolutionary relationships, virulence, and genetic adaptations to specific environmental conditions.
3.3. EO Composition
The volatile profile of O. vulgare EO is characterized by a complex mixture of monoterpene and sesquiterpene molecules (Table 1). Carvacrol (50.44%) and thymol (7.55%), both phenolic monoterpenes, are the main representatives of the oxygenated monoterpenes (62.04%) chemical class. These substances have a hydroxylated aromatic ring in common, which gives them a higher polarity than other monoterpenes. p-Cymene (17.54%) and γ-terpinene (8.52%) are the most prevalent monoterpene hydrocarbons that constitute 33.43% of the O. vulgare EO volatile content. Other detected monoterpenes were (+)-4-carene (2.52%), a bicyclic monoterpene with a fused cyclopropane-cyclohexane structure, and β-myrcene (2.16%), a linear acyclic monoterpene with a conjugated diene system. Caryophyllene (0.88%) is the most prevalent of the sesquiterpene hydrocarbons, which make up 1.69% of the overall composition. Our previous study [26] on O. vulgare EO has also demonstrated the prevalence of carvacrol as the main constituent of EO. In comparison to our previous investigation, the current study brings more identified compounds within the volatile profile of O. vulgare. Comparative study of numerous EOs from Europe indicated that they are rich in monoterpenes such as sabinene, myrcene, p-cymene, 1,8-cineole, β-ocimene, γ-terpinene, sabinene hydrate, linalool, α-terpineol, carvacrol methyl ether, linalyl acetate, thymol, and carvacrol. Sesquiterpenes such as β-caryophyllene, germacrene D, germacrene D-4-ol, spathulenol, caryophyllene oxide, and oplopanone were also notable constituents [27]. Subsequent studies are needed to reveal the possible impact of storage duration and different conditions on the chemical composition of EO, since it may affect its constituents and subsequent bioactive properties.

Table 1.
Chemical composition (% v/v) of O. vulgare EO.
3.4. In Vitro Antifungal Activity of O. vulgare EO
Antifungal activity of O. vulgare EO towards different M. perniciosa strains was strong and highlighted with MIQ in the range of 0.1–2.0 µL/disc and MFQ in the range of 0.5–5.0 µL/disc (Table 2). The oil of O. vulgare possessed the strongest antifungal activity against M. perniciosa MPS with a minimal inhibitory quantity of 0.1 μL/disc and a minimal fungicidal quantity of 0.5 μL/disc (Table 2). The fungicide prochloraz-Mn, which we used as a control, showed much lower antifungal activity than O. vulgare EO towards all strains tested, with MIQ 5.0 μL/disc and MFQ 50.0 μL/disc or ≥50.0 μL/disc. It was thought that vapor treatment would be very effective against M. perniciosa, allowing the use of only a limited amount of EO. Despite similar sensitivity of the examined fungal strains to prochloraz-Mn treatment, their susceptibility to O. vulgare EO varied greatly. We can observe a similar pattern of EO sensitivity based on the strain’s geographical origin, with the highest sensitivity recorded for the strain from Bosnia and Herzegovina (MPS, MIQ 0.1 µL/disc), followed by two strains from the Netherlands (MPH1 and MPH2 with MIQ 0.3 and 0.2 µL/disc, respectively), while the highest resistance can be attributed to the two strains from Serbia (MPPS and MPR with MIQ 1.0 and 2.0 µL/disc, respectively).

Table 2.
Antifungal activity of O. vulgare EO towards M. perniciosa strains; MIQ—minimal inhibitory quantity; MFQ—minimal fungicidal quantity. The results are presented as mean value ± standard deviation of three replicates, with the asterisks representing statistical significance, **** p < 0.0001, between the O. vulgare EO and Prochloraz-treated group.
O. vulgare EO is a well-known antifungal agent. Its ability to limit the growth of microbial pathogens has been previously explored towards Botrytis cinerea, where the in vivo vapor contact assay suggested that oil had antifungal activity at 250 mg/L and it reduced the decay of cherry tomatoes by 96.39% [14]. Its antibacterial potential is also well-known [28], implying the wide antimicrobial application of this natural product. Carvacrol and thymol were found as the dominant constituents of O. vulgare EO examined in this study along with the p-cymene and γ-terpinene and might be the underlying reason for the observed antifungal activity. The carvacrol antifungal mechanism was investigated previously towards Candida albicans, and it involved binding to sterols in the fungal membrane [29]. Furthermore, both thymol and carvacrol inhibit the growth of B. cinerea by interfering with membrane permeability and destroying the cell membrane structure [30]. Thymol induced a similar protective effect as the commercial fungicide (Chronos 450 SC) on A. bisporus by inhibiting the growth of M. perniciosa while at the same time having minimal effect on A. bisporus [31]. Antimicrobial properties of p-cymene are being linked with its ability to impact the stability of microbial cell membranes [32], while the study of Bursera morelensis Ramirez EO found its promising anti-Candida potential, possibly due to interaction among the dominant molecules α-pinene and γ-terpinene [33]. Even the compounds found in lower abundances in O. vulgare EO (Table 1) have been known as antifungal agents. This is the case with linalool, a compound that inhibits the growth of the pathogenic fungus Fusarium oxysporum f. sp. radicis-lycopersici by interfering with cell membrane integrity and enhancing levels of reactive oxygen species [34].
The goal of out future research is to link the mechanisms of antifungal activity for the O. vulgare EO dominant molecules with the antifungal mode of action for O. vulgare towards M. perniciosa. Likewise, including additional M. perniciosa strains when assessing O. vulgare EO antifungal activity would provide compeling evidence for the potential wide application of this natural product and additional connection of the susceptibility to EO with the geographical origin of the strains. Future studies might be focused on elucidating the nature of interaction between O. vulgare EO and Prochloraz Mn with the goal of revealing potential synergistic effects that might improve antifungal efficacy while at the same time reducing the dosages of synthetic fungicides.
3.5. Investigation of O. vulgare EO In Situ
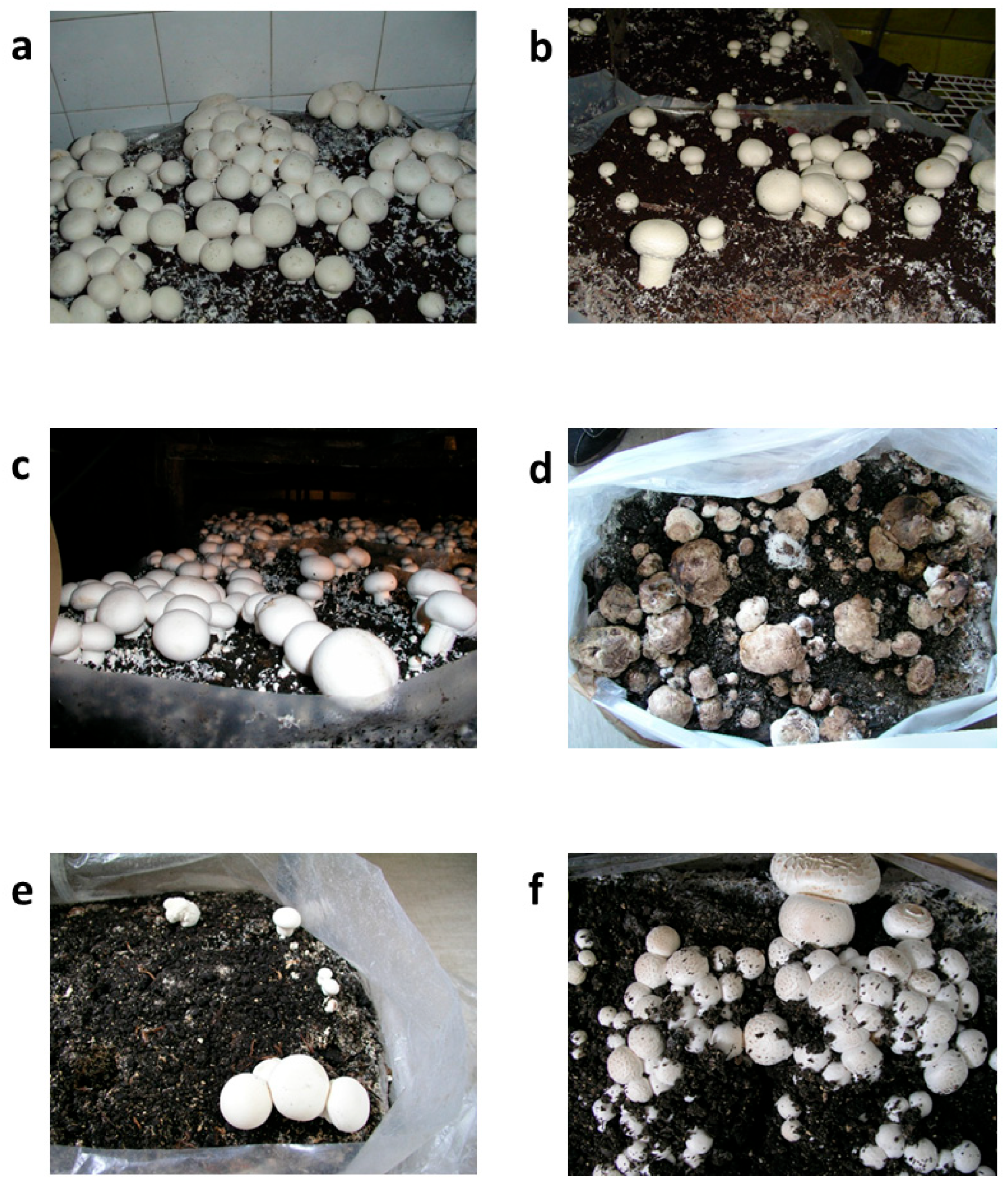
Strain M. perniciosa MPS was selected for the assessment of in situ sensitivity to O. vulgare EO due to its higher sensitivity to the in vitro treatment in comparison with other tested M. perniciosa strains. The morphological traits of A. bisporus fruiting bodies under different treatment settings after 20 days are depicted in Figure 4. This information offers insight into the course of the disease and the effectiveness of the treatment, with consequences for yield and economic viability. Both the prochloraz-Mn-treated group (Figure 4b) and the untreated control group (Figure 4a) showed completely grown, morphologically normal fruiting bodies, which can be considered as a sign of ideal yield performance conditions. The size, color, and integrity of the fruiting bodies treated with 2% O. vulgare EO (Figure 4c) were similar to those in the control groups, indicating that O. vulgare EO had no detrimental effects on biomass accumulation or growth. At this point, it is worth noting that in this sample, establishment of primordia was detected at higher levels when compared to the control groups 1 and 2. According to this observation, O. vulgare EO maintained yield at levels similar to those of conventional fungicidal treatments when applied as a protective treatment (Table 3).
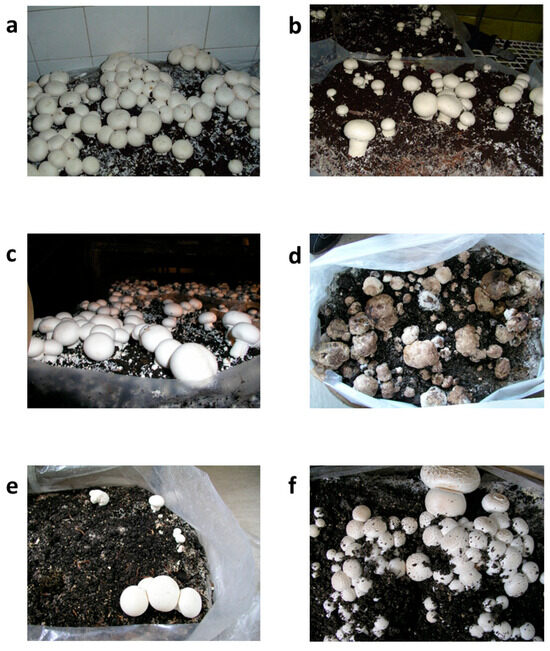
Figure 4.
Appearance of Agaricus bisporus fruiting bodies after 20 days: (a) untreated control; (b) treated with prochloraz-Mn; (c) treated with 2% O. vulgare EO; (d) inoculated with the pathogen Mycogone perniciosa; (e) inoculated with M. perniciosa followed by O. vulgare EO application 4 h before inoculation; and (f) simultaneously inoculated with M. perniciosa and treated with O. vulgare EO.

Table 3.
Yield performance of Agaricus bisporus fruiting bodies under different treatment conditions. Results are presented as the mean of three replicates ± SD, with the different letters in each row representing significant differences among the samples.
On the other hand, the Mycogone perniciosa-inoculated group (Figure 4d) that received no treatment showed obvious symptoms of infection, such as structural deterioration and tissue softening, which are traits of WBD. Given this morphology, a significant yield loss is anticipated in terms of both marketable quality—a crucial component of commercial mushroom production—and overall biomass.
Fruiting body integrity appeared to be partially intact in the group that received O. vulgare EO four hours before pathogen inoculation (Figure 4e), which indicated a less severe case of the disease. Nonetheless, there has still been some deformation or delayed growth, which has led to a slight yield drop (Table 3). However, when early indications of infection are found, this condition may serve as a salvage tactic, possibly averting complete crop loss. Fruiting bodies that received O. vulgare EO and M. perniciosa inoculation at the same time (Figure 4f) were completely healthy and comparable to the untreated group. The structure’s preservation implies that early intervention or O. vulgare EO co-treatment may prevent early pathogen establishment, preserving yields that are more in line with those of untreated or fungicide-treated groups. This preventative measure may provide a natural and efficient way to manage disease, especially in low-chemical or organic production systems.
The data in Table 3 demonstrate how the yield and quality of Agaricus bisporus fruiting bodies are affected by M. perniciosa infection and subsequent O. vulgare EO treatment. All fruiting bodies in the untreated control group were healthy and obtained the highest visual grading score (5), resulting in a total yield of 1.19 kg per bag. Prochloraz’s effectiveness in preserving crop health and appearance was further demonstrated by the slightly greater yield (1.21 kg/bag) and 100% healthy fruiting bodies in the group treated with the fungicide. The fruiting bodies treated with 2% O. vulgare EO alone produced the maximum output of any sample (1.24 kg/bag), indicated by a intact and healthy fruiting body and supperior visual appeal. This implies that under typical, uninfected conditions, O. vulgare EO may have a minor yield-promoting effect in addition to having no detrimental effects on mushroom development.
The group that was injected with M. perniciosa alone (Sample 4) had a significant decrease in yield (0.75 kg/bag), with just 8% of fruiting bodies staying healthy and the lowest visual grading (1), indicating the fungus’s extreme pathogenicity. Partial protection was seen when O. vulgare EO was applied four hours before inoculation (Sample 5). The visual grade was lower (3); suggesting that some degree of damage or deformation occurred after treatment, even though the overall yield increased to 0.97 kg/bag and 87% of the fruiting bodies remained healthy. Sample 6 showed the greatest results among the infected groups, with a total output of 1.04 kg/bag, 100% viable fruiting bodies, and the highest visual grade (5) due to simultaneous M. perniciosa inoculation and O. vulgare EO treatment. These results imply that O. vulgare EO might successfully prevent infection and preserve yield and quality when used in conjunction with pathogen exposure, possibly outperforming synthetic fungicides. The information suggests that O. vulgare EO is a viable natural remedy for controlling M. perniciosa infections in A. bisporus production, particularly when used early or in conjunction with pathogen exposure. We have observed a higher percentage of healthy fruiting bodies when O. vulgare EO was applied simultaneously with the pathogen, rather than when it was applied prior to infection. On the other hand, the total yield remained similar in both cases. This might be due to the rapid dissipation of active volatile components before pathogen contact when applied too early, with further research needed to confirm this. Moreover, further research might explore the antifungal activity of encapsulated oil, since encapsulation might slow down the release of active volatile components and provide a prolonged bioactive effect [35], which should be additionally studied.
The in situ experiment was based on the effects of one selected M. perniciosa strain. Future evaluation should reveal protective effects of O. vulgare EO when A. bisporus is exposed to additional M. perniciosa strains. Also, only two O. vulgare EO application timings were evaluated, limiting the understanding of how treatment timing affects infection and its inhibition. Additionally, a larger-scale field trial could provide more firm evidence for the observed protective effect of O. vulgare EO on spread of the disease.
A representative and high-stakes model for researching pathogen resistance, sanitation practices, and the effectiveness of biocontrol agents in A. bisporus farming is offered by concentrating on M. perniciosa. When this pathogen is successfully controlled, it frequently leads to more extensive advancements in disease prevention and cleanliness practices, which can lower the risk of other infections.
Previous investigations of different chemical fungicides found their promising antifungal activity, especially in the case of prochloraz-Mn. On the other hand, the same study indicated the low effect of bio-fungicides based on Bacillus strains [36]. It seems that the antifungal activity of EOs is not limited to certain types of pathogens since their inhibiting efficacy is noted towards human pathogens [37], animal pathogens [38], and causes of plant diseases [39]. These natural products should be further explored by including additional EOs in the analysis of their protective effects on A. bisporus and by optimizing EO delivery methods.
A number of studies have examined the effect of essential oils on the pathogens of button mushrooms [40]. It has been proven that essential oils not only have a contact effect, but also, by vaporizing, the active agents can block the vegetative growth of pathogens [41].
The in vivo trial using M. perniciosa-inoculated casings revealed that the preventative use of lemon, verbena, or thyme oils was able to control the development of the WBD. The two of their main components (nerol and thymol) revealed that none of these treatments were detrimental to the growth of the A. bisporus [31].
Our experiment has not included button mushroom mycelia in the tested main components (Carvacrol, Thymol, p-Cymene, and γ-Terpinene). The World Health Organization (WHO, 2014) confirmed that thymol and carvacrol residues in food are harmless to consumers as long as they do not exceed 50 mg kg−1 [42]. Additionally, O. vulgare EO has been regarded as safe in different studies. The study by Llana-Ruiz-Cabello et al. evaluated the effect of Origanum vulgare L. virens EO on the rodents during the 90 days application and found no mortality and no treatment-related adverse effects at 200 mg/kg b.w. in Wistar rats [43]. Another study found the IC50 value of O. vulgare EO towards HEK293 cells, a human embryonic kidney cell line, to be at 310 µg/µL [44]. On the other hand, research on Zea Mays seeds found that O. vulgare EO has genotoxic activity by affecting both DNA and proteins [45]. The previous findings highlight the need for additional toxicological evaluations of O. vulgare EO to fully elucidate its safety profile across different biological systems.
4. Conclusions
In this study we have observed both morphological and genetic diversity among M. perniciosa strains from different origins, indicating that more detailed studies are needed to explore additional links between colony morphology and genetic characteristics with virulence and susceptibility to fungicides. Taking into account the toxicity of synthetic fungicides, which reach humans through the food chain, there is an increasing need to find alternative fungicidal agents. Current knowledge indicates that plant metabolites show good antifungal activity and can be considered less harmful in comparison with synthetic fungicides. Additionally, we have provided strong evidence for O. vulgare’s potential to limit growth of M. perniciosa both in vitro and in situ. This natural product, along with its dominant constituent, carvacrol, should be further explored in order to reveal its antifungal mechanism and potential to be applied in basement cultivation of A. bisporus, especially in organically certified mushroom production farms.
Author Contributions
Conceptualization, J.G. and M.S.; methodology, J.G., M.S., S.N., D.M. and D.S.; formal analysis, J.G., S.N., D.M., D.S. and A.Ć.; investigation, J.G., S.N., D.M., M.I. and D.S.; resources, J.G., S.N., I.M., D.S. and A.Ć.; data curation, J.G., M.S., S.N., D.M. and D.S.; writing—original draft preparation, J.G., S.N., A.Ć., M.I. and D.S.; writing—review and editing, J.G., M.S., A.Ć., M.I. and D.S.; visualization, J.G., M.S., A.Ć., M.I. and D.S.; supervision, J.G., M.S., A.Ć., M.I. and D.S.; project administration, J.G., M.S., A.Ć., M.I. and D.S.; funding acquisition, J.G., M.S., A.Ć., S.N., I.M., D.M., M.I. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (Grant No. 451-03-136/2025-03/200007) and aligns with the United Nations Sustainable Development Goal (UNSDG) 3: Good Health and Well-Being.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Ivanka Milenković was employed by the company Ekofungi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Živković, J.; Ivanov, M.; Stojković, D.; Glamočlija, J. Ethnomycological Investigation in Serbia: Astonishing Realm of Mycomedicines and Mycofood. J. Fungi 2021, 7, 349. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Milinčić, D.D.; Doroški, A.; Lević, S.; Stanojević, S.P.; Kostić, A.Ž.; Minić, D.A.P.; Vidović, B.B.; Plećić, A.; et al. Comparative Chemical Analysis and Bioactive Properties of Aqueous and Glucan-Rich Extracts of Three Widely Appreciated Mushrooms: Agaricus bisporus (J.E.Lange) Imbach, Laetiporus sulphureus (Bull.) Murill and Agrocybe aegerita (V. Brig.) Vizzini. Pharmaceuticals 2024, 17, 1153. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, J.; Stojković, D.; Nikolić, M.; Ćirić, A.; Reis, F.S.; Barros, L.; Ferreira, I.C.F.R.; Soković, M. A Comparative Study on Edible Agaricus Mushrooms as Functional Foods. Food Funct. 2015, 6, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Schilla, F.; Mumladze, G.; Soukupova, J.; Smutka, L. Economic Analysis of Agaricus bisporus Mushrooms Production and the Perspective of Sharing Economy. Front. Sustain. Food Syst. 2024, 8, 1415291. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Savoie, J.M. Microbially Induced Diseases of Agaricus bisporus: Biochemical Mechanisms and Impact on Commercial Mushroom Production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, J.; Soković, M.; Ljaljević-Grbić, M.; Vukojević, J.; Milenković, I.; Van Griensven, L. Morphological Characteristics and Mycelial Compatibility of Different Mycogone perniciosa Isolates. J. Microsc. 2008, 232, 489–492. [Google Scholar] [CrossRef]
- Novikova, I.; Titova, J. Antifungal Activity of Industrial Bacillus Strains against Mycogone perniciosa, the Causative Agent of Wet Bubble Disease in White Button Mushrooms. Microorganisms 2023, 11, 2056. [Google Scholar] [CrossRef]
- Li, D.; Sossah, F.L.; Yang, Y.; Liu, Z.; Dai, Y.; Song, B.; Fu, Y.; Li, Y. Genetic and Pathogenic Variability of Mycogone perniciosa Isolates Causing Wet Bubble Disease on Agaricus bisporus in China. Pathogens 2019, 8, 179. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Sc, B.; Gaze, R.H.; White, P.F.; Rinker, D.; Eicker, A.; Grogan, H. Mushroom Pest and Disease Control: A Colour Handbook; CRC Press: London, UK, 2007. [Google Scholar] [CrossRef]
- Sharma, M.; Maheshwari, N.; Khan, F.H.; Mahmood, R. Carbendazim Toxicity in Different Cell Lines and Mammalian Tissues. J. Biochem. Mol. Toxicol. 2022, 36, e23194. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.C.; Navarro, M.J. Efficacy and Effects on Yield of Different Fungicides for Control of Wet Bubble Disease of Mushroom Caused by the Mycoparasite Mycogone perniciosa. Crop Prot. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.H.; Ye, M.; Wang, K.B.; Fan, L.M.; Su, F.W. Chemical Composition and Antifungal Activity of Essential Oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef] [PubMed]
- Cid-Chevecich, C.; Müller-Sepúlveda, A.; Jara, J.A.; López-Muñoz, R.; Santander, R.; Budini, M.; Escobar, A.; Quijada, R.; Criollo, A.; Díaz-Dosque, M.; et al. Origanum vulgare L. Essential Oil Inhibits Virulence Patterns of Candida Spp. and Potentiates the Effects of Fluconazole and Nystatin in Vitro. BMC Complement. Med. Ther. 2022, 22, 39. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Sakr, S.; De Martino, L.; Mattia, C.A.; De Feo, V.; Camele, I. Antifungal Activity of Some Constituents of Origanum vulgare L. Essential Oil Against Postharvest Disease of Peach Fruit. J. Med. Food 2015, 18, 929–934. [Google Scholar] [CrossRef]
- Booth, C. Fungal Culture Media. In Methods in Microbiology; Norris, J.R., Ribbons, D.W., Eds.; Academic Press: London, UK; New York, NY, USA, 1971; Volume 4, pp. 49–94. [Google Scholar]
- Brady, B.L.K.; Gibson, I.A.S. Mycogone Perniciosa; CMI Descriptions of Pathogenic Fungi and Bacteria N 499; Commonwealth Agricultural Bureaux: London, UK, 1976. [Google Scholar]
- Qi, T.; Romaine, C.P.; Schlagnhaufer, B. Genetic analysis of different isolates of Mycogone perniciosa Mang. using Random Amplified Polymorphic DNA (RAPD) marcers. Acta Eulis Fungi 1996, 3, 52–56. [Google Scholar] [CrossRef]
- Mello, A.; Nosenzo, C.; Meotto, F.; Bonfante, P. Rapid Typing of Truffle Mycorrhizal Roots by PCR Amplification of the Ribosomal DNA Spacers. Mycorrhiza 1996, 6, 417–421. [Google Scholar] [CrossRef]
- Zaharieva, A.; Rusanov, K.; Rusanova, M.; Paunov, M.; Yordanova, Z.; Mantovska, D.; Tsacheva, I.; Petrova, D.; Mishev, K.; Dobrev, P.I.; et al. Uncovering the Interrelation between Metabolite Profiles and Bioactivity of In Vitro- and Wild-Grown Catmint (Nepeta nuda L.). Metabolites 2023, 13, 1099. [Google Scholar] [CrossRef]
- Amvam Zollo, P.H.; Biyiti, L.; Tchoumbougnang, F.; Menut, C.; Lamaty, G.; Bouchet, P. Aromatic Plants of Tropical Central Africa. Part XXXII. Chemical Composition and Antifungal Activity of Thirteen Essential Oils from Aromatic Plants of Cameroon. Flavour. Fragr. J. 1998, 13, 107–114. [Google Scholar] [CrossRef]
- Du, Y.; Shi, N.; Ruan, H.; Chen, F. Three Mycogone Species, Including a New Species, Cause Wet Bubble Disease of Agaricus bisporus in China. Plant Dis. 2021, 105, 3780–4145. [Google Scholar] [CrossRef]
- Szumigaj-Tarnowska, J.; Slusarski, C.; Ulinski, Z. Pathogenicity of Mycogone perniciosa isolates collected on Polish mushroom farms. J. Hortic. Res. 2015, 23, 87–92. [Google Scholar] [CrossRef]
- Zhou, C.; Li, D.; Chen, L.; Li, Y. Genetic Diversity Analysis of Mycogone perniciosa Causing Wet Bubble Disease of Agaricus bisporus in China Using SRAP. J. Phytopathol. 2016, 164, 271–275. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J.L.D. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Stojković, D.; Glamočlija, J.; Ćirić, A.; Nikolić, M.; Ristić, M.; Šiljegović, J.; Soković, M. Investigation on Antibacterial Synergism of Origanum vulgare and Thymus vulgaris Essential Oils. Arch. Biol. Sci. 2013, 65, 639–644. [Google Scholar] [CrossRef]
- Lima, I.O.; De Oliveira Pereira, F.; De Oliveira, W.A.; De Oliveira Lima, E.; Menezes, E.A.; Cunha, F.A.; De Fátima Formiga Melo Diniz, M. Antifungal Activity and Mode of Action of Carvacrol against Candida albicans Strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal Activity of Thymol and Carvacrol against Postharvest Pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S. In Vitro and in Vivo Screening of Essential Oils for the Control of Wet Bubble Disease of Agaricus bisporus. S. Afr. J. Bot. 2010, 76, 681–685. [Google Scholar] [CrossRef]
- Pyo, Y.; Jung, Y.J. Microbial Fermentation and Therapeutic Potential of P-Cymene: Insights into Biosynthesis and Antimicrobial Bioactivity. Fermentation 2024, 10, 488. [Google Scholar] [CrossRef]
- Rivera-Yañez, C.R.; Terrazas, L.I.; Jimenez-Estrada, M.; Campos, J.E.; Flores-Ortiz, C.M.; Hernandez, L.B.; Cruz-Sanchez, T.; Garrido-Fariña, G.I.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene—An In Vitro Study. Molecules 2017, 22, 2095. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Li, H.; Wang, X.; Zhang, R.; Yang, X.; Jiang, Q.; Shi, Q. Revealing the Mechanisms for Linalool Antifungal Activity against Fusarium oxysporum and Its Efficient Control of Fusarium Wilt in Tomato Plants. Int. J. Mol. Sci. 2023, 24, 458. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Esteban-Rubio, S.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Oregano Essential Oil Micro- and Nanoencapsulation with Bioactive Properties for Biotechnological and Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 703684. [Google Scholar] [CrossRef]
- Navarro, M.J.; Santos, M.; Diánez, F.; Gea, F.J. Chemical and Biological Control of Wet Bubble Disease (Hypomyces perniciosus) in Mushroom Crops. Agronomy 2023, 13, 1672. [Google Scholar] [CrossRef]
- Kostić, M.; Ivanov, M.; Markovic, T.; Sanković Babić, S.; Barros, L.; Calhelha, R.; Sokovic, M.; Ciric, A. An in Vitro Study of the Origanum minutiflorum O. Schwarz & P. H. Davis and Coriandrum sativum L. Essential Oils as Chronic Tonsillitis Therapeutics: Antibacterial, Antibiofilm, Antioxidant, and Cytotoxic Activities. J. Essent. Oil Res. 2022, 34, 533–543. [Google Scholar] [CrossRef]
- Ebani, V.V.; Mancianti, F. Use of Essential Oils in Veterinary Medicine to Combat Bacterial and Fungal Infections. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol Potential of Essential Oils in Organic Horticulture Systems: From Farm to Fork. Front. Nutr. 2022, 8, 805138. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, I.; Todorović, B.; Đurović-Pejčev, R.; Stepanović, M.; Emil Rekanović, E.; Svetlana Milijašević-Marčić, S. Antimicrobial activity of biochemical substances against pathogens of cultivated mushrooms in Serbia. Pestic. Phytomed. 2016, 31, 19–27. [Google Scholar] [CrossRef]
- Geosel, A.; Szabo, A.; Akan, O.; Szarvas, J. Effect of essential oils on mycopathogens of Agaricus bisporus. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; pp. 530–535. [Google Scholar]
- World Health Organization Website (WHO). Available online: http://apps.who.int/medicinedocs/en/d/Js2200e/28.html (accessed on 21 January 2014).
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Pichardo, S.; Jos, A.; Moyano, R.; Cameán, A.M. A Subchronic 90-Day Oral Toxicity Study of Origanum vulgare Essential Oil in Rats. Food Chem. Toxicol. 2017, 101, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum vulgare L. on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of Its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef]
- Bozari, S.; Agar, G.; Yanmis, D. Chemical Content, and Toxic Effects of Essential Oil of Origanum vulgare L. Ssp Vulgare Against to Zea mays Seedlings. J. Essent. Oil-Bear. Plants 2014, 17, 67–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).