Abstract
Dehydroabietic acid (DHA) is a secondary metabolite isolated from rosin, which has certain antifungal activity, but its inhibitory effects against Alternaria alternata are unclear. In the present study, we found that DHA inhibited the mycelial growth of A. alternata, Botrytis cinerea, Valsa mali, Pestalotiopsis neglecta, and Fusarium oxysporum in a concentration-dependent manner, with the best inhibitory effect against A. alternata. Moreover, DHA can also inhibit the spore germination of A. alternata. Then, in vivo inoculation experiments showed that the leaf lesions of Populus alba gradually decreased with the increase in DHA concentration. The disease of P. alba leaves inoculated with A. alternata was not obvious after treatment with 800 mg/L DHA. The scanning electron microscopy showed that the mycelial morphology was abnormal, with crinkles and depressions. Meanwhile, the relative conductivity, soluble protein content, malondialdehyde content and hydrogen peroxide content of A. alternata were significantly increased after DHA treatment, which affected the integrity of the cell membrane and increased the permeability of A. alternata, resulting in a large leakage of intracellular substances, exacerbating the degree of lipid peroxidation of the cell membrane of A. alternata and causing oxidative damage to cells. The enzyme activity assay showed that treatment with 56.015 mg/L (EC50) DHA significantly reduced the activities of antioxidant enzymes (superoxide dismutase, catalase, peroxidase) and cell-wall-degrading enzymes (endoglucanase, polygalacturonase, pectin lyase) in A. alternata (p < 0.05), resulting in a decrease in the activity of pathogenic fungi, as well as a reduction in the ability of the A. alternata to degrade the cell wall of the host plant, which led to a decrease in the ability of the A. alternata to infest the host plant. Moreover, the decrease in the relative expression of defense-related enzyme genes (AaSOD, AaPOD, AaCAT) and pathogenicity-related enzyme genes (AaPL, AaPG) was consistent with the enzyme activity results. Thus, the present study revealed the fungicidal activity and mechanism of DHA against A. alternata and the potential of DHA to be developed as a plant-derived antifungal agent was established.
1. Introduction
Populus spp. (poplar), a keystone genus in global afforestation programs, plays a vital role in ecological restoration, soil conservation, and sustainable timber production due to its rapid growth and environmental adaptability [1,2]. However, poplar cultivation faces persistent phytopathological threats, with over 300 documented fungal pathogens causing diseases ranging from stem cankers to foliar necrosis [3]. Among these, leaf spot disease has emerged as a particularly destructive issue in northeastern China, severely affecting nursery stocks of economically important species including P. alba, P. tomentosa, P. simonii, and P. pseudosimonii [4]. Field observations reveal characteristic symptoms of brown necrotic lesions on foliar tissues, which progress to premature defoliation, growth retardation, and irreversible wood quality degradation under epidemic conditions. The pathogenicity of this foliar disease has been conclusively linked to A. alternata, a cosmopolitan asexual fungus renowned for its broad host range spanning 380+ plant species across agricultural and forestry systems [5,6]. As a polyphagous pathogen, A. alternata exhibits exceptional ecological plasticity, capable of infecting diverse plant organs including roots, stems, leaves, and fruits, where it induces tissue maceration, blight, and rot [7,8]. Its infection mechanisms in poplar involve the production of host-specific toxins and cell-wall-degrading enzymes, enabling rapid colonization of leaf parenchyma and vascular tissues [4]. Current management strategies predominantly rely on synthetic fungicides such as mancozeb, daconil, and captafol. While long-term use of fungicides causes pesticide residues as well as resistance problems, which have a negative impact on human health and the environment [9,10,11]. Given the various drawbacks of using chemical pesticides, as well as the strengthening of people’s awareness of environmental protection, the trend of modern pesticide development tends to develop new highly efficient, low-toxicity and environmentally friendly pesticides, and people are searching for new substitutes in renewable natural resources.
Rosin is a renewable natural advantageous resource obtained through the distillation process of turpentine [12,13]. Dehydroabietic acid (DHA), also known as dehydrorosin acid, is one of the constituents of rosin and the main component of disproportionated rosin. According to the literature, DHA is an important natural tricyclic diterpene resin acid, which is stable, has strong antioxidant capacity, good biocompatibility and biodegradability [14]. It has a variety of biological activities, such as anti-microbial, insecticidal, anti-carcinogenic, and anti-aging [15,16,17,18]. Soderberg et al. [19] found that DHA has bacteriostatic activity against Gram-positive and Gram-negative bacteria. The inhibitory effect on bacteria may be due to the biofilm-targeted nature of DHA, which inhibits bacterial proliferation and therefore also acts as a natural bacterial biofilm inhibitor [20,21]. Due to the advantages of the structure of DHA, the modification based on the benzene ring and carboxyl group is the current hotspot in the study of DHA [22]. Gao et al. [23] synthesized two series of dehydroabietyl oxime ester derivatives using DHA as the lead compound; an in vitro antifungal test showed that these compounds had a better inhibitory effect on six plant pathogenic fungi—one of the compounds had the strongest activity on tomato gray mold fungus—and further study found that the compound could change the morphology and ultrastructure of Botrytis cinerea mycelium, leading to the dysfunction of nucleus and mitochondria, thus causing apoptosis of B. cinerea cells, and it had good protective and therapeutic effects on tomato. However, relatively few studies have explored the inhibitory effect of DHA on pathogenic fungi and its mechanism.
Therefore, the present study confirmed that DHA has different inhibitory effects on five common pathogenic fungi and took A. alternata as the research object to investigate the inhibitory mechanism of DHA on A. alternata, which made up the gap of DHA in this field. Meanwhile, the control effect of DHA on P. alba leaf spot provided a theoretical basis for developing DHA as a substitute for chemical pesticides.
2. Materials and Methods
2.1. Pathogens and Spore Suspensions
The phytopathogenic fungi A. alternata, Pestalotiopsis neglecta, B. cinerea, Fusarium oxysporum, and Valsa mali were provided by the Key Laboratory of Forest Protection of Heilongjiang Province (Northeast Forestry University, Harbin, China). These pathogenic fungi were cultured on potato dextrose agar (PDA) medium at 25 °C.
The spores of A. alternata were collected with reference to the method described by Li et al. [24]. A. alternata was inoculated onto PDA medium, and the medium was inverted and incubated in a constant temperature incubator at 25 °C for 7 d under dark conditions. Sterile water was poured into the Petri dishes after 7 d in small amounts and several times, and mycelium and spores were collected by gently scraping the surface of the medium with a sterile inoculation loop, and the mycelium was filtered off using sterilized skimmed cotton to obtain the spore suspension. The concentration of the spore suspension was adjusted to 1 × 106 spores/mL using a haemocytometer plate under a 400× inverted optical microscope.
2.2. Reagents and Plant Samples
DHA was purchased from Jingmen Dongxin Biotechnology Co., Ltd., (Jingmen, China). The kits for the determination of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), endo-β-1,4 glucanase (EG), polygalacturonase (PG), pectin lyase (PL), malondialdehyde (MDA), and hydrogen peroxide (H2O2) were purchased from Suzhou Grace Biotechnology Co., Ltd. (Suzhou, China). The RNA extraction kit was RNA pure Plant Kit (DNase I), purchased from Jiangsu Kangwei Century Biotechnology Co., Ltd. (Taizhou, China). The reverse transcription reagent was Prime ScriptTM RT reagent Kit with gRNA Eraser (Perfect Real Time), and the fluorescence quantification kit was TB Green Premix Ex TaqTM II (Tli RNase H Plus), both of which were purchased from Beijing TaKaRa Biotechnology Co., Ltd. (Beijing, China). Other analytical grade reagents and solvents were obtained from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China) and were not further purified before use.
The poplar leaves used in the experiment were collected in the on-campus forest of Northeast Forestry University, from which healthy, intact and similar-sized leaves were selected.
2.3. Effect of DHA on Mycelial Growth of Five Pathogenic Fungi
The inhibitory effect of DHA on five pathogenic fungi was determined with reference to the mycelial growth inhibition method described by Li et al. [25]. DHA was added to the PDA medium using DMSO as a co-solvent (final concentration of 2%) such that the final concentration of DHA was 800, 400, 200, 100, 50, 25, and 12.5 mg/L. The medium with equal amounts of sterile water and DMSO was used as a blank and solvent control. Mycelial plugs 5 mm in diameter were inoculated in the center of 90 mm diameter Petri dishes, then sealed and inverted for incubation in a constant temperature incubator at 25 °C. The radial growth diameter of the pathogen was determined when the control group was fully grown and the mycelial growth inhibition rate was calculated, which was repeated three times for each group. The growth inhibition rate (%) was calculated as follows:
2.4. Effect of DHA on Spore Germination of A. alternata
The effect of DHA on spore germination of A. alternata was investigated using the suspension-drop method described by Qiu et al. [26]. A 20 μL suspension of spores was mixed with 20 μL of a solution containing DHA at concentrations of 9.966 mg/L (EC30) and 56.015 mg/L (EC50), and the control group was added with equal amounts of sterile water and DMSO. After dropping 40 μL of liquid into the depression of the haemocytometer plate and incubating in the constant temperature incubator at 25 °C for 24 h, the number of spore germination was observed randomly in five fields of view using a microscope, and the inhibition of spore germination was calculated, which was repeated three times for each group. Spore germination rate (%) and germination inhibition rate (%) were calculated as follows:
2.5. In Vivo Control Effect
The leaves were surface-disinfected in a 1% sodium hypochlorite solution for 45 s, rinsed in sterile water, soaked in a 75% ethanol solution for 30 s, and rinsed twice in sterile water. After being cleaned and sterilized, the surface of each leaf was pricked three times using a sterilized inoculation needle, and then each leaf was sprayed with 5 mL of DHA solution (containing 2% DMSO), with a DHA concentration of 56.015 mg/L (EC50), 400 mg/L, and 800 mg/L, and the control group was sprayed with 2% DMSO solution. After the air-drying of the medicinal solution on the surface of P. alba leaves, A. alternata mycelial plugs (7.5 mm in diameter) were inoculated at the punctures of the leaves and wrapped around the petioles using sterilized skimmed cotton dipped in sterile water to provide moisture, and the inoculated leaves were moisturized and cultured in a thermostatic incubator at 25 °C [27]. The onset of disease was observed after 5 d of incubation and the lesion area was determined using Image J 1.54k. Three replicates were set up for each group of treatments, with five leaves in each replicate.
2.6. Morphological Observation of A. alternata Mycelium
Scanning electron microscopy (SEM) was used to study the effect of DHA on mycelial morphology during the growth of A. alternata. Mycelial plugs (7.5 mm in diameter) were inoculated on PDA plates containing EC50-level DHA, with parallel controls using untreated PDA for blank and DMSO-contained PDA for solvent effects. Sterilized coverslips were inserted obliquely into the medium at an angle of 45°, about 2–3 cm from the centrally positioned mycelial plugs, sealed and incubated in an inverted position in a constant temperature incubator at 25 °C, modifying the method described by Li et al. [28]. When the mycelium grew onto the coverslips, the coverslips with attached hyphae were removed and immediately immersed in 2.5% (v/v) glutaraldehyde PBS fixative for 4 h at room temperature. After fixation, the specimens were rinsed with PBS buffer of identical concentration, followed by sequential dehydration through an ethanol gradient series (30%, 50%, 70%, 80%, 90%, 95%, and 100%) prepared in PBS. Subsequently, the samples underwent vacuum freeze-drying for 12 h before being mounted, sputter-coated with gold-palladium, and examined under a SEM at 5000× magnification.
2.7. Effect of DHA on Extracellular Proteins and Electrical Conductivity in A. alternata
A mixture of DHA dissolved in DMSO was added to the PDB medium (total system 100 mL) with final concentrations of DHA of 9.966 mg/L (EC30) and 56.015 mg/L (EC50). Erlenmeyer flasks containing PDB medium were filled with an equal amount of sterile water as a blank control and an equal amount of DMSO as a solvent control. Then, five mycelial plugs of A. alternata (5 mm in diameter) were placed in Erlenmeyer flasks, sealed and placed in an oscillating incubator at 25 °C and 150 rpm, and three replicates were set up for each group of treatments, and the mycelial samples and supernatants were collected at 48 h, 54 h, 60 h, 66 h, and 72 h after incubation, respectively. Mycelium samples were filtered using sterile silk cloth, rinsed three times with 0.9% NaCl solution, and then stored at −80 °C after blotting with sterile filter paper. The supernatant obtained after centrifugation of the filtrate was used for subsequent experimental determinations and was also stored at −80 °C.
The supernatant was removed to measure the extracellular conductivity (μS/cm) of A. alternata using the DDS-11 digital conductivity meter (Shanghai Precision Scientific Instrument Co., Ltd., Shanghai, China) and soluble protein leakage was determined according to the method described by Bradford [29]. Three replicates were set for each group of treatments.
2.8. Effect of DHA on MDA and H2O2 in A. alternata
To assess the extent of A. alternata cell membrane damage by DHA, the content of malondialdehyde (MDA) and hydrogen peroxide (H2O2) was determined in A. alternata using the corresponding kits, respectively. Samples of A. alternata mycelium were prepared according to the method mentioned in Section 2.7, processed according to the instructions of the kit, and then set at the corresponding wavelengths using a Super Max 3000 AL multifunctional microplate reader (Shanghai Shanpu Biotechnology Co., Ltd., Shanghai, China) to read the absorbance values, and then calculate the contents of MDA and H2O2. Three replicates were set for each group of treatments.
2.9. Effect of DHA on the Enzyme Activities
The mycelium samples and supernatant samples of A. alternata were prepared according to the Section 2.7 and operated according to the instructions of the enzyme activity determination commercial kits of SOD, POD, CAT, EG, PG and PL. SOD, POD and CAT activities were determined using A. alternata mycelium samples, and EG, PG, and PL activities were determined using A. alternata supernatant samples. The Super Max 3000 AL multifunctional microplate reader (Shanghai Shanpu Biotechnology Co., Ltd.) was used for the determination of antioxidant enzymes and cell-wall-degrading enzymes activities in A. alternata, and three replicates were set up for each group of treatments.
2.10. Effect of DHA on the Gene Expression
Samples of A. alternata mycelium cultured for 72 h were prepared according to 2.6, total RNA of A. alternata was extracted using the corresponding kit, and reverse transcription was performed to synthesize cDNA, which was stored at −20 °C for subsequent experiments. The gene sequences were obtained from the NCBI website, AaBenA was selected as the reference gene, and primer design was performed using Bioxm 2.7.1 (Table 1). Changes in the expression of defense enzyme genes (AaSOD, AaPOD, AaCAT) and pathogenic enzyme genes (AaPL, AaPG) in A. alternata were determined using the CFX96 Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA, USA) according to the instructions of the quantification kit, and three independent biological replicates were performed for each treatment. The expression of the tested genes was calculated using the 2−ΔΔCT method [30].

Table 1.
Primer sequence.
2.11. Statistical Analysis
The significance of differences was analyzed by one-way ANOVA using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA) and p < 0.05 was considered statistically significant, while the toxicity equations as well as EC30 and EC50 concentrations were calculated by probit analysis. GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) was used for graphics creation.
3. Results
3.1. Effect of DHA on Mycelial Growth Inhibition of Five Pathogenic Fungi
The inhibitory effects of DHA on the mycelial growth of five pathogenic fungi are shown in Figure 1. DHA showed concentration-dependent inhibitory effects on different forest pathogenic fungi, and there was a certain degree of variability between different concentrations (p < 0.05). Among them, DHA showed the best inhibitory effect on A. alternata and the worst inhibitory effect on F. oxysporum. When the concentration of DHA was 800 mg/L, the inhibition rates of A. alternata, B. cinerea, V. mali, P. neglecta and F. oxysporum were 80.33%, 75.67%, 75.33%, 63.76% and 57.94%, respectively.
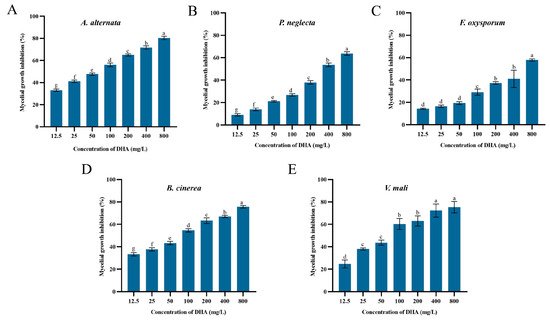
Figure 1.
The inhibition rate of the mycelial growth of A. alternata (A), P. neglecta (B), F. oxysporum (C), B. cinerea (D), V. mali (E) by different concentrations of dehydroabietic acid. The bars represent the standard error of the mean (n = 3), and the letters a–g indicate significant differences between different concentrations (p < 0.05).
Probit analysis was performed using SPSS 21.0 to determine the toxicity equations of DHA against the five pathogenic fungi as well as the values of EC50 and EC30 (Table 2). The toxicity equation of DHA against A. alternata was Y = 0.7X − 1.23 (R2 = 0.991), EC30 against A. alternata mycelial growth was 9.966 mg/L, and the value of EC50 was 56.015 mg/L, which were lower than those of the other four pathogenic fungi. Therefore, DHA had an inhibitory effect on all five pathogenic fungi, but the best inhibitory effect on A. alternata.

Table 2.
Toxicity equations of dehydroabietic acid against five pathogenic fungi.
3.2. Effect of DHA on Spore Germination of A. alternata
The effects of DHA on the mycelial growth and spore germination of A. alternata at different concentrations are shown in Figure 2. Compared with the control group, the growth of A. alternata mycelium in the DHA-treated group was significantly inhibited (p < 0.05), and the mycelium in the treated group grew slowly, the color changed, and the inhibitory effect gradually increased with the increase in DHA concentration (Figure 2A). DHA could significantly inhibit the germination of A. alternata spores (p < 0.05), and the inhibition effect was concentration-dependent, and the inhibition rate of DHA on the spore germination of A. alternata was 44.49% and 70.55% at the concentrations of EC30 and EC50 (Figure 2B).
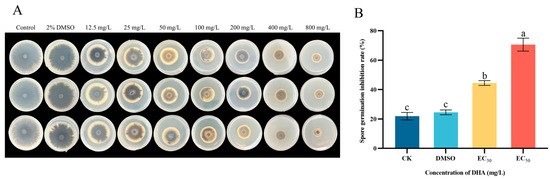
Figure 2.
The inhibitory effect of different concentrations of dehydroabietic acid on mycelial growth (A) and spore germination (B) of A. alternata. (A) The inhibition of mycelial growth of A. alternata after different concentrations of DHA; (B) The inhibition of A. alternata after treatment with EC30 and EC50 concentrations of DHA. The bars represent the standard error of the mean (n = 3), and the letters a–c indicate significant differences between different concentrations (p < 0.05).
3.3. In Vivo Control Effect
The protective effect of DHA on P. alba leaves was investigated through the triple interaction of DHA-P. alba leaves-A. alternata (Figure 3). The disease severity of P. alba leaves inoculated with A. alternata after DHA treatment is shown in Figure 3A. In the control group, the poplar leaves inoculated with A. alternata showed irregular brown spots with a large area and high severity, and when 800 mg/L DHA was used for the treatment, the inoculation position lesions of poplar leaves were not obvious and showed almost no disease. After measuring the lesion area, it was found that there was a significant difference between the disease area of A. alternata inoculated P. alba leaves treated with different concentrations of DHA and that of the control group (p < 0.05), and the lesion area gradually decreased with the increase in the concentration of DHA; the lesion area of the blank control group was about 17.59 cm2. When the concentration of DHA was EC50, the disease area was 8.72 cm2, which decreased by 50.43% compared with the control group, and when the concentration of DHA was 800 mg/L, the disease area was 1.03 cm2, which decreased by 94.14% compared with the control group (Figure 3B). It can be seen that DHA can inhibit the infestation of A. alternata on P. alba leaves and reduce the incidence area of poplar leaves, which has a desirable protective effect.
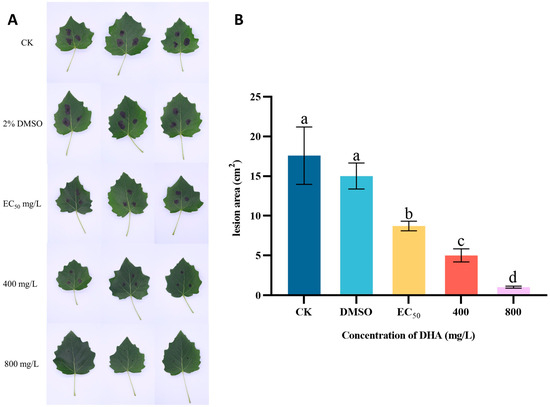
Figure 3.
In vivo effect of different concentrations of dehydroabietic acid on poplar leaf spot. (A) The disease symptoms of P. alba leaves inoculated with A. alternata after spraying different concentrations of DHA. (B) The lesion areas of P. alba leaves inoculated with A. alternata. The bars represent the standard error of the mean (n = 3), and the letters a–d indicate significant differences between different concentrations (p < 0.05). CK, a blank control treated with sterile water.
3.4. Effect of DHA on Mycelial Morphology
To further observe the effect of DHA on the morphology and structure of A. alternata mycelia, the morphology of mycelia in the blank control group, solvent control group, and the DHA-treated group (EC50) was observed by SEM (Figure 4). The results showed that both the mycelium of the two control groups were thick and full, with a smooth surface and normal morphology and structure (Figure 4A,B), but after DHA treatment, the mycelium structure was abnormal, and the surface of the mycelium became rough and twisted and appeared to be wrinkled and concave (Figure 4C). Therefore, it can be shown that DHA can destroy the normal morphology and structure of mycelium, thus affecting the normal growth of A. alternata.
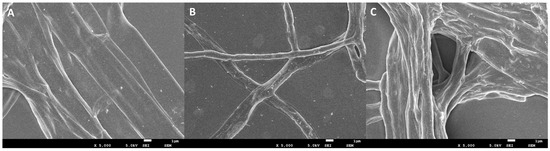
Figure 4.
SEM images. (A) Mycelial morphology of A. alternata cultured under normal conditions (blank control); (B) mycelial morphology of A. alternata after treatment with DMSO (solvent control); (C) mycelial morphology of A. alternata after treatment with 56.015 mg/L (EC50) DHA. Bar = 1 μm.
3.5. Effect of DHA on Extracellular Proteins and Conductivity of A. alternata
Conductivity measurements can be used to assess the changes in the permeability of the cell membrane of pathogenic fungi, the changes in the extracellular conductivity of A. alternata under different concentrations of DHA treatment are shown in Figure 5A. With the increase in the incubation time, the conductivities of the experimental groups showed an increasing tendency, but that of the control group was slowly increased and maintained at a lower level. The conductivity of the EC50-treated group reached a maximum of 1953.67 μS/cm at 72 h of incubation, which was 1.24 times higher than that of the EC30-treated group, and 2.6 times higher than that of the blank control group. Therefore, DHA caused damage to the cell membrane structure of A. alternata, leading to an increase in cell membrane permeability and extravasation of intracellular substances, which resulted in an increase in conductivity.
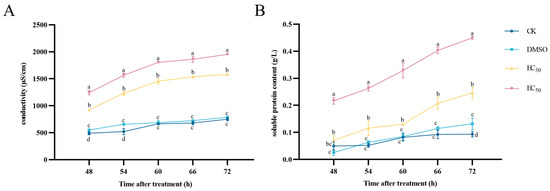
Figure 5.
The effect of dehydroabietic acid at different concentrations (0 mg/mL and DMSO as control, EC30, and EC50) on the extracellular conductivity (A) and soluble protein content (B) of A. alternata. The bars represent the standard error of the mean (n = 3), and the letters a–d indicate significant differences between different concentrations (p < 0.05).
With the increase in treatment time, the extracellular protein content of A. alternata showed a rising trend in all experimental groups, the extracellular protein content of mycelium treated with DHA at concentrations of EC30 and EC50 was higher than that of the blank control group and the DMSO control group, and the higher the concentration was, the higher the extracellular protein content was (Figure 5B). At 72 h of incubation, the protein content of EC30 and EC50 treatment groups were 2.62 and 4.78 times higher than that of the blank control group, so it can be seen that DHA caused the abnormal cell membrane of A. alternata in a concentration-dependent manner, and the leakage of soluble protein occurred.
3.6. Effect of DHA on MDA and H2O2 Contents of A. alternata
The peroxidation of cell membranes occurs, resulting in the production of MDA. The MDA content in the pathogens treated with EC30 and EC50 concentrations of DHA was higher than that in the control group. At 48–60 h of incubation, the MDA content in the treated groups showed an increasing trend, after which it gradually stabilized. The highest content of the EC50-treated group was 22.46 nmol/g at 60 h of incubation, which was 1.39 times higher than that of the EC30-treated group and 2.55 times higher than that of the blank control group (Figure 6A). Therefore, the treatment of DHA led to a large accumulation of MDA in A. alternata, and this result also indicates a deepening of lipid peroxidation of the cell membrane of the A. alternata and a further increase in the oxidative damage of the cell membrane.
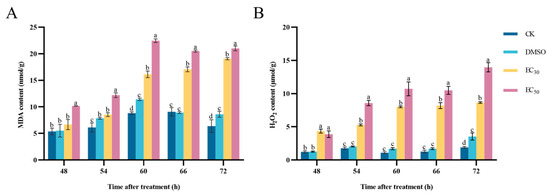
Figure 6.
The effect of dehydroabietic acid at different concentrations (0 mg/mL and DMSO as control, EC30, and EC50) on the MDA content (A) and H2O2 content (B) of A. alternata. The bars represent the standard error of the mean (n = 3), and the letters a–d indicate significant differences between different concentrations (p < 0.05).
H2O2 is one of the most abundant reactive oxygen species (ROS) in the cell, which can be used as a key signaling molecule to regulate the growth and development of organisms and resist adversity stress. The H2O2 content in A. alternata after DHA treatment increased with the increase in incubation time, and the H2O2 content in mycelium reached the maximum at 72 h of incubation, and the H2O2 content in the EC50-treated group was 1.61 times higher than that in the EC30-treated group and 7.29 times higher than that in the blank control group (Figure 6B). It can be seen that the DHA can lead to the increase in H2O2 content in A. alternata, and when H2O2 accumulates to a certain extent it will cause some oxidative damage to the cells.
3.7. Effect of DHA on Antioxidant Enzyme Activities of A. alternata
The results of the determination of the effect of DHA on the enzyme activities of SOD, CAT and POD in A. alternata are shown in Figure 7. In all experimental groups, the activity of SOD enzyme in A. alternata showed an overall increasing trend with the increase in incubation time. The higher the concentration of DHA, the lower the enzyme activity of SOD, and the enzyme activity of the control group was always higher than that of the treated group, and there was a significant difference (p < 0.05). After 72 h of incubation, the enzyme activity of SOD in the blank control group was 1.89 times higher than that in the EC30-treated group and 3.11 times higher than that in the EC50-treated group (Figure 7A).
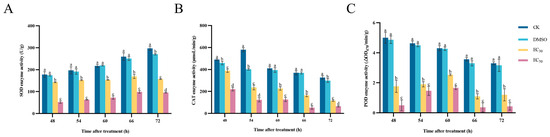
Figure 7.
The effect of dehydroabietic acid at different concentrations (0 mg/mL and DMSO as control, EC30, and EC50) on the SOD activity (A), CAT activity (B) and POD activity (C) of A. alternata. The bars represent the standard error of the mean (n = 3), and the letters a–d indicate significant differences between different concentrations (p < 0.05).
After treatment with DHA at concentrations of EC30 and EC50, the CAT enzyme activity in A. alternata was always lower than that of the control group and was significantly different from the control group (p < 0.05). With the increase in incubation time, the CAT enzyme activity gradually decreased, and when incubated for 66 h, the CAT activity of the EC50-treated group was maximally inhibited to 53.28 μmoL/min/g, and the enzyme activity of the blank control group was 370.19 μmoL/min/g, which was 6.95 times higher than that of the EC50-treated group. (Figure 7B).
With the increase in incubation time, the POD activity in mycelium under the treatment of EC30 and EC50 concentrations showed an increase and then a decrease, and gradually stabilized after 66 h. When incubated for 66 h, EC50-treated group showed the lowest POD activity, which was 89.4% lower compared with the blank control group (Figure 7C). Therefore, it can be seen that DHA can significantly inhibit the activity of antioxidant enzymes in A. alternata, which reduces the pathogen’s own defense ability and thus exerts an inhibitory effect.
3.8. Effect of DHA on Cell-Wall-Degrading Enzymes Activities of A. alternata
The results of the determination of the effect of DHA on the enzyme activities of EG, PG and PL of A. alternata are shown in Figure 8. With the increase in incubation time, the enzyme activity of EG showed an overall decreasing trend, and the EC50-treated group had the lowest EG activity. When incubated for 72 h, the activity of EG at EC50 concentration was 24.75 μg/h/mL, and that of the blank control group was 153.84 μg/h/mL, which was 6.22 times higher than that of the EC50-treated group (Figure 8A).
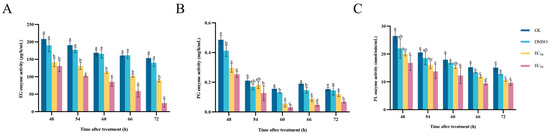
Figure 8.
The effect of dehydroabietic acid at different concentrations (0 mg/mL and DMSO as control, EC30, and EC50) on the EG activity (A), PG activity (B) and PL activity (C) of A. alternata. The bars represent the standard error of the mean (n = 3), and the letters a–d indicate significant differences between different concentrations (p < 0.05).
The higher the concentration of DHA, the stronger the inhibition effect on PG enzyme activity in A. alternata, and the PG activity of the blank control group was always significantly higher than that of the EC50-treated group (p < 0.05). When the incubation time was 60 h, the PG activity of the control group was 4.92 times that of the EC50-treated group (Figure 8B).
Compared with the control, DHA was able to significantly reduce the enzyme activity of PL in a concentration-dependent manner. At 66 h of incubation, the lowest PL activity was 9.49 nmol/min/mL in A. alternata treated with EC50 DHA, and the blank control group was 1.61 times higher than that of the EC50-treated group. After that, the PL activity gradually stabilized, but the PL activity of the DHA-treated group was always lower than that of the control group (Figure 8C). It can be seen that DHA can effectively inhibit the activity of cell-wall-degrading enzymes, destroy the infestation ability of A. alternata, and decrease the pathogenicity, thus reducing the damage of A. alternata to plants.
3.9. Effect of DHA on the Gene Expression of Defense-Related Enzymes in A. alternata
The effect of DHA on genes encoding A. alternata defense-related enzymes was shown in Figure 9. Compared with the control group, genes encoding SOD, POD, and CAT were significantly down-regulated (p < 0.05) by DHA treatment, in which the expression of AaSOD in the control group was 1.32 and 2.86 times higher than that of the EC30 and EC50-treated groups, respectively; the expression of AaPOD in the control group was 1.73 and 3.76 times higher than that of the treated group, respectively; and the expression of AaCAT in the control group was 1.29 and 1.52 times higher than that of the treated group, respectively. The results showed that the expression of the three protective enzyme genes was significantly down-regulated after DHA treatment, but the expression of the gene encoding POD was more obviously regulated, and the lowest expression was found after EC50 concentration DHA treatment.
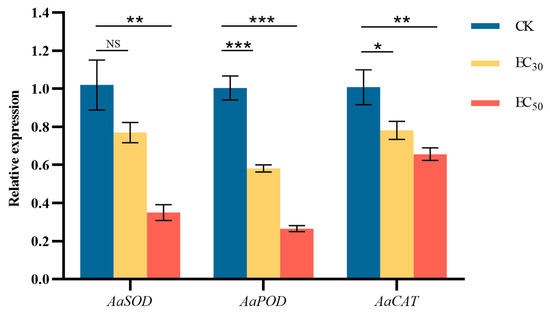
Figure 9.
The effect of dehydroabietic acid at different concentrations (0 mg/mL as control, EC30, and EC50) on the expression of defense-related enzyme genes of A. alternata. The bars represent the standard error of the mean (n = 3), NS indicates that there is no significant difference in the relative expression of AaSOD gene after treatment with EC30 concentrations of DHA (p > 0.05), the asterisks *, ** and *** indicate significant differences in the relative expression of defense-related enzyme genes after DHA treatment (p < 0.05, p < 0.01 and p < 0.001, respectively).
3.10. Effect of DHA on the Gene Expression of Pathogenicity-Related Enzyme in A. alternata
The effect of DHA on genes encoding A. alternata pathogenicity-related enzymes was shown in Figure 10. The difference between the expression of genes encoding PL and PG in A. alternata after treatment with DHA at the concentration of EC50 and that of the control group was highly significant (p < 0.001). The expression of AaPL in the control group was 1.37 and 2.37 times higher than that in the EC30 and EC50-treated groups, respectively; and the expression of AaPG in the control group was 1.68 and 2.18 times higher than that in the treated groups, respectively. The results showed that DHA treatment could significantly inhibit the expression of the two pathogenic enzyme genes, and the higher the concentration of DHA, the lower the relative expression of AaPL and AaPG.
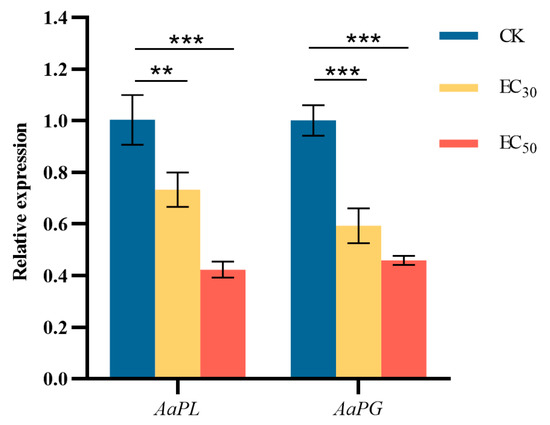
Figure 10.
The effect of dehydroabietic acid at different concentrations (0 mg/mL as control, EC30, and EC50) on the expression of pathogenicity-related enzyme genes of A. alternata. The bars represent the standard error of the mean (n = 3), the asterisks ** and *** indicate significant differences in the relative expression of pathogenicity-related enzyme genes after DHA treatment (p < 0.01 and p < 0.001, respectively).
4. Discussion
Fungal diseases cause huge economic losses to global agriculture and forestry every year. Although the widespread use of chemical pesticides is the main way to control fungal diseases in production, the overuse of chemical pesticides poses a threat to the ecosystem and human health, and the development of plant-derived pesticides will help to reduce the negative impacts of chemical pesticide-induced residues, environmental pollution, and drug resistance [31,32,33]. DHA, a natural product of rosin, can be isolated from resinous acids, which have some antifungal activity in their own right [34]. In the present study, DHA was found to have different levels of fungicidal activity against A. alternata, P. neglecta, B. cinerea, F. oxysporum, and V. mali. Zhai et al. [35] found that DHA and dehydroabietylamine have certain inhibitory activity on Alternaria brassicae, B. cinerea, Fusarium solani, Fusarium graminearum. Chen et al. [36] introduced 1,3,4-thiadiazole-thiazolidinone into the DHA skeleton, and synthesized novel DHA derivatives, which showed good antifungal activity against Gibberella zeae, with a maximum inhibition rate of 91.3%. Therefore, DHA and its derivatives have the potential to be developed into plant-derived pesticides as well as potential lead compounds for new pesticides due to their excellent and broad-spectrum antimicrobial properties.
The cell membrane, which plays a barrier protection function for pathogenic fungi, is the target of the action of a variety of fungicides [37]. When the cell membrane structure of mycelium is damaged by the external environment, the cell membrane is unable to perform the barrier function, the disruption of cellular homeostasis results in the leakage of intracellular components (including cytoplasm, soluble proteins, sugars, and nucleic acids) into the surrounding medium, which leads to an increase in the extracellular conductivity; thus, changes in conductivity are related to leakage of intracellular components and electrolyte efflux from the mycelium. Yuan et al. [38] found that the treatment of Colletotrichum gloeosporioides with rosemary essential oil increased the extracellular conductivity and the leakage of nucleic acids, soluble proteins and soluble reducing sugars of C. gloeosporioides, indicating that the plasma membrane and cell wall of the pathogenic fungi were disrupted, thus releasing the cellular contents. Xue et al. [39] found that total alkaloids and berberine of Coptidis rhizoma led to metabolic disorders in the fungal by increasing the permeability of the cell membrane, and the greater the membrane permeability, the better the fungal inhibition effect. In the present study, the conductivity and soluble protein content were significantly increased in A. alternata after DHA treatment, suggesting that the integrity of A. alternata cell membrane was disrupted, and the contents were extravasated, which ultimately led to a reduction in the physiological functions of A. alternata.
Increased cell membrane permeability caused by lipid peroxidation is one of the main mechanisms of antifungal agents [40]. MDA is one of the main products of lipid peroxidation, so it can be used to assess whether lipid peroxidation is increased in cell membranes [41]. Lin et al. [42] found that extracts of Lysurus mokusin led to oxidative stress in P. neglecta, with a large increase in mycelial MDA content, exacerbating cell membrane peroxidation. ROS are substances produced by oxygen metabolism in the body, which are mainly composed of oxygen-containing substances such as H2O2, OH−, O2−, etc. At low concentrations, ROS perform signaling functions and are involved in the regulation of organismal growth and development, as well as in responding to external stresses. When a large amount of ROS accumulates, cellular components of organisms such as lipids, nucleic acids, and proteins may be subjected to destructive oxidation, ultimately leading to cell death [43,44,45]. Therefore, in the present study, the MDA and H2O2 contents in A. alternata were significantly increased after DHA treatment, which further reflected the degree of cell membrane damage. DHA may have further increased the degree of lipid peroxidation of fungal cell membranes, and caused some oxidative damage, which led to the accumulation of ROS.
Under normal conditions, the production and removal of ROS in organisms are in a state of dynamic equilibrium, and when subjected to external stress, this equilibrium is disrupted in the cells, and the production of large amounts of ROS leads to oxidative stress, loss of cellular function, and ultimately apoptosis or necrosis [46]. All aerobic organisms have an antioxidant system to control ROS accumulation and maintain redox homeostasis [47]. SOD, CAT, and POD, as key enzymes of the antioxidant system, are critical for promoting ROS scavenging [48]. In this study, SOD, CAT, and POD activities were inhibited in A. alternata after treatment with DHA, indicating that the antioxidant capacity of A. alternata was decreased, and a large amount of ROS was accumulated in the organism after A. alternata was subjected to oxidative damage, and DHA inhibited A. alternata by inhibiting the activity of the protective enzymes and decreasing the ability of the pathogenic fungi to scavenge ROS. Similarly to the present study, Bao et al. [49] found that SOD, POD, CAT, SDH, and NAD-MDH activities decreased to varying degrees in Botrytis cinerea after piperine treatment, and mycelial viability was decreased and thus inhibited. Pan et al. [50] suggested that matrine exerts antifungal effects by affecting membrane lipid peroxidation and ROS accumulation and attributed the decrease in SOD, CAT, and POD in Botryosphaeria dothidea to cellular damage. Therefore, in this study, DHA was able to inhibit the activity of protective enzymes in A. alternata, leading to a reduction in the viability of the pathogenic fungus, and this result coincided with the changes in the membrane permeability of the A. alternata cells as described above. The overall trend of SOD showed an upward trend, which is likely to be the response of A. alternata to the oxidative stress caused by DHA.
The plant cell wall is the first barrier of the plant against pathogenic fungi and consists mainly of cellulose and pectin [51]. Pathogenic fungi successfully infect plants by disrupting the host plant cell wall through the secretion of cell-wall-degrading enzymes [52]. Common enzymes such as cellulases and pectinases are cell-wall-degrading enzymes and their activities is positively correlated with the infestation ability of pathogenic fungi [53,54]. Yang et al. [55] found that the higher the enzyme concentration, the more severe the damage of rice leaves after using cell-wall-degrading enzymes to treat rice leaves. Zhou et al. [56] showed that the crude extract of Brevibacillus brevis inhibited both extracellular pectinase and cellulase of Alternaria solani, which has potential in green control of tomato early blight. Li et al. [57] investigated the inhibitory effect of phytic acid on three cell-wall-degrading enzymes (EG, PG, PL) of F. oxysporum, and it had a good control of seedling blight of Pinus sylvestris var. mongolica. In this study, the enzyme activities of EG, PG, and PL in A. alternata were significantly reduced by DHA, indicating that DHA reduced the ability of A. alternata to degrade the cell wall of the host plant, which in turn affected the infestation of the host plant. Therefore, the changes in the incidence area of poplar leaf spot after infestation using P. alba leaves further verified that DHA weakened the pathogenicity of A. alternata.
The regulation of the expression of genes encoding antioxidant enzymes and cell-wall-degrading enzymes in A. alternata by DHA was also investigated. The results showed that the expression of antioxidant enzyme genes AaSOD, AaPOD, and AaCAT as well as cell-wall-degrading enzyme genes AaPL and AaPG in A. alternata were significantly down-regulated in A. alternata. Zhang et al. [58] found that Fusarium pseudograminearum down-regulated SOD and POD genes and up-regulated CAT gene under the action of the biocontrol agent Bacillus velezensis YB-185 by transcriptome analysis, suggesting that F. pseudograminearum may maintain normal cellular functions by enhancing antioxidant responses. However, the reduced scavenging activity of free radicals (ABTS+) was determined, indicating that the overall antioxidant capacity was decreased and insufficient to prevent cellular damage. In this study, the down-regulation of three antioxidant enzyme genes also proved that DHA induced oxidative stress in A. alternata. In the enzyme activity assay, the activity of SOD showed a gradual increase, but the overall was lower than that of the control group, which also confirmed that A. alternata made an antioxidant response to DHA, but the response was insufficient, which still led to the accumulation of free radicals and even cell death. Zhang et al. [59] analyzed the enzyme activity and gene expression of four cell-wall-degrading enzymes of A. alternata during the infestation of melons and found that these index quantities were higher in infestation of 86-1 melons. Combined with the melon disease, it indicated that A. alternata was more aggressive in infesting 86-1 melon, which shows that the activity and gene expression of cell-wall-degrading enzymes can indicate the infestation ability of the pathogen to a certain extent. Yang et al. [60] found that the expression of three cell-wall-degrading enzyme-related genes (PnEG, PnBG and PnPG) were inhibited in P. neglecta after 60 min of action using different concentrations of sodium pheophorbide a treatment. Paccanaro et al. [61] found that the pathogenicity of F. graminearum on wheat was significantly reduced when xyrl, a major regulatory gene encoding PG, was knocked out. Therefore, in this study, it is likely that DHA affects the pathogenicity of A. alternata by inhibiting the expression of the genes for these cell-wall-degrading enzymes, while the changes in the expression of this range of genes confirmed the enzyme activity results.
5. Conclusions
The present study revealed the fungicidal activity and mechanism of DHA against A. alternata and identified the potential of DHA to be developed as a plant-derived antifungal agent. DHA could destroy the morphology of A. alternata mycelium, increase the permeability of the cell membrane, lead to a large leakage of intracellular substances, and at the same time cause oxidative damage to the mycelial cells, inhibit the activities of the defense-related enzymes and pathogenicity-related enzymes in the pathogenic fungi, reduce the viability and pathogenicity of A. alternata, and thus exert antifungal activity. Therefore, it is of practical significance to develop DHA into a new low-toxicity and high-efficiency plant-derived pesticide for the control of poplar leaf spot caused by A. alternata.
Author Contributions
Investigation, Y.-Z.C., Y.-D.Z., C.C. and Q.-E.S.; formal analysis, Y.-Z.C., Y.-D.Z.; validation, C.C. and Q.-E.S.; writing—original draft preparation, Y.-Z.C.; writing—review and editing, Y.-D.Z. and J.Y.; funding acquisition, Y.-Z.C. and J.Y.; methodology, J.Y.; supervision, J.Y. and G.-C.Z.; resources, G.-C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Guizhou Provincial Science and Technology Project (grant number QKHJC[2024]youth041), Guizhou Forestry Scientific Research Project (grant number QLKH[2024]21) and Natural Science Special (Special Post) Research Fund of Guizhou University (grant number [2022]02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DHA | Dehydroabietic acid |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| EG | Endoglucanase |
| PG | Polygalacturonase |
| PL | Pectin lyase |
| PDA | Potato dextrose agar |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| DMSO | Dimethyl sulfoxide |
| SEM | Scanning electron microscopy |
| RNA | Ribonucleic acid |
References
- Xi, B.; Clothier, B.; Coleman, M.; Duan, J.; Hu, W.; Li, D.; Di, N.; Liu, Y.; Fu, J.; Li, J.; et al. Irrigation management in poplar (Populus spp.) plantations: A review. For. Ecol. Manag. 2021, 494, 119330. [Google Scholar]
- Liu, W. Research progress on physiologic and ecologic characteristics of popular. World For. Res. 2010, 23, 50–55. [Google Scholar]
- Zeng, D. Review of current situation of research and control on poplar diseases in China. For. Pest Dis. 2002, 21, 20–26. [Google Scholar]
- Xiang, C.; Zhu, H. Control tactics of poplar diseases in China. J. For. Res. 2000, 11, 252. [Google Scholar]
- Demers, M. Alternaria alternata as endophyte and pathogen. Microbiology 2022, 168, 001153. [Google Scholar]
- Harish, B.N.; Nagesha, S.N.; Ramesh, B.N.; Shyamalamma, S.; Nagaraj, M.S.; Girish, H.C.; Pradeep, C.; Shiva Kumar, K.S.; Tharun Kumar, K.S.; Pavan, S.N.; et al. Molecular characterization and antifungal activity of lipopeptides produced from Bacillus subtilis against plant fungal pathogen Alternaria alternata. BMC Microbiol. 2023, 23, 179. [Google Scholar]
- Thomma, B. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar]
- Feng, L.; Zhao, D.; Jiao, L.; Song, L.; Peng, R. Polygenic identification of Poplar Leaf Blight pathogen and its pathogenicity to different poplar varieties. Mol. Plant Breed. 2022, 20, 6097–6103. [Google Scholar]
- Yang, L.N.; He, M.H.; Ouyang, H.B.; Zhu, W.; Pan, Z.C.; Sui, Q.J.; Shang, L.P.; Zhan, J. Cross-resistance of the pathogenic fungus Alternaria alternata to fungicides with different modes of action. BMC Microbiol. 2019, 19, 205. [Google Scholar]
- Leskovac, A.; Petrovic, S. Pesticide use and degradation strategies: Food safety, challenges and perspectives. Foods 2023, 12, 2709. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, H.; Yue, Y.; Zhou, T.; Zhu, Z.; Wang, C. Integrated transcriptomic and transgenic analyses reveal potential mechanisms of poplar resistance to Alternaria alternata infection. BMC Plant Biol. 2022, 22, 413. [Google Scholar]
- Liu, P.; Liu, X.; Saburi, T.; Kubota, S.; Huang, P.; Wada, Y. Thermal stability evaluation of resin acids and rosin modified resins. Acs Omega 2020, 5, 29102–29109. [Google Scholar] [PubMed]
- Sarria-Villa, R.A.; Gallo-Corredor, J.A.; Benitez-Benitez, R. Characterization and determination of the quality of rosins and turpentines extracted from Pinus oocarpa and Pinus patula resin. Heliyon 2021, 7, e07834. [Google Scholar] [PubMed]
- Wang, X.; Pang, F.; Li, F. Review on the structural modification and biological activities of dehydroabietic acid. Nat. Prod. Res. Dev. 2017, 29, 1621–1625. [Google Scholar]
- Savluchinske-Feio, S.; Nunes, L.; Pereira, P.T.; Silva, A.M.; Roseiro, J.C.; Gigante, B.; Marcelo Curto, M.J. Activity of dehydroabietic acid derivatives against wood contaminant fungi. J. Microbiol. Methods 2007, 70, 465–470. [Google Scholar]
- Kamaya, Y.; Tokita, N.; Suzuki, K. Effects of dehydroabietic acid and abietic acid on survival, reproduction, and growth of the crustacean Daphnia magna. Ecotoxicol. Environ. Saf. 2005, 61, 83–88. [Google Scholar]
- Minami, T.; Wada, S.; Tokuda, H.; Tanabe, G.; Muraoka, O.; Tanaka, R. Potential antitumor-promoting diterpenes from the cones of Pinus luchuensis. J. Nat. Prod. 2002, 65, 1921–1923. [Google Scholar]
- Kim, J.; Kang, Y.G.; Lee, J.Y.; Choi, D.H.; Cho, Y.U.; Shin, J.M.; Park, J.S.; Lee, J.H.; Kim, W.G.; Seo, D.B.; et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol. Cell. Endocrinol. 2015, 412, 216–225. [Google Scholar]
- Söderberg, T.A.; Gref, R.; Holm, S.; Elmros, T.; Hallmans, G. Antibacterial activity of rosin and resin acids in vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 199–205. [Google Scholar]
- Kusumoto, N.; Zhao, T.; Swedjemark, G.; Ashitani, T.; Takahashi, K.; Borg-Karlson, A.-K. Antifungal properties of terpenoids in Picea abies against Heterobasidion parviporum. For. Pathol. 2014, 44, 353–361. [Google Scholar]
- Fallarero, A.; Skogman, M.; Kujala, J.; Rajaratnam, M.; Moreira, V.M.; Yli-Kauhaluoma, J.; Vuorela, P. (+)-Dehydroabietic acid, an abietane-type diterpene, inhibits Staphylococcus aureus biofilms in vitro. Int. J. Mol. Sci. 2013, 14, 12054–12072. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ma, L.; Mo, X.; Lei, L. Advances in structural modification of dehydroabietic phenanthrene ring and activity of derivatives. Yunnan Chem. Technol. 2022, 49, 1–5. (In Chinese) [Google Scholar]
- Gao, Y.; Xu, R.; Gu, S.; Chen, K.; Li, J.; He, X.; Shang, S.; Song, Z.; Song, J. Discovery of natural rosin derivatives containing oxime ester moieties as potential antifungal agents to control tomato gray mold caused by Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 5551–5560. [Google Scholar] [PubMed]
- Li, W.; Yuan, S.; Li, Q.; Sang, W.; Cao, J.; Jiang, W. Methyl p-coumarate inhibits black spot rot on jujube fruit through membrane damage and oxidative stress against Alternaria alternata. Postharvest Biol. Technol. 2018, 145, 230–238. [Google Scholar]
- Li, R.Y.; Wu, X.M.; Yin, X.H.; Long, Y.H.; Li, M. Naturally produced citral can significantly inhibit normal physiology and induce cytotoxicity on Magnaporthe grisea. Pestic. Biochem. Physiol. 2015, 118, 19–25. [Google Scholar]
- Qiu, M.; Wu, C.; Ren, G.; Liang, X.; Wang, X.; Huang, J. Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem. 2014, 155, 105–111. [Google Scholar]
- Liu, B.; Han, J.; Zhang, H.; Li, Y.; An, Y.; Ji, S.; Liu, Z. The regulatory pathway of transcription factor MYB36 from Trichoderma asperellum Tas653 resistant to poplar leaf blight pathogen Alternaria alternata Aal004. Microbiol. Res. 2024, 282, 127637. [Google Scholar] [CrossRef]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [PubMed]
- Yoon, M.; Cha, B.; Kim, J. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [PubMed]
- Gonzalez, M.A. Synthetic derivatives of aromatic abietane diterpenoids and their biological activities. Eur. J. Med. Chem. 2014, 87, 834–842. [Google Scholar] [CrossRef]
- Zhai, Z.; Lv, P.; Zhao, P.; Song, Z.; Shang, S.; Rao, X. Antibacterial activity of dehydroabietyl nitrogen derivatives. Biomass Chem. Eng. 2020, 54, 1–7. (In Chinese) [Google Scholar]
- Chen, N.; Duan, W.; Lin, G.; Liu, L.; Zhang, R.; Li, D. Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers. 2016, 20, 897–905. [Google Scholar]
- Peng, Y.; Chen, B. Role of cell membrane homeostasis in the pathogenicity of pathogenic filamentous fungi. Virulence 2024, 15, 2299183. [Google Scholar]
- Yuan, T.; Hua, Y.; Zhang, D.; Yang, C.; Lai, Y.; Li, M.; Ding, S.; Li, S.; Chen, Y. Efficacy and antifungal mechanism of rosemary essential oil against Colletotrichum gloeosporioides. Forests 2024, 15, 377. [Google Scholar] [CrossRef]
- Xue, D.; Zou, Z.; Chen, B.; Wang, Y.; Wu, H.; Ye, X.; Li, X. Study on membrane injury mechanism of total alkaloids and berberine from Coptidis rhizoma on Aeromonas hydrophila. China J. Chin. Mater. Medica 2015, 40, 1787–1792. [Google Scholar]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yang, J.; Zhang, G.C.; Zhang, X.; Wang, T.; Wu, D. Evaluation of the antifungal activity of Lysurus mokusin extract against Pestalotiopsis neglecta and GC-MS analysis of the active components. J. Plant Pathol. 2021, 103, 1295–1305. [Google Scholar] [CrossRef]
- Martinez-Finley, E.J.; Gavin, C.E.; Aschner, M.; Gunter, T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free. Radic. Biol. Med. 2013, 62, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Serrano, M.; L’Haridon, F.; Tjamos, S.E.; Metraux, J.P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 2015, 112, 54–62. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Na, M.K.; Jang, T.S.; Choi, J.Y.; Lee, S.H.; Bae, K.H. Protective Effect of Some Medicinal Plants on tert-Butyl Hydroperoxide-Induced Oxidative Stress in Human Keratinocytes. Nat. Prod. Sci. 2008, 14, 244–248. [Google Scholar]
- Taheri, P.; Kakooee, T. Reactive oxygen species accumulation and homeostasis are involved in plant immunity to an opportunistic fungal pathogen. J. Plant Physiol. 2017, 216, 152–163. [Google Scholar] [CrossRef]
- Al-Issawi, M.; Rihan, H.Z.; Al-Shmgani, H.; Fuller, M.P. Molybdenum application enhances antioxidant enzyme activity and COR15a protein expression under cold stress in wheat. J. Plant Interact. 2016, 11, 5–10. [Google Scholar] [CrossRef]
- Bao, H.; Li, K.; Zhong, M.; Hao, Z. Inhibitory effect of piperine on Botrytis cinerea and its mechanism. J. China Agric. Univ. 2022, 27, 117–124. [Google Scholar]
- Pan, J.; Hao, X.; Yao, H.; Ge, K.; Ma, L.; Ma, W. Matrine inhibits mycelia growth of Botryosphaeria dothidea by affecting membrane permeability. J. For. Res. 2019, 30, 1105–1113. [Google Scholar] [CrossRef]
- Wan, J.; He, M.; Hou, Q.; Zou, L.; Yang, Y.; Wei, Y.; Chen, X. Cell wall associated immunity in plants. Stress Biol. 2021, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yamamoto, R.; Sakurai, N.; Nakano, Y.; Takeda, T. Fungal hemicellulose-degrading enzymes cause physical property changes concomitant with solubilization of cell wall polysaccharides. Planta 2015, 241, 359–370. [Google Scholar] [PubMed]
- Wang, D.; Kanyuka, K.; Papp-Rupar, M. Pectin: A critical component in cell-wall-mediated immunity. Trends Plant Sci. 2023, 28, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Noutoshi, Y. The function of the plant cell wall in plant-microbe interactions. Plant Physiol. Biochem. 2022, 192, 273–284. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Zheng, L.; Li, M.; Zhou, E. Component analysis, activity measurement and pathogenic effect of cell wall degrading enzymes from rice sheath blight pathogen Rhizoctonia solani. Chin. J. Rice Sci. 2012, 26, 600–606. [Google Scholar]
- Kim, B.; Nguyen, M.V.; Park, J.; Kim, Y.S.; Han, J.W.; Lee, J.-Y.; Jeon, J.; Son, H.; Choi, G.J.; Kim, H. The antifungal activity and mechanism of Brevibacillus brevis on plant pathogenic fungus. J. Plant Prot. 2016, 43, 600–607. [Google Scholar]
- Li, N.; Wu, Y.X.; Zhang, Y.D.; Wang, S.R.; Zhang, G.C.; Yang, J. Phytic acid is a new substitutable plant-derived antifungal agent for the seedling blight of Pinus sylvestris var. mongolica caused by Fusarium oxysporum. Pestic. Biochem. Physiol. 2023, 191, 105341. [Google Scholar]
- Zhang, J.; Zhu, W.; Goodwin, P.H.; Lin, Q.; Xia, M.; Xu, W.; Sun, R.; Liang, J.; Wu, C.; Li, H.; et al. Response of Fusarium pseudograminearum to biocontrol agent Bacillus velezensis YB-185 by phenotypic and transcriptome analysis. J. Fungi 2022, 8, 763. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Y.; Liu, Y.; Tang, M.; Saibihai, Y.; Feng, Z. Gene expression and activity change analysis of cell wall degrading enzyme in postharvest melon infected by Alternaria alternata. Sci. Technol. Food Ind. 2024, 45, 291–299. [Google Scholar]
- Yang, J.; Ji, J.Y.; Zhang, B.W.; Chen, Y.Z.; Wang, S.R.; Zhang, G.C.; Zhang, J. Transcriptome and cell wall degrading enzyme-related gene analysis of Pestalotiopsis neglecta in response to sodium pheophorbide a. Pestic. Biochem. Physiol. 2020, 169, 104639. [Google Scholar] [CrossRef]
- Paccanaro, M.C.; Sella, L.; Castiglioni, C.; Giacomello, F.; Martínez-Rocha, A.L.; D’Ovidio, R.; Schäfer, W.; Favaron, F. Synergistic effect of different plant cell wall-degrading enzymes is important for virulence of Fusarium graminearum. Mol. Plant Microbe Interact. 2017, 30, 886–895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).